Following the Action of Atypical Antipsychotic Clozapine and Possible Prediction of Treatment Response in Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Details of Clozapine Action in the Brain Affected by Schizophrenia
3.2. Increased Cerebral Blood Flow
3.3. Hypofunction of Ribosomal Protein S6
3.4. Inhibition of Kynurenine 3-Monooxygenase (KMO)
3.5. Neurobiology of Clozapine-Resistant Patients
3.6. Treatment of Clozapine-Resistant Patients
3.6.1. Augmentation of Clozapine with Other Antipsychotics
Augmentation with Amisulpride
Augmentation with Paliperidone
3.6.2. Augmentation of Clozapine with Anti-Inflammatory Medication
Minocycline
3.6.3. Augmentation of Clozapine with Other Active Substances
N-Acetylcysteine (NAC)
Memantine
3.6.4. Alternative Therapies in Clozapine Augmentation
Repetitive Transcranial Magnetic Stimulation (rTMS)
Electroconvulsive Therapy (ECT)
3.7. Effect of Clozapine on Cannabis Use in Schizophrenia
3.8. Possible Methods for Predicting Effectiveness of Antipsychotic Treatment
3.8.1. Plasma Levels of Clozapine N-Oxide (CNO) and N-Desmethylclozapine (NDMC)
3.8.2. Serum Levels of Neurotrophins and Glutamate
3.8.3. Morphometry
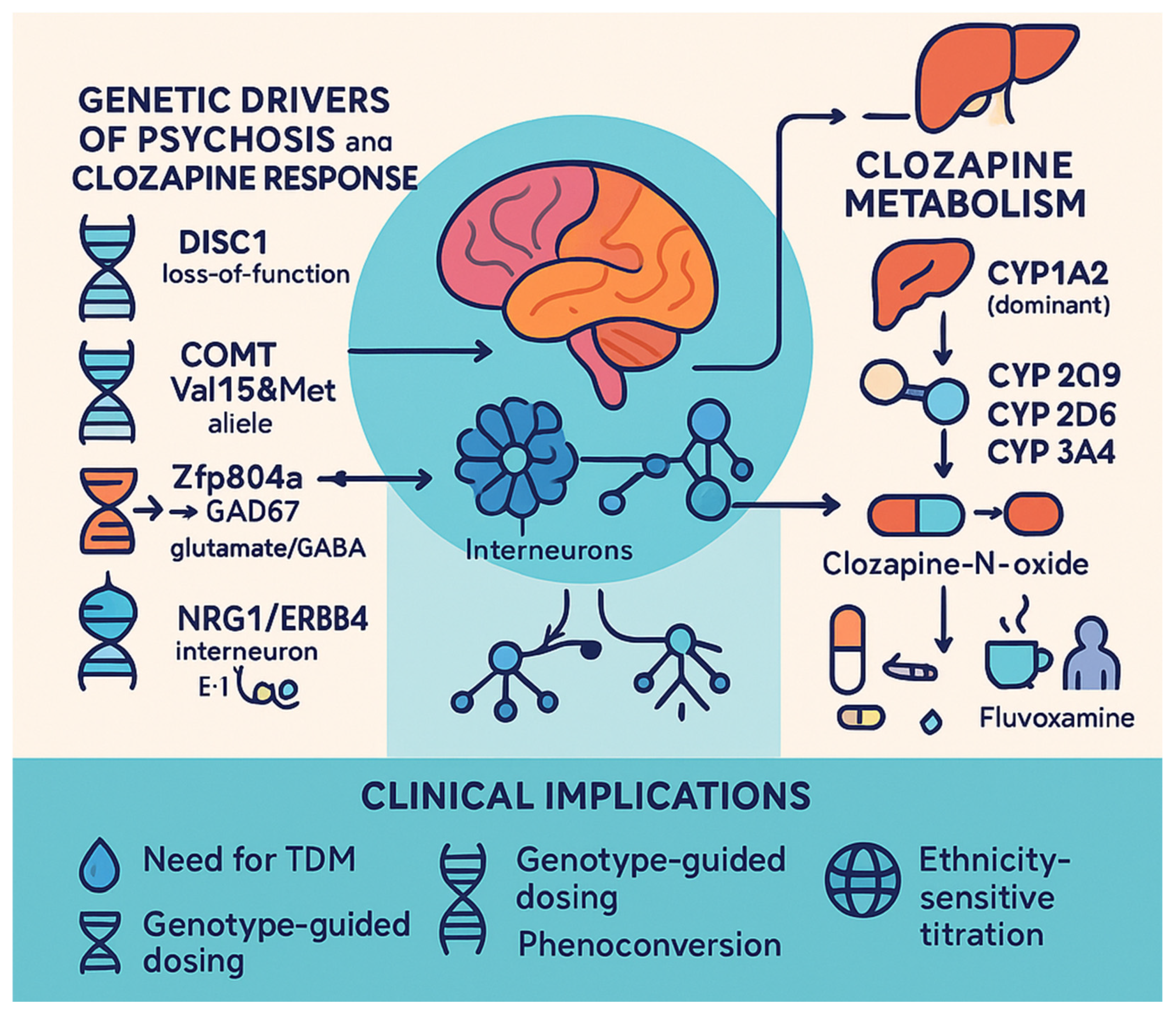
3.8.4. Genetic Testing
3.8.5. Identifying Persistent Negative Symptoms
3.9. Side Effects
3.9.1. Cardiotoxicity of Clozapine
3.9.2. Metabolic Abnormalities
3.9.3. Clozapine-Induced Hypersalivation (CIH)
3.9.4. Blood Disorders
3.9.5. Weight Gain
3.9.6. Clozapine in Pregnancy and the Pediatric Population
3.9.7. Gender Differences in Clozapine Response in Males Versus Females
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Littrell, R.A.; Schneiderhan, M. The neurobiology of schizophrenia. Pharmacotherapy 1996, 16 Pt 2, 143S–147S; discussion 166S–168S. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, T.R.; Dt, C.; Novais, F. Exploring the Hypothesis of a Schizophrenia and Bipolar Disorder Continuum: Biological, Genetic and Pharmacologic Data. CNS Neurol. Disord. Drug Targets 2023, 22, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Nikam, S.S.; Awasthi, A.K. Evolution of schizophrenia drugs: A focus on dopaminergic systems. Curr. Opin. Investig. Drugs 2008, 9, 37–46. [Google Scholar] [PubMed]
- Griffa, A.; Mach, M.; Dedelley, J.; Gutierrez-Barragan, D.; Gozzi, A.; Allali, G.; Grandjean, J.; Van De Ville, D.; Amico, E. Evidence for increased parallel information transmission in human brain networks compared to macaques and male mice. Nat. Commun. 2023, 14, 8216. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef]
- Seeman, P. Clozapine: A fast-off-D2 antipsychotic. ACS Chem. Neurosci. 2014, 5, 24–29. [Google Scholar] [CrossRef]
- Bruno, V.; Valiente-Gómez, A.; Alcoverro, O. Clozapine and Fever: A Case of Continued Therapy with Clozapine. Clin. Neuropharmacol. 2015, 38, 151–153. [Google Scholar] [CrossRef]
- Laux, G. Clozapine in the Treatment of Psychosis. In NeuroPsychopharmacotherapy; Springer International Publishing: Cham, Switzerland, 2022; pp. 1837–1846. [Google Scholar] [CrossRef]
