Osteochondral Alterations in Patients Treated with Total Knee Arthroplasty Due to Rheumatoid Arthritis and Primary Osteoarthritis: Cross-Sectional Study with Focus on Elucidating Effects of Knee Malalignment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection Process
2.2. Bone Sampling Process and Storage
2.3. High-Resolution 3D Histomorphometric Assessment of Knee Osteochondral Properties
2.4. Statistical Analysis
3. Results
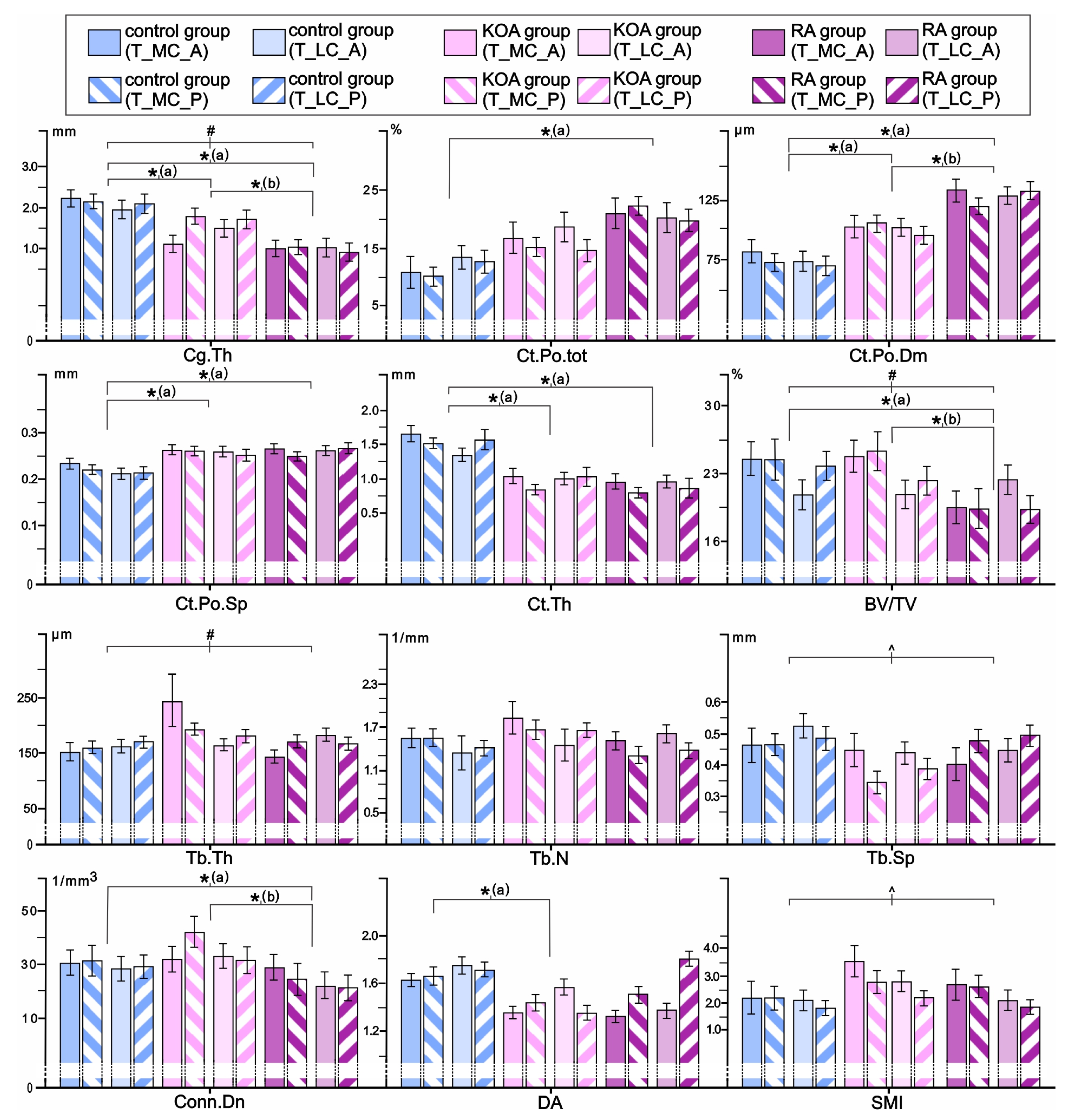
3.1. Comparisons of Femoral Osteochondral Microstructure in Individuals with RA, Individuals with KOA, and Controls
3.2. Inter-Site Differences in Femoral Osteochondral Parameters as Indicators of Medial-to-Lateral Load Distribution in the Knee Joint of Individuals with RA and KOA Depending on Knee Alignment
3.3. Comparisons of Tibial Osteochondral Microstructure in Individuals with RA, Individuals with KOA, and Controls
3.4. Inter-Site Differences in Tibial Osteochondral Microstructural Parameters as Indicators of Medial-to-Lateral Load Distribution in the Knee Joint of Individuals with RA and KOA Depending on Knee Alignment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KOA | Knee osteoarthritis |
| RA | Rheumatoid arthritis of the knee |
| SB | Subchondral bone |
| SBP | Subchondral bone plate |
| STB | Subchondral trabecular bone |
| TKA | Total knee arthroplasty |
| F | Female (women) |
| M | Male (men) |
| BMI | Body mass index |
| HKA | Hip–knee–ankle angle |
| N.A. | Not assessed (not applicable) |
| CAD | Coronary artery disease |
| PVD | Peripheral vascular disease |
| HLP | Hyperlipidemia |
| T2DM | Type 2 diabetes mellitus |
| HT | Hypothyroidism |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| F_MC | Medial femoral condyle |
| F_LC | Lateral femoral condyle |
| T_MC_A | The anterior part of the medial tibial condyle |
| T_MC_P | The posterior part of the medial tibial condyle |
| T_LC_A | The anterior part of the lateral tibial condyle |
| T_LC_P | The posterior part of the lateral tibial condyle |
| Ct.Po.tot | Total cortical porosity |
| Ct.Po.Dm | Cortical pore diameter |
| Ct.Po.Sp | Cortical pore separation |
| Ct.Th | Cortical thickness |
| BV/TV | Bone volume fraction |
| Tb.Th | Trabecular thickness |
| Tb.N | Trabecular number |
| Tb.Sp | Trabecular separation |
| Conn.Dn | Connectivity density |
| FD | Fractal dimension |
| DA | Degree of anisotropy |
| SMI | Structure model index |