- Krajner, F.; Hadaya, L.; McQueen, G.; Sendt, K.-V.; Gillespie, A.; Avila, A.; Lally, J.; Hedges, E.P.; Diederen, K.; Howes, O.D.; et al. Subcortical volume reduction and cortical thinning 3 months after switching to clozapine in treatment resistant schizophrenia. Schizophrenia 2022, 8, 13. [Google Scholar] [CrossRef]
- Asenjo Lobos, C.; Komossa, K.; Rummel-Kluge, C.; Hunger, H.; Schmid, F.; Schwarz, S.; Leucht, S. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 2010, 11, CD006633. [Google Scholar] [CrossRef]
- Mishra, B.R.; Agrawal, K.; Biswas, T.; Mohapatra, D.; Nath, S.; Maiti, R. Comparison of Acute Followed by Maintenance ECT vs. Clozapine on Psychopathology and Regional Cerebral Blood Flow in Treatment-Resistant Schizophrenia: A Randomized Controlled Trial. Schizophr. Bull. 2022, 48, 814–825. [Google Scholar] [CrossRef]
- Ibarra-Lecue, I.; Diez-Alarcia, R.; Morentin, B.; Meana, J.J.; Callado, L.F.; Uriguen, L. Ribosomal Protein S6 Hypofunction in Postmortem Human Brain Links mTORC1-Dependent Signaling and Schizophrenia. Front. Pharmacol. 2020, 11, 344. [Google Scholar] [CrossRef]
- Kim, S.H.; Yu, H.S.; Park, H.G.; Park, S.; Seo, M.S.; Jeon, W.J.; Ahn, Y.M.; Ha, K.; Shin, S.Y.; Kim, Y.S. Role of MKP-1 (DUSP1) in clozapine-induced effects on the ERK1/2 signaling pathway in the rat frontal cortex. Psychopharmacology 2013, 230, 425–437. [Google Scholar] [CrossRef]
- Pereira, A.; Zhang, B.; Malcolm, P.; Sundram, S. Clozapine regulation of p90RSK and c-Fos signaling via the ErbB1-ERK pathway is distinct from olanzapine and haloperidol in mouse cortex and striatum. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson-Alm, M.; Schwieler, L.; Schwarcz, R.; Goiny, M.; Erhardt, S.; Engberg, G. Importance of kynurenine 3-monooxygenase for spontaneous firing and pharmacological responses of midbrain dopamine neurons: Relevance for schizophrenia. Neuropharmacology 2018, 138, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. N-Methyl-D-aspartate receptors as a target for improved antipsychotic agents: Novel insights and clinical perspectives. Psychopharmacology 2005, 179, 30–53. [Google Scholar] [CrossRef]
- Roerig, J.L. Clozapine augmentation strategies. Ment. Health Clin. 2019, 9, 336–348. [Google Scholar] [CrossRef]
- Schrader, J.M.; Irving, C.M.; Octeau, J.C.; Christian, J.A.; Aballo, T.J.; Kareemo, D.J.; Conti, J.; Camberg, J.L.; Lane, J.R.; Javitch, J.A.; et al. The differential actions of clozapine and other antipsy-chotic drugs on the translocation of dopamine D2 receptors to the cell surface. J. Biol. Chem. 2019, 294, 5604–5615. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, T.; Shmelkov, E.; Felsovalyi, K.; Swetnam, J.; Butler, T.; Malaspina, D.; Shmelkov, S.V. Chemistry-based molecular signature underlying the atypia of clozapine. Transl. Psychiatry 2017, 7, e1036. [Google Scholar] [CrossRef][Green Version]
- Haidary, H.A.; Padhy, R.K. Clozapine; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535399/ (accessed on 12 February 2025).