| Cg.Th | Cartilage thickness |
| ANCOVA | Analysis of covariance |
| SD | Standard deviation |
| SE | Standard error |
References
- Holzer, L.A.; Kraiger, M.; Talakic, E.; Fritz, G.A.; Avian, A.; Hofmeister, A.; Leithner, A.; Holzer, G. Microstructural Analysis of Subchondral Bone in Knee Osteoarthritis. Osteoporos. Int. 2020, 31, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral Bone in Osteoarthritis: Insight into Risk Factors and Microstructural Changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Liu, D.; Chen, P.; Wang, S.; Li, F.; Chen, Q.; Lv, S.; Li, F.; Chen, C.; et al. Pathological Progression of Osteoarthritis: A Perspective on Subchondral Bone. Front. Med. 2024, 18, 237–257. [Google Scholar] [CrossRef]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The Basic Science of the Subchondral Bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef]
- Evans, L.A.E.; Pitsillides, A.A. Structural Clues to Articular Calcified Cartilage Function: A Descriptive Review of This Crucial Interface Tissue. J. Anat. 2022, 241, 875–895. [Google Scholar] [CrossRef]
- Luo, P.; Yuan, Q.L.; Yang, M.; Wan, X.; Xu, P. The Role of Cells and Signal Pathways in Subchondral Bone in Osteoarthritis. Bone Jt. Res. 2023, 12, 536–545. [Google Scholar] [CrossRef]
- Li, Q.; Miramini, S.; Smith, D.W.; Gardiner, B.S.; Zhang, L. Osteochondral Junction Leakage and Cartilage Joint Lubrication. Comput. Methods Programs Biomed. 2023, 230, 107353. [Google Scholar] [CrossRef]
- Han, X.; Cui, J.; Xie, K.; Jiang, X.; He, Z.; Du, J.; Chu, L.; Qu, X.; Ai, S.; Sun, Q.; et al. Association between Knee Alignment, Osteoarthritis Disease Severity, and Subchondral Trabecular Bone Microarchitecture in Patients with Knee Osteoarthritis: A Cross-Sectional Study. Arthritis Res. Ther. 2020, 22, 203. [Google Scholar] [CrossRef]
- Li, G.; Ma, Y.; Cheng, T.S.; Landao-Bassonga, E.; Qin, A.; Pavlos, N.J.; Zhang, C.; Zheng, Q.; Zheng, M.H. Identical Subchondral Bone Microarchitecture Pattern with Increased Bone Resorption in Rheumatoid Arthritis as Compared to Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 2083–2092. [Google Scholar] [CrossRef]
- Schadow, J.E.; Maxey, D.; Smith, T.O.; Finnilä, M.A.J.; Manske, S.L.; Segal, N.A.; Wong, A.K.O.; Davey, R.A.; Turmezei, T.; Stok, K.S. Systematic Review of Computed Tomography Parameters Used for the Assessment of Subchondral Bone in Osteoarthritis. Bone 2024, 178, 116948. [Google Scholar] [CrossRef] [PubMed]