- Mlambo, R.; Liu, J.; Wang, Q.; Tan, S.; Chen, C. Receptors Involved in Mental Disorders and the Use of Clozapine, Chlorpromazine, Olanzapine, and Aripiprazole to Treat Mental Disorders. Pharmaceuticals 2023, 16, 603. [Google Scholar] [CrossRef]
- Okubo, R.; Okada, M.; Motomura, E. Dysfunction of the NMDA Receptor in the Patho-physiology of Schizophrenia and/or the Pathomechanisms of Treatment-Resistant Schizophrenia. Biomolecules 2024, 14, 1128. [Google Scholar] [CrossRef]
- Nucifora, F.C., Jr.; Mihaljevic, M.; Lee, B.J.; Sawa, A. Clozapine as a Model for Antipsychotic Development. Neurotherapeutics 2017, 14, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, A.; Pylvänäinen, J.; Lehtiniemi, H.; Hirvonen, N.; Corripio, I.; Koponen, H.; Seppälä, J.; Ahmed, A.; Isohanni, M.; Miettunen, J.; et al. Predictors of response to pharmacological treatments in treatment-resistant schizophrenia–A systematic review and meta-analysis. Schizophr. Res. 2021, 236, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chan, H.Y.; Hsu, C.C.; Chen, F.C. Temporal trends in clozapine use at time of discharge among people with schizophrenia at two public psychiatric hospitals in Taiwan, 2006–2017. Sci. Rep. 2020, 10, 17984. [Google Scholar] [CrossRef]
- Zhu, M.H.; Liu, Z.J.; Hu, Q.Y.; Yang, J.Y.; Jin, Y.; Zhu, N.; Huang, Y.; Shi, D.H.; Liu, M.J.; Tan, H.Y.; et al. Amisulpride augmentation therapy improves cognitive performance and psychopathology in clozapine-resistant treatment-refractory schizophrenia: A 12-week randomized, double-blind, placebo-controlled trial. Mil. Med. Res. 2022, 9, 59. [Google Scholar] [CrossRef]
- Barnes, T.R.; Leeson, V.C.; Paton, C.; Marston, L.; Davies, L.; Whittaker, W.; Osborn, D.; Kumar, R.; Keown, P.; Zafar, R.; et al. Amisulpride augmentation in clozapine-unresponsive schizophrenia (AMICUS): A double-blind, placebo-controlled, randomised trial of clinical effectiveness and cost-effectiveness. Health Technol. Assess. 2017, 21, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.R.E.; Leeson, V.; Paton, C.; Marston, L.; Osborn, D.P.; Kumar, R.; Keown, P.; Zafar, R.; Iqbal, K.; Singh, V.; et al. Amisulpride augmentation of clozapine for treatment-refractory schizophrenia: A double-blind, placebo-controlled trial. Ther. Adv. Psychopharmacol. 2018, 8, 185–197. [Google Scholar] [CrossRef]
- Bioque, M.; Parellada, E.; García-Rizo, C.; Amoretti, S.; Fortea, A.; Oriolo, G.; Palau, P.; Boix-Quintana, E.; Safont, G.; Bernardo, M. Clozapine and paliperidone palmitate antipsychotic combination in treatment-resistant schizophrenia and other psychotic disorders: A retrospective 6-month mirror-image study. Eur. Psychiatry 2020, 63, e71. [Google Scholar] [CrossRef]
- Cotes, R.O. Minocycline Augmentation to Clozapine. Emory University. Available online: https://clinicaltrials.gov/study/NCT02124811 (accessed on 27 December 2023).
- Kelly, D.L. Treatment of Schizophrenia With L-tetrahydropalmatine (l-THP): A Novel Dopamine Antagonist with Anti-Inflammatory and Antiprotozoal Activity. University of Maryland, Baltimore. Available online: https://clinicaltrials.gov/study/NCT02118610 (accessed on 27 December 2023).