- Colyn, W.; Azari, F.; Bellemans, J.; van Lenthe, G.H.; Scheys, L. Microstructural Adaptations of the Subchondral Bone Are Related to the Mechanical Axis Deviation in End Stage Varus OA Knees. Eur. Cell Mater. 2023, 45, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Kasaeian, A.; Roemer, F.W.; Ghotbi, E.; Ibad, H.A.; He, J.; Wan, M.; Zbijewski, W.B.; Guermazi, A.; Demehri, S. Subchondral Bone in Knee Osteoarthritis: Bystander or Treatment Target? Skelet. Radiol. 2023, 52, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Rapagna, S.; Roberts, B.C.; Solomon, L.B.; Reynolds, K.J.; Thewlis, D.; Perilli, E. Relationships between Tibial Articular Cartilage, in Vivo External Joint Moments and Static Alignment in End-Stage Knee Osteoarthritis: A Micro-CT Study. J. Orthop. Res. 2022, 40, 1125–1134. [Google Scholar] [CrossRef]
- Roberts, B.C.; Solomon, L.B.; Mercer, G.; Reynolds, K.J.; Thewlis, D.; Perilli, E. Relationships between in Vivo Dynamic Knee Joint Loading, Static Alignment and Tibial Subchondral Bone Microarchitecture in End-Stage Knee Osteoarthritis. Osteoarthr. Cartil. 2018, 26, 547–556. [Google Scholar] [CrossRef]
- Renault, J.B.; Carmona, M.; Tzioupis, C.; Ollivier, M.; Argenson, J.N.; Parratte, S.; Chabrand, P. Tibial Subchondral Trabecular Bone Micromechanical and Microarchitectural Properties Are Affected by Alignment and Osteoarthritis Stage. Sci. Rep. 2020, 10, 3975. [Google Scholar] [CrossRef]
- Reina, N.; Cavaignac, E.; Pailhé, R.; Pailliser, A.; Bonnevialle, N.; Swider, P.; Laffosse, J.M. BMI-Related Microstructural Changes in the Tibial Subchondral Trabecular Bone of Patients with Knee Osteoarthritis. J. Orthop. Res. 2017, 35, 1653–1660. [Google Scholar] [CrossRef]
- Kaspiris, A.; Hadjimichael, A.C.; Lianou, I.; Iliopoulos, I.D.; Ntourantonis, D.; Melissaridou, D.; Savvidou, O.D.; Papadimitriou, E.; Chronopoulos, E. Subchondral Bone Cyst Development in Osteoarthritis: From Pathophysiology to Bone Microarchitecture Changes and Clinical Implementations. J. Clin. Med. 2023, 12, 815. [Google Scholar] [CrossRef]
- Johnston, J.D.; Burnett, W.D.; Kontulainen, S.A. Subchondral Bone Features and Mechanical Properties as Biomarkers of Osteoarthritis. Expo. Health 2016, 2016, 1–24. [Google Scholar] [CrossRef]
- Rapagna, S.; Roberts, B.C.; Solomon, L.B.; Reynolds, K.J.; Thewlis, D.; Perilli, E. Tibial Cartilage, Subchondral Bone Plate and Trabecular Bone Microarchitecture in Varus- and Valgus-Osteoarthritis versus Controls. J. Orthop. Res. 2021, 39, 1988–1999. [Google Scholar] [CrossRef]
- Roberts, B.C.; Thewlis, D.; Solomon, L.B.; Mercer, G.; Reynolds, K.J.; Perilli, E. Systematic Mapping of the Subchondral Bone 3D Microarchitecture in the Human Tibial Plateau: Variations with Joint Alignment. J. Orthop. Res. 2017, 35, 1927–1941. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Wang, Z.; Zhang, X.N.; Xu, J.; Hu, Y.C. Rheumatoid Arthritis of Knee Joints: MRI–Pathological Correlation. Orthop. Surg. 2018, 10, 247–254. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Si, L.; Shen, H.; Wang, Q.; Yao, W. Relationship between Subchondral Bone Microstructure and Articular Cartilage in the Osteoarthritic Knee Using 3T MRI. J. Magn. Reson. Imaging 2018, 48, 669–679. [Google Scholar] [CrossRef]
- Andjelic, U.; Djuric, M.; Jadzic, J. Methodological Diversity in Micro-CT Evaluation of Bone Micro-Architecture: Importance for Inter-Study Comparability. Med. Res. 2024, 57, 13–21. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Jadzic, J.; Mijucic, J.; Nikolic, S.; Djuric, M.; Djonic, D. The Comparison of Age- and Sex-Specific Alteration in Pubic Bone Microstructure: A Cross-Sectional Cadaveric Study. Exp. Gerontol. 2021, 150, 111375. [Google Scholar] [CrossRef]
- Jadzic, J.; Andjelic, U.; Milovanovic, P.; Zivkovic, V.; Nikolic, S.; Djonic, D.; Djuric, M. Improved Femoral Micro-Architecture in Adult Male Individuals with Overweight: Fracture Resistance Due to Regional Specificities. Int. J. Obes. 2023, 48, 202–208. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, F.; Lin, F.; Zhang, F.; Zhang, S. Bone Quality, and the Combination and Penetration of Cement-Bone Interface a Comparative Micro-Ct Study of Osteoarthritis and Rheumatoid Arthritis. Medicine 2018, 97, e11987. [Google Scholar] [CrossRef]
- Simon, D.; Mascarenhas, R.; Saltzman, B.M.; Rollins, M.; Bach, B.R.; MacDonald, P. The Relationship between Anterior Cruciate Ligament Injury and Osteoarthritis of the Knee. Adv. Orthop. 2015, 2015, 928301. [Google Scholar] [CrossRef]
- Huang, C.C.; Jiang, C.C.; Hsieh, C.H.; Tsai, C.J.; Chiang, H. Local Bone Quality Affects the Outcome of Prosthetic Total Knee Arthroplasty. J. Orthop. Res. 2016, 34, 240–248. [Google Scholar] [CrossRef]