- Neill, E.; Rossell, S.L.; Yolland, C.; Meyer, D.; Galletly, C.; Harris, A.; Siskind, D.; Berk, M.; Bozaoglu, K.; Dark, F.; et al. N-Acetylcysteine (NAC) in Schizophrenia Resistant to Clozapine: A Double-Blind, Randomized, Placebo-Controlled Trial Targeting Negative Symptoms. Schizophr. Bull. 2022, 48, 1263–1272. [Google Scholar] [CrossRef]
- Veerman, S.R.; Schulte, P.F.; Smith, J.D.; de Haan, L. Memantine augmentation in clozapine-refractory schizophrenia: A randomized, double-blind, placebo-controlled crossover study. Psychol. Med. 2016, 46, 1909–1921. [Google Scholar] [CrossRef]
- Veerman, S.R.; Schulte, P.F.; Deijen, J.B.; de Haan, L. Adjunctive memantine in clozapine-treated refractory schizophrenia: An open-label 1-year extension study. Psychol. Med. 2017, 47, 363–375. [Google Scholar] [CrossRef]
- Wagner, E.; Wobrock, T.; Kunze, B.; Langguth, B.; Landgrebe, M.; Eichhammer, P.; Frank, E.; Cordes, J.; Wölwer, W.; Winterer, G.; et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr. Res. 2019, 208, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Petrides, G.; Malur, C.; Braga, R.J.; Bailine, S.H.; Schooler, N.R.; Malhotra, A.K.; Kane, J.M.; Sanghani, S.; Goldberg, T.E.; John, M.; et al. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: A prospective, randomized study. Am. J. Psychiatry 2015, 172, 52–58. [Google Scholar] [CrossRef]
- Green, A.I. Clozapine for Cannabis Use Disorder in Schizophrenia. Dartmouth-Hitchcock Medical Center. Available online: https://clinicaltrials.gov/study/NCT01639872 (accessed on 27 December 2023).
- Machielsen, M.W.J.; Veltman, D.J.; van den Brink, W.; de Haan, L. Comparing the effect of clozapine and risperidone on cue reactivity in male patients with schizophrenia and a cannabis use disorder: A randomized fMRI study. Schizophr. Res. 2018, 194, 32–38. [Google Scholar] [CrossRef]
- Machielsen, M.W.; Veltman, D.J.; van den Brink, W.; de Haan, L. The effect of clozapine and risperidone on attentional bias in patients with schizophrenia and a cannabis use disorder: An fMRI study. J. Psychopharmacol. 2014, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Fabrazzo, M.; La Pia, S.; Monteleone, P.; Esposito, G.; Pinto, A.; De Simone, L.; Bencivenga, R.; Maj, M. Is the time course of clozapine response correlated to the time course of clozapine plasma levels? A one-year prospective study in drug-resistant patients with schizophrenia. Neuropsychopharmacology 2002, 27, 1050–1055. [Google Scholar] [CrossRef]
- Krivoy, A.; Hochman, E.; Sendt, K.V.; Hollander, S.; Vilner, Y.; Selakovic, M.; Weizman, A.; Taler, M. Association between serum levels of glutamate and neurotrophic factors and response to clozapine treatment. Schizophr. Res. 2018, 192, 226–231. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, H.; Liu, S.; Zhang, B.; Zhao, G.; Zhang, Z.; Li, S.; Li, H.; Yu, X.; Deng, H. Brain Structure Measurements Predict Individualized Treatment Outcome of 12-Week Antipsychotic Monotherapies in First-episode Schizophrenia. Schizophr. Bull. 2023, 49, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Trifu, S.C.; Vlăduţi, A.; Trifu, A.I. Genetic aspects in schizophrenia. Receptoral theories. Metabolic theories. Rom. J. Morphol. Embryol. 2020, 61, 25–32. [Google Scholar] [CrossRef]
- Wulaer, B.; Nagai, T.; Sobue, A.; Itoh, N.; Kuroda, K.; Kaibuchi, K.; Nabeshima, T.; Yamada, K. Repetitive and compulsive-like behaviors lead to cognitive dysfunction in Disc1Δ2-3/Δ2-3 mice. Genes Brain Behav. 2018, 17, e12478. [Google Scholar] [CrossRef]
- Bosia, M.; Lorenzi, C.; Pirovano, A.; Guglielmino, C.; Cocchi, F.; Spangaro, M.; Bramanti, P.; Smeraldi, E.; Cavallaro, R. COMT Val158Met and 5-HT1A-R -1019 C/G polymorphisms: Effects on the negative symptom response to clozapine. Pharmacogenomics 2015, 16, 35–44. [Google Scholar] [CrossRef]
- Rajagopal, V.M.; Rajkumar, A.P.; Jacob, K.S.; Jacob, M. Gene-gene interaction between DRD4 and COMT modulates clinical response to clozapine in treatment-resistant schizophrenia. Pharmacogenetics Genom. 2018, 28, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Wu, S.S.; Wang, P.J.; Zhang, R.; Valenzuela, R.K.; Shang, S.S.; Wan, T.; Ma, J. Schizophrenia-Like Deficits and Impaired Glutamate/Gamma-aminobutyric acid Homeostasis in Zfp804a Conditional Knockout Mice. Schizophr. Bull. 2024, 50, 1411–1426. [Google Scholar] [CrossRef]
- Wan, C.; Xia, Y.; Yan, J.; Lin, W.; Yao, L.; Zhang, M.; Gaisler-Salomon, I.; Mei, L.; Yin, D.M.; Chen, Y. nNOS in Erbb4-positive neurons regulates GABAergic transmission in mouse hippocampus. Cell Death Diseas 2024, 15, 167. [Google Scholar] [CrossRef]