- Zhang, A.R.; Cheng, Q.H.; Yang, Y.Z.; Yang, X.; Zhang, Z.Z.; Guo, H.Z. Meta-Analysis of Outcomes after Total Knee Arthroplasty in Patients with Rheumatoid Arthritis and Osteoarthritis. Asian J. Surg. 2024, 47, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, F.; Zhang, L.; Song, X.; Yu, X.; Zhang, H.; Liu, P.; Zhou, S. A Systematic Review and Meta-Analysis Comparing Outcomes Following Total Knee Arthroplasty for Rheumatoid Arthritis versus for Osteoarthritis. BMC Musculoskelet. Disord. 2023, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cui, J.; Chu, L.; Zhang, W.; Xie, K.; Jiang, X.; He, Z.; Du, J.; Ai, S.; Sun, Q.; et al. Abnormal Subchondral Trabecular Bone Remodeling in Knee Osteoarthritis under the Influence of Knee Alignment. Osteoarthr. Cartil. 2022, 30, 100–109. [Google Scholar] [CrossRef]
- Robbins, S.M.; Abram, F.; Boily, M.; Pelletier, J.P.; Martel-Pelletier, J. Relationship between Alignment and Cartilage Thickness in Patients with Non-Traumatic and Post-Traumatic Knee Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 630–637. [Google Scholar] [CrossRef]
- Takeda, R.; Matsumoto, T.; Omata, Y.; Inui, H.; Taketomi, S.; Nagase, Y.; Nishikawa, T.; Oka, H.; Tanaka, S. Changes in Knee Joint Destruction Patterns among Patients with Rheumatoid Arthritis Undergoing Total Knee Arthroplasty in Recent Decades. Clin. Rheumatol. 2023, 42, 2341–2352. [Google Scholar] [CrossRef]
- Ter Voert, C.E.M.; Kour, R.Y.N.; van Teeffelen, B.C.J.; Ansari, N.; Stok, K.S. Contrast-Enhanced Micro-Computed Tomography of Articular Cartilage Morphology with Ioversol and Iomeprol. J. Anat. 2020, 237, 1062–1071. [Google Scholar] [CrossRef]
- Jadzic, J.; Zagorac, S.; Djuric, M.; Djonic, D. Hip Structure Analysis and Femoral Osteodensitometry in Aged Postmenopausal Women with Hip Osteoarthritis and Femoral Neck Fracture. Int. Orthop. 2022, 46, 2747–2755. [Google Scholar] [CrossRef]
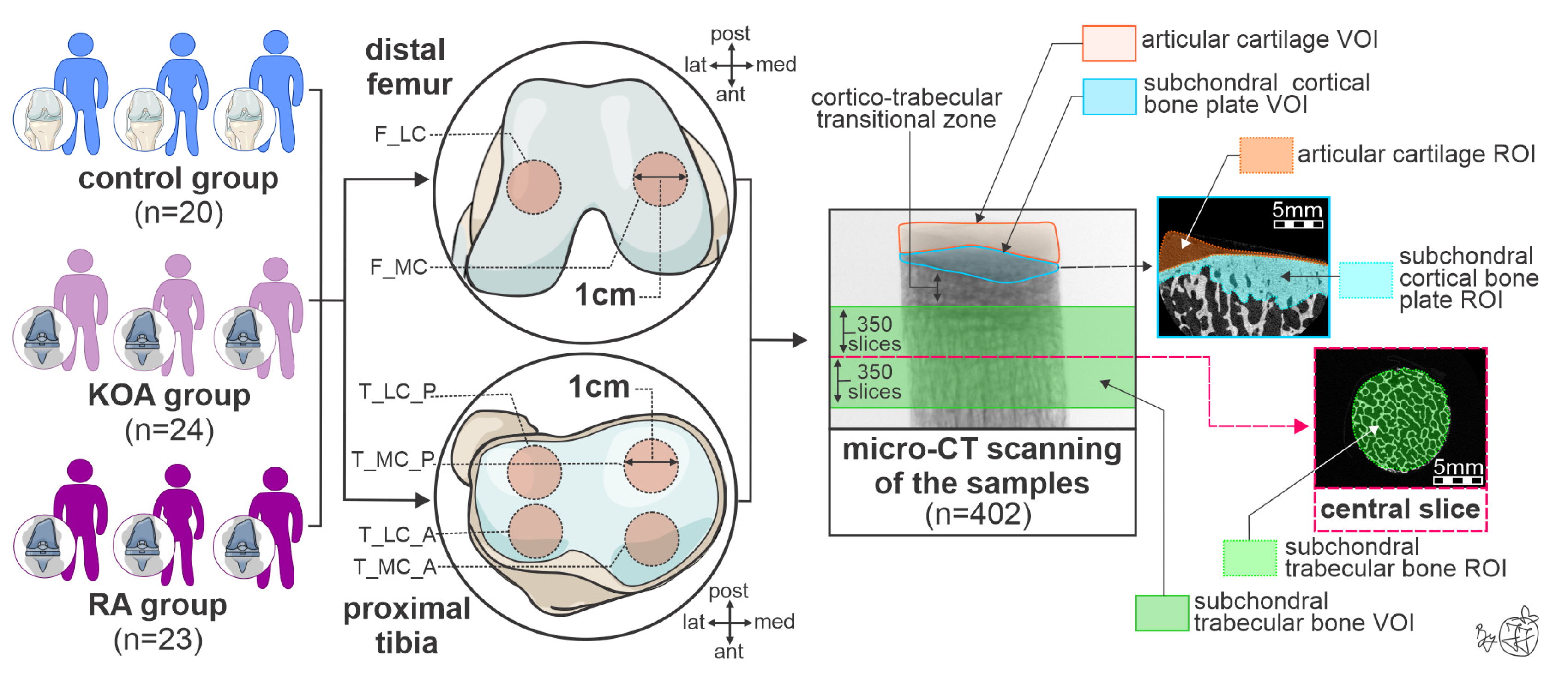

| Control Group (n = 20) | KOA Group (n = 24) | RA Group (n = 23) | |
|---|---|---|---|
| Basic anthropometric characteristics | |||
| Age (years) | 67 ± 11 | 71 ± 9 | 61 ± 10 |
| Height (cm) | 172 ± 10 | 169 ± 10 | 163 ± 8 |
| Weight (kg) | 75 ± 22 | 85 ± 14 | 74 ± 13 |
| BMI (kg/m2) | 25.1 ± 6.8 | 29.8 ± 4.3 | 28.1 ± 5.1 |
| Sex | 12/20 F 8/20 M | 17/24 F 7/24 M | 19/23 F 4/23 M |
| Radiographic characteristics | |||
| Kellgren–Lawrence classification | N.A. | 0/24 grade 1 1/24 grade 2 10/24 grade 3 13/24 grade 4 | 0/23 grade 1 1/23 grade 2 8/23 grade 3 14/23 grade 4 |
| Varus knee alignment | N.A. | 14/24 | 8/23 |
| Valgus knee alignment | N.A. | 10/24 | 15/23 |
| HKA angle (°) | N.A. | −2.27 ± 9.19 | 4.42 ± 12.45 |
| Chronic comorbidities | |||
| Hypertension | 16/20 | 23/24 | 13/23 |
| CAD | 13/20 | 4/24 | 2/23 |
| PVD | 4/20 | 4/24 | 1/23 |
| HLP | N.A. | 3/24 | 3/23 |
| T2DM | 3/20 | 7/24 | 5/23 |
| Thyroid disorders | 0/20 | 5/24 HT | 3/23 HT |
| Antirheumatic pharmacotherapy | |||
| Glucocorticoids | N.A. | N.A. | 9/23 |
| Methotrexate and other DMARDs | N.A. | N.A. | 20/23 |
| Biological treatment | N.A. | N.A. | 6/23 |
| Skeletal Site | KOA Group Valgus Knee (Mean ± SE) | KOA Group Varus Knee (Mean ± SE) | RA Group Valgus Knee (Mean ± SE) | RA Group Varus Knee (Mean ± SE) | p-Value | |
|---|---|---|---|---|---|---|
| Cg.Th (mm) | F_MC | 0.67 ± 0.12 | 0.46 ± 0.11 | 0.55 ± 0.11 | 0.44 ± 0.13 | subgroup-p = 0.169 site-p = 0.471 site × subgroup-p = 0.003 |
| F_LC | 0.93 ± 0.14 | 1.08 ± 0.12 | 0.39 ± 0.13 | 0.96 ± 0.15 | ||
| Medial-to-lateral ratio of Cg.Th | 0.72 ± 0.23 | 0.43 ± 0.22 | 1.41 ± 0.19 | 0.46 ± 0.26 | subgroup-p = 0.107 | |
| Ct.Po.tot (%) | F_MC | 19.73 ± 3.48 | 19.86 ± 2.99 | 22.58 ± 3.18 | 28.72 ± 3.73 | subgroup-p = 0.378 site-p = 0.079 site × subgroup-p = 0.001 |
| F_LC | 28.74 ± 3.10 | 16.58 ± 2.66 | 19.24 ± 2.83 | 17.42 ± 3.32 | ||
| Ct.Po.Dm (µm) | F_MC | 113 ± 12 | 113 ± 10 | 134 ± 11 | 137 ± 12 | subgroup-p = 0.200 site-p = 0.440 site × subgroup-p = 0.175 |
| F_LC | 131 ± 14 | 102 ± 12 | 142 ± 13 | 118 ± 15 | ||
| Ct.Th (mm) | F_MC | 0.96 ± 0.19 | 1.50 ± 0.15 | 0.92 ± 0.16 | 1.29 ± 0.18 | subgroup-p = 0.416 site-p = 0.101 site × subgroup-p = 0.050 |
| F_LC | 0.92 ± 0.18 | 0.91 ± 0.14 | 1.06 ± 0.15 | 0.70 ± 0.16 | ||
| Medial-to-lateral ratio of Ct.Th | 1.29 ± 0.31 | 2.04 ± 0.29 | 0.88 ± 0.26 | 1.52 ± 0.36 | subgroup-p = 0.101 | |
| Tb.Th (µm) | F_MC | 160 ± 13 | 179 ± 11 | 192 ± 11 | 167 ± 14 | subgroup-p = 0.817 site-p = 0.663 site × subgroup-p = 0.015 |
| F_LC | 174 ± 12 | 151 ± 10 | 163 ± 12 | 181 ± 13 | ||
| Tb.N (1/mm) | F_MC | 1.625 ± 0.142 | 1.993 ± 0.122 | 1.519 ± 0.177 | 1.353 ± 0.152 | subgroup-p = 0.014 site-p = 0.317 site × subgroup-p = 0.351 |
| F_LC | 1.585 ± 0.194 | 1.895 ± 0.207 | 1.248 ± 0.130 | 1.292 ± 0.207 | ||
| Tb.Sp (mm) | F_MC | 0.330 ± 0.053 | 0.292 ± 0.045 | 0.526 ± 0.048 | 0.378 ± 0.057 | subgroup-p = 0.033 site-p = 0.494 site × subgroup-p = 0.189 |
| F_LC | 0.348 ± 0.043 | 0.371 ± 0.037 | 0.440 ± 0.039 | 0.425 ± 0.046 | ||