- Mostaid, M.S.; Lee, T.T.; Chana, G.; Sundram, S.; Shannon Weickert, C.; Pantelis, C.; Everall, I.; Bousman, C. Elevated peripheral expression of neuregulin-1 (NRG1) mRNA isoforms in clozapine-treated schizophrenia patients. Transl. Psychiatry 2017, 7, 1280. [Google Scholar] [CrossRef] [PubMed]
- Pardiñas, A.F.; Nalmpanti, M.; Pocklington, A.J.; Legge, S.E.; Medway, C.; King, A.; Jansen, J.; Helthuis, M.; Zammit, S.; MacCabe, J.; et al. Pharmacogenomic Variants and Drug Interactions Identified Through the Genetic Analysis of Clozapine Metabolism. Am. J. Psychiatry 2019, 176, 477–486. [Google Scholar] [CrossRef]
- Ammar, H.; Chadli, Z.; Mhalla, A.; Khouadja, S.; Hannachi, I.; Alshaikheid, M.; Slama, A.; Ben Fredj, N.; Ben Fadhel, N.; Ben Romdhane, H.; et al. Clinical and genetic influencing factors on clozapine pharmacokinetics in Tunisian schizophrenic patients. Pharmacogenomics J. 2021, 21, 551–558. [Google Scholar] [CrossRef]
- Ortega-Vázquez, A.; Mayen-Lobo, Y.G.; Dávila-Ortiz de Montellano, D.J.; Tristán-López, L.; Aviña-Cervantes, C.L.; Ríos, C.; López-López, M.; Monroy-Jaramillo, N. Alcohol intake potentiates clozapine adverse effects associated to CYP1A2*1C in patients with refractory psychosis. Drug Dev. Res. 2021, 82, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Maciukiewicz, M.; Freeman, N.; Huang, E.; Tiwari, A.; Mulsant, B.H.; Pollock, B.G.; Remington, G.; Kennedy, J.L.; Müller, D.J.; et al. Contributions of cholinergic receptor muscarinic 1 and CYP1A2 gene variants on the effects of plasma ratio of clozapine/N-desmethylclozapine on working memory in schizophrenia. J. Psychopharmacol. 2021, 35, 31–39. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, S.; Park, K.; Yu, U.; Jang, Y.; Kim, B.H.; Lee, J.H.; Kim, E. Predictors of clozapine concentration and psychiatric symptoms in patients with schizophrenia. PLoS ONE 2025, 20, e0319037. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Semedo, A.T.; Neri, H.F.D.S.; Vianello, R.P.; Galaviz-Hernández, C.; Sosa-Macías, M.; de Brito, R.B.; Ghedini, P.C. The CYP2C19*2 and CYP2C19*17 Polymorphisms Influence Responses to Clozapine for the Treatment of Schizophrenia. Neuropsychiatr. Dis. Treat. 2020, 16, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Lesche, D.; Mostafa, S.; Everall, I.; Pantelis, C.; Bousman, C.A. Impact of CYP1A2, CYP2C19, and CYP2D6 genotype- and phenoconversion-predicted enzyme activity on clozapine exposure and symptom severity. Pharmacogenomics J. 2020, 20, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.K.; Determan, M.L.; Wright, J.A.; Matey, E.; Leung, J.G. The potential influence of estrogen-containing oral contraception on clozapine metabolism in a patient with known pharmacogenomic status. Ment. Health Clin. 2024, 14, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.; Kane, M. Clozapine Therapy and CYP Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Butcher, N.J.; Fung, W.L.; Fitzpatrick, L.; Guna, A.; Andrade, D.M.; Lang, A.E.; Chow, E.W.; Bassett, A.S. Response to clozapine in a clinically identifiable subtype of schizophrenia. Br. J. Psychiatry 2015, 206, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Bucci, P.; Mucci, A.; van Rossum, I.W.; Aiello, C.; Arango, C.; Baandrup, L.; Buchanan, R.W.; Dazzan, P.; Demjaha, A.; Díaz-Caneja, C.M.; et al. Persistent negative symptoms in recent-onset psychosis: Relationship to treatment response and psychosocial functioning. Eur. Neuropsychopharmacol. 2020, 34, 76–86. [Google Scholar] [CrossRef]
- Curto, M.; Comparelli, A.; Ciavarella, G.M.; Gasperoni, C.; Lionetto, L.; Corigliano, V.; Uccellini, A.; Mancinelli, I.; Ferracuti, S.; Girardi, P.; et al. Impairment of left ventricular function early in treatment with clozapine: A preliminary study. Int. Clin. Psychopharmacol. 2015, 30, 282–289. [Google Scholar] [CrossRef]
- Ishikawa, S.; Yamamura, R.; Hashimoto, N.; Okubo, R.; Sawagashira, R.; Ito, Y.M.; Sato, N.; Kusumi, I. The type rather than the daily dose or number of antipsychotics affects the incidence of hyperglycemic progression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 113, 110453. [Google Scholar] [CrossRef]
- Qurashi, I.; Chu, S.; Husain, N.; Drake, R.J.; Chaudhry, I.; Deakin, J.F. Glycopyrrolate in comparison to hyoscine hydrobromide and placebo in the treatment of hypersalivation induced by clozapine (GOTHIC1): Study protocol for a randomised controlled feasibility study. Trials 2016, 17, 553. [Google Scholar] [CrossRef]
- Kelly, D.L. Biomarker and Safety Study of Clozapine in Patients with Benign Ethnic Neutropenia (BEN). University of Maryland, Baltimore. ClinicalTrials.gov. Identifier: NCT02404155. Available online: https://clinicaltrials.gov/study/NCT02404155 (accessed on 27 December 2023).