| BV/TV (%) | F_MC | 27.57 ± 2.96 | 34.42 ± 2.54 | 27.38 ± 2.40 | 23.24 ± 3.17 | subgroup-p = 0.059 site-p = 0.979 site × subgroup-p = 0.011 |
| F_LC | 29.03 ± 2.63 | 25.96 ± 2.25 | 19.87 ± 2.70 | 22.42 ± 2.81 | ||
| Medial-to-lateral ratio of BV/TV | 0.94 ± 0.13 | 1.32 ± 0.12 | 1.37 ± 0.11 | 1.04 ± 0.15 | subgroup-p = 0.050 | |
| Skeletal Site | KOA Group Valgus Knee (Mean ± SE) | KOA Group Varus Knee (Mean ± SE) | RA Group Valgus Knee (Mean ± SE) | RA Group Varus Knee (Mean ± SE) | p-Value | |
|---|---|---|---|---|---|---|
| Cg.Th (mm) | T_MC_A | 1.37 ± 0.22 | 0.63 ± 0.19 | 1.61 ± 0.20 | 0.39 ± 0.29 | subgroup-p = 0.004 site-p = 0.059 site × subgroup-p = 0.001 |
| T_MC_P | 2.08 ± 0.24 | 1.44 ± 0.20 | 1.31 ± 0.22 | 0.64 ± 0.26 | ||
| T_LC_A | 0.62 ± 0.21 | 2.25 ± 0.18 | 0.61 ± 0.19 | 2.01 ± 0.23 | ||
| T_LC_P | 0.80 ± 0.23 | 2.40 ± 0.19 | 0.39 ± 0.21 | 2.00 ± 0.24 | ||
| Medial-to-lateral ratio of Cg.Th | 2.43 ± 0.53 | 0.48 ± 0.05 | 3.12 ± 0.59 | 0.52 ± 0.08 | subgroup-p = 0.001 | |
| Ct.Po.tot (%) | T_MC_A | 19.47 ± 4.48 | 14.63 ± 3.84 | 22.12 ± 4.09 | 17.34 ± 4.80 | subgroup-p = 0.676 site-p = 0.255 site × subgroup-p = 0.150 |
| T_MC_P | 14.91 ± 3.84 | 14.19 ± 2.31 | 23.84 ± 2.46 | 19.80 ± 2.89 | ||
| T_LC_A | 15.70 ± 4.20 | 23.92 ± 3.60 | 18.44 ± 3.84 | 20.48 ± 4.50 | ||
| T_LC_P | 17.02 ± 3.22 | 12.56 ± 2.76 | 20.04 ± 2.94 | 19.34 ± 3.45 | ||
| Ct.Po.Dm (µm) | T_MC_A | 110 ± 14 | 98 ± 12 | 124 ± 13 | 136 ± 15 | subgroup-p = 0.102 site-p = 0.651 site × subgroup-p = 0.804 |
| T_MC_P | 104 ± 9 | 101 ± 8 | 117 ± 9 | 120 ± 10 | ||
| T_LC_A | 103 ± 12 | 108 ± 10 | 127 ± 11 | 111 ± 13 | ||
| T_LC_P | 105 ± 13 | 91 ± 11 | 133 ± 12 | 114 ± 14 | ||
| Ct.Th (mm) | T_MC_A | 0.96 ± 0.16 | 1.26 ± 0.14 | 0.64 ± 0.15 | 1.40 ± 0.18 | subgroup-p = 0.030 site-p = 0.874 site × subgroup-p = 0.001 |
| T_MC_P | 0.84 ± 0.10 | 0.99 ± 0.08 | 0.63 ± 0.09 | 0.95 ± 0.11 | ||
| T_LC_A | 1.40 ± 0.10 | 0.66 ± 0.09 | 1.10 ± 0.09 | 0.63 ± 0.11 | ||
| T_LC_P | 1.53 ± 0.20 | 0.72 ± 0.17 | 0.89 ± 0.18 | 0.66 ± 0.21 | ||
| Medial-to-lateral ratio of Ct.Th | 0.72 ± 0.11 | 1.25 ± 0.36 | 0.69 ± 0.04 | 2.09 ± 0.48 | subgroup-p = 0.001 | |
| Tb.Th (µm) | T_MC_A | 337 ± 196 | 167 ± 67 | 193 ± 16 | 195 ± 21 | subgroup-p = 0.195 site-p = 0.130 site × subgroup-p = 0.060 |
| T_MC_P | 217 ± 18 | 146 ± 15 | 169 ± 17 | 169 ± 20 | ||
| T_LC_A | 183 ± 17 | 180 ± 16 | 179 ± 42 | 171 ± 19 | ||
| T_LC_P | 182 ± 19 | 182 ± 19 | 171 ± 17 | 167 ± 20 | ||
| Tb.N (1/mm) | T_MC_A | 1.639 ± 0.204 | 1.625 ± 0.175 | 1.526 ± 0.186 | 1.479 ± 0.219 | subgroup-p = 0.627 site-p = 0.422 site × subgroup-p = 0.282 |
| T_MC_P | 1.827 ± 0.208 | 1.634 ± 0.178 | 1.350 ± 0.190 | 1.246 ± 0.223 | ||
| T_LC_A | 1.471 ± 0.377 | 1.646 ± 0.324 | 1.366 ± 0.344 | 2.561 ± 0.404 | ||
| T_LC_P | 1.614 ± 0.177 | 1.743 ± 0.152 | 1.421 ± 0.161 | 1.283 ± 0.189 | ||
| BV/TV (%) | T_MC_A | 25.34 ± 3.22 | 18.96 ± 2.57 | 23.20 ± 2.73 | 19.78 ± 3.44 | subgroup-p = 0.046 site-p = 0.975 site × subgroup-p = 0.016 |
| T_MC_P | 34.49 ± 3.38 | 19.70 ± 2.99 | 20.70 ± 3.08 | 16.88 ± 3.62 | ||
| T_LC_A | 22.50 ± 2.99 | 24.24 ± 2.76 | 19.40 ± 2.94 | 22.10 ± 3.20 | ||
| T_LC_P | 23.27 ± 2.79 | 20.65 ± 2.39 | 20.69 ± 2.54 | 18.28 ± 2.98 | ||
| Medial-to-lateral ratio of BV/TV | 1.47 ± 0.13 | 0.86 ± 0.15 | 1.10 ± 0.05 | 0.98 ± 0.12 | subgroup-p = 0.004 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baljozovic, A.; Lekovic, A.; Nikolic, S.; Djonic, D.; Djuric, M.; Bascarevic, Z.; Jadzic, J. Osteochondral Alterations in Patients Treated with Total Knee Arthroplasty Due to Rheumatoid Arthritis and Primary Osteoarthritis: Cross-Sectional Study with Focus on Elucidating Effects of Knee Malalignment. Life 2025, 15, 818. https://doi.org/10.3390/life15050818
Baljozovic A, Lekovic A, Nikolic S, Djonic D, Djuric M, Bascarevic Z, Jadzic J. Osteochondral Alterations in Patients Treated with Total Knee Arthroplasty Due to Rheumatoid Arthritis and Primary Osteoarthritis: Cross-Sectional Study with Focus on Elucidating Effects of Knee Malalignment. Life. 2025; 15(5):818. https://doi.org/10.3390/life15050818
Chicago/Turabian StyleBaljozovic, Andreja, Aleksa Lekovic, Slobodan Nikolic, Danijela Djonic, Marija Djuric, Zoran Bascarevic, and Jelena Jadzic. 2025. "Osteochondral Alterations in Patients Treated with Total Knee Arthroplasty Due to Rheumatoid Arthritis and Primary Osteoarthritis: Cross-Sectional Study with Focus on Elucidating Effects of Knee Malalignment" Life 15, no. 5: 818. https://doi.org/10.3390/life15050818
APA StyleBaljozovic, A., Lekovic, A., Nikolic, S., Djonic, D., Djuric, M., Bascarevic, Z., & Jadzic, J. (2025). Osteochondral Alterations in Patients Treated with Total Knee Arthroplasty Due to Rheumatoid Arthritis and Primary Osteoarthritis: Cross-Sectional Study with Focus on Elucidating Effects of Knee Malalignment. Life, 15(5), 818. https://doi.org/10.3390/life15050818