- Smith, R.C.; Maayan, L.; Wu, R.; Youssef, M.; Jing, Z.; Sershen, H.; Szabo, V.; Meyers, J.; Jin, H.; Zhao, J.; et al. Betahistine effects on weight-related measures in patients treated with antipsychotic medications: A double-blind placebo-controlled study. Psychopharmacology 2018, 235, 3545–3558. [Google Scholar] [CrossRef]
- Kulkarni, J.; De Chellis, A.; Gilbert, H.; Gavrilidis, E.; Mu, E.; Karimi, L.; Li, Q. Clozapine Safety in Pregnancy: A Clinical Study. Schizophr. Bull. 2024, sbae132. [Google Scholar] [CrossRef]
- Thanigaivel, R.; Bretag-Norris, R.; Amos, A.; McDermott, B. A systematic review of maternal and infant outcomes after clozapine continuation in pregnancy. Int. J. Psychiatry Clin. Pract. 2022, 26, 178–182. [Google Scholar] [CrossRef] [PubMed]
- De Las Cuevas, C.; Sanz, E.J.; de Leon, J. Pharmacovigilance in Action: Utilizing VigiBase Data to Improve Clozapine Safety. Patient Prefer. Adherence 2024, 18, 2261–2280. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.R.; Damkier, P.; Pedersen, L.H.; Fenger-Gron, J.; Mikkelsen, R.L.; Nielsen, R.E.; Linde, V.J.; Knudsen, H.E.; Skaarup, L.; Videbech, P.; et al. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr. Scand. 2015, 445, 1–28. [Google Scholar] [CrossRef]
- Tuncturk, M.; Ermis, C.; Buyuktaskin, D.; Turan, S.; Saglam, Y.; Alarslan, S.; Guler, D.; Sut, E.; Unutmaz, G.; Guzel, A.B.; et al. Electroconvulsive therapy or clozapine for adolescents with treatment-resistant schizophrenia: An explorative analysis on symptom dimensions. Int. J. Psychiatry Clin. Pract. 2023, 27, 257–263. [Google Scholar] [CrossRef]
- Mutlu, C.; Güneş, S.; Turan, S.; Çaml, Ş.E. Clozapine-induced myocarditis: An adolescent girl with very early-onset schizophrenia. Psychiatr. Danub. 2024, 36, 123–125. [Google Scholar] [CrossRef]
- Pimenta de Figueiredo, T.; de Almeida, I.R.; de Freitas, F.A.C.; Kubrusly, C.H.C.; Alvim-Soares Júnior, A.M.; de Miranda, D.M. Beyond the Off-Label: A Systematic Review of What We Know About Clozapine Use for Children. J. Child Adolesc. Psychopharmacol. 2024, 34, e419–e426. [Google Scholar] [CrossRef] [PubMed]
- Wellesley Wesley, E.; Patel, I.; Kadra-Scalzo, G.; Pritchard, M.; Shetty, H.; Broadbent, M.; Segev, A.; Patel, R.; Downs, J.; MacCabe, J.H.; et al. Gender disparities in clozapine prescription in a cohort of treatment-resistant schizophrenia in the South London and Maudsley case register. Schizophr. Res. 2021, 232, 68–76. [Google Scholar] [CrossRef]
- Ferrara, M.; Domenicano, I.; Bellagamba, A.; Zaffarami, G.; Benini, L.; Sorio, C.; Gentili, E.; Srihari, V.H.; Grassi, L. Sex differences in clozapine prescription: Results from an Italian 30-year health records registry. J. Psychiatr. Res. 2025, 185, 215–223. [Google Scholar] [CrossRef]
- Mayerova, M.; Ustohal, L.; Jarkovsky, J.; Pivnicka, J.; Kasparek, T.; Ceskova, E. Influence of dose, gender, and cigarette smoking on clozapine plasma concentrations. Neuropsychiatr. Dis. Treat. 2018, 14, 1535–1543, Erratum in Neuropsychiatr. Dis. Treat. 2019, 2019, 655–656. https://doi.org/10.2147/NDT.S205205. [Google Scholar] [CrossRef]
- Flanagan, R.J.; Hunter, S.; Obee, S.J.; Reeves, S. Clozapine: Dose, Sex, Ethnicity, Smoking Habit, Age, Body Weight, and Plasma Clozapine and N -Desmethylclozapine (Norclozapine) Concentrations in Clinical Practice. J. Clin. Psychopharmacol. 2023, 43, 131–138. [Google Scholar] [CrossRef]
- Anderson, S.G.; Livingston, M.; Couchman, L.; Smith, D.J.; Connolly, M.; Miller, J.; Flanagan, R.J.; Heald, A.H. Sex differences in plasma clozapine and norclozapine concentrations in clinical practice and in relation to body mass index and plasma glucose concentrations: A retrospective survey. Ann. Gen. Psychiatry 2015, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Alberich, S.; Fernández-Sevillano, J.; González-Ortega, I.; Usall, J.; Sáenz, M.; González-Fraile, E.; González-Pinto, A. A systematic review of sex-based differences in effectiveness and adverse effects of clozapine. Psychiatry Res. 2019, 280, 112506. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.; Spangaro, M.; Buonocore, M.; Bechi, M.; Cocchi, F.; Guglielmino, C.; Bianchi, L.; Sapienza, J.; Agostoni, G.; Mastromatteo, A.; et al. Clozapine tolerability in Treatment Resistant Schizophrenia: Exploring the role of sex. Psychiatry Res. 2021, 297, 113698. [Google Scholar] [CrossRef] [PubMed]
- Asif, S.; Pattnaik, J.I.; Debata, I.; Das, S.K.; Ravan, J.R.; Samantaray, S.; Swain, R. Predictors of clozapine efficacy in treatment-resistant schizophrenia: A cross-sectional analysis of sociodemographic, clinical, biochemical, and electrophysiological EEG changes. Arch. Ment. Health 2024, 25, 102–106. [Google Scholar] [CrossRef]
- van der Horst, M.Z.; Meijer, Y.; de Boer, N.; Guloksuz, S.; Hasan, A.; Siskind, D.; Wagner, E.; CLOZIN Consortium; Okhuijsen-Pfeifer, C.; Luykx, J.J. Comprehensive dissection of prevalence rates, sex differences, and blood level-dependencies of clozapine-associated adverse drug reactions. Psychiatry Res. 2023, 330, 115539. [Google Scholar] [CrossRef]
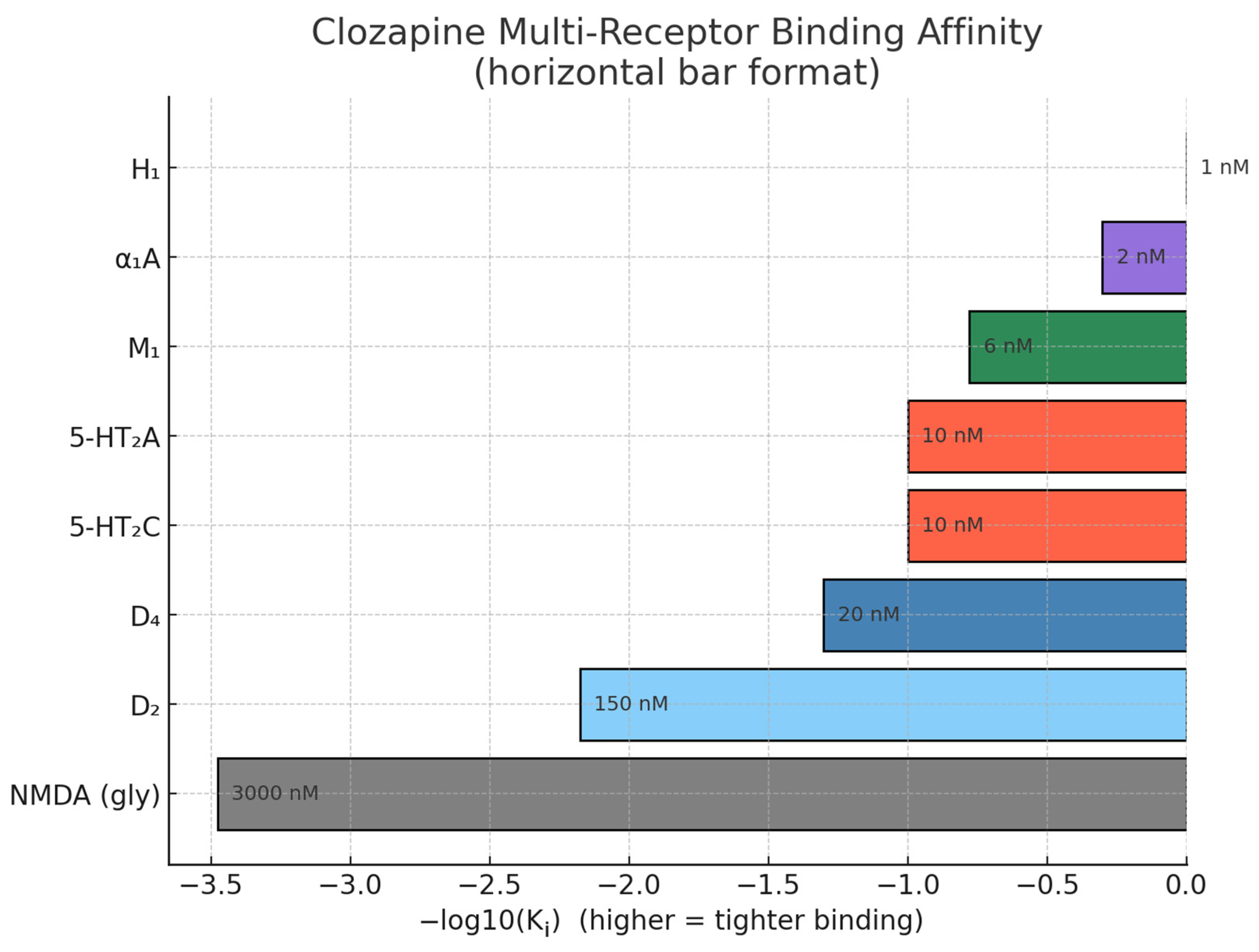
| Receptor/Target | Clozapine Action | Effect/Implication | Reference |
|---|---|---|---|
| Dopamine receptors (D1–D5; high affinity D4) | Competitive antagonist (low-affinity, fast-off at D2; higher affinity at D4) | Modulation of cognitive, motor, and behavioural functions and underlies antipsychotic efficacy with fewer extrapyramidal effects | [18,19] |
| Serotonin receptors (5-HT2A/2C) | Antagonist | Augments dopamine modulation in cortex/limbic areas, contributing to efficacy for negative symptoms | [19] |
| Muscarinic receptors (M1, M2, M3, M5) | Antagonist | Responsible for anticholinergic side effects and pro-cognitive metabolite activity | [19,20] |
| Histamine H1 receptor | Antagonist | May cause sedation and weight gain | [20] |
| Alpha-1 adrenergic receptor | Antagonist | Impacts vascular tone, may cause orthostatic hypotension | [20] |
| NMDA receptor | Functional (indirect) partial agonism via metabolite NDMC; clinical interest in glycine-site augmentation | Potentially reduces dopaminergic hyperactivity | [16] |
| mTOR pathway (ribosomal protein S6) | Regulatory modulation of protein synthesis/neuroplasticity | Impacts protein synthesis, cell survival, neuroplasticity | [19] |
| Kynurenine 3-Monooxygenase (KMO) | Inhibition | Reduces dopamine neuron hyperactivity via NMDA/α7 nicotinic modulation | [15] |
| Regional cerebral blood flow | Increase (esp. right inferior frontal gyrus and other cortical areas) | Improved perfusion in specific brain areas to reduced impulsivity/aggression and potential structural recovery | [11] |
| Augmentation Agent/Therapy | Mechanism/Role | Effect | Considerations |
|---|---|---|---|
| Amisulpride | D2/D3 antagonist | Improves negative and cognitive symptoms | Risk of cardiac side effects |
| Paliperidone | D2 antagonist, serotonin antagonist | Effective, safer compared to amisulpride | Requires more randomized trials |
| Minocycline | Anti-inflammatory, neuroprotective | Reduces inflammation, well-tolerated | Small sample studies, needs replication |
| N-Acetylcysteine (NAC) | Antioxidant, glutathione precursor | Minimal improvement in schizophrenia symptoms | Limited efficacy observed |
| Memantine | NMDA receptor antagonist | Improves memory, executive function, negative symptoms | Promising, well-tolerated |
| Repetitive Transcranial Magnetic Stimulation (rTMS) | Non-invasive brain stimulation | Enhances antipsychotic efficacy, reduces psychotic symptoms | Requires more research |
| Electroconvulsive Therapy (ECT) | Electrical stimulation therapy | Effective in treatment-resistant cases | Long-term efficacy needs more studies |
| Predictive Factor | Details/Indicators | Relevance to Response |
|---|---|---|
| Plasma Levels | Lower clozapine and clozapine N-oxide; higher N-desmethylclozapine in non-responders | May predict poor response |
| Serum Neurotrophins and Glutamate | Higher BDNF, VEGF, NGF, GDNF, and glutamate levels in responders | Possible biomarkers |
| Brain Morphometry | Grey matter volume in PFC, parietal cortex, amygdala | May predict symptom progression and drug response |
| Polygenic Risk Score | High PRS linked to clozapine prescription | May assist in early intervention strategies |
| Genetic Testing | Specific polymorphisms in DISC1, COMT, MAO-A/B, GAD67, NRG1; 22q11.2 deletion | Potential influence on responsiveness |
| Persistent Negative Symptoms (PNSs) | Poor response when present | Early identification aids treatment planning |
| Adverse Effect | Cause/Mechanism | Mitigation Strategy |
|---|---|---|
| Cardiotoxicity | Impaired left ventricular function | Monitoring, dose adjustment |
| Hyperglycemia | Blockade of H1, M1, M3, 5-HT2C receptors | Metabolic monitoring, insulin-sensitizing agents |
| Hypersalivation (CIH) | Muscarinic receptor involvement | Glycopyrrolate preferred over hyoscine hydrobromide |
| Agranulocytosis/Neutropenia | Possibly related to genetic polymorphisms (DARC gene in AA population) | Monitoring ANC, cautious use in at-risk groups |
| Weight Gain | Strong H1 receptor antagonism | Betahistine (H1 agonist, H3 antagonist) administration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Năstase, M.-G.; Vasile, A.I.; Pietreanu, A.C.; Trifu, S. Following the Action of Atypical Antipsychotic Clozapine and Possible Prediction of Treatment Response in Schizophrenia. Life 2025, 15, 830. https://doi.org/10.3390/life15060830
Năstase M-G, Vasile AI, Pietreanu AC, Trifu S. Following the Action of Atypical Antipsychotic Clozapine and Possible Prediction of Treatment Response in Schizophrenia. Life. 2025; 15(6):830. https://doi.org/10.3390/life15060830
Chicago/Turabian StyleNăstase, Mihai-Gabriel, Antonia Ioana Vasile, Arina Cipriana Pietreanu, and Simona Trifu. 2025. "Following the Action of Atypical Antipsychotic Clozapine and Possible Prediction of Treatment Response in Schizophrenia" Life 15, no. 6: 830. https://doi.org/10.3390/life15060830
APA StyleNăstase, M.-G., Vasile, A. I., Pietreanu, A. C., & Trifu, S. (2025). Following the Action of Atypical Antipsychotic Clozapine and Possible Prediction of Treatment Response in Schizophrenia. Life, 15(6), 830. https://doi.org/10.3390/life15060830






