Flaws in Estrus Synchronization Protocols Increase Vaginal Prolapse and Hydrometra Risk in Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Farm Selection Criteria
- (i)
- Housing system: Exclusive use of fully indoor intensive housing, characterized by permanent confinement and feeding with total mixed rations composed of commercial concentrates and locally produced forages (hay and/or ensilage).
- (ii)
- Flock size: A minimum of 500 reproductive females, corresponding to the economic threshold for viability in intensive Greek dairy sheep operations [5].
- (iii)
- Estrus synchronization protocols: Routine application of hormonal estrus synchronization using progestagen-impregnated intravaginal sponges (inserted for 12–14 days), followed by an intramuscular injection of 500 IU of eCG at sponge removal [7].
- (iv)
- Ultrasonographic pregnancy diagnosis: Regular use of transabdominal ultrasonography between 45 and 60 days post-mating, as part of standard reproductive monitoring procedures.
2.3. Animals
2.4. Data Collection
2.4.1. Reproductive History
- Annual milk production per flock;
- Age group classification of reproductive females (hoggets or ewes);
- Dates of estrus synchronization procedures and ram introduction for natural mating.
2.4.2. Clinical Examination
2.4.3. Ultrasonographic Examination
- Fetal numbers were determined via ultrasound and later confirmed or corrected based on parturition outcomes or post-mortem findings in cases of pregnancy loss.
- (i)
- Presence of anechoic or hypoechoic intrauterine fluid;
- (ii)
- Absence of fetal or embryonic structures;
- (iii)
- Visualization of fine echogenic septations within the fluid, when present.
2.5. Data Management and Statistical Analysis
- The prevalence of vaginal prolapse and hydrometra was calculated for the entire study population.
- Associations between categorical variables (e.g., hoggets vs. ewes) were analyzed using the Chi-square (χ2) test.
- Differences among multiple groups (e.g., based on the number of estrus synchronization applications or parity) were assessed using one-way analysis of variance (ANOVA).
- A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Study Population Overview
3.2. Vaginal Prolapse
3.3. Hydrometra
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDO | Protected Designation of Origin |
| ES | estrus synchronization |
| eCG | equine chorionic gonadotropin |
References
- Peters, G.M.; Rowley, H.V.; Wiedemann, S.; Tucker, R.; Short, M.D.; Schulz, M. Red meat production in Australia: Life cycle assessment and comparison with overseas studies. Environ. Sci. Technol. 2010, 44, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). GLEAM 2.0–Global Livestock Environmental Assessment Model: Model Description; FAO: Rome, Italy, 2017; Available online: https://www.fao.org/gleam/resources/en/ (accessed on 17 February 2025).
- Jones Family Farm. Farming Impact on the Environment: Cows vs. Sheep. 2022. Available online: https://jonesfamilyfarm.co.nz/farming-impact-on-the-environment-cows-vs-sheep/ (accessed on 17 February 2025).
- Ali, S.; Zhao, Z.; Zhen, G.; Kang, J.Z.; Yi, P.Z. Reproductive problems in small ruminants (Sheep and goats): A substantial economic loss in the world. Large Anim. Rev. 2019, 25, 215–223. [Google Scholar]
- Papanikolopoulou, V.; Vouraki, S.; Priskas, S.; Theodoridis, A.; Dimitriou, S.; Arsenos, G. Economic Performance of Dairy Sheep Farms in Less-Favoured Areas of Greece: A Comparative Analysis Based on Flock Size and Farming System. Sustainability 2023, 15, 1681. [Google Scholar] [CrossRef]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2017, 101, 6715–6729. [Google Scholar] [CrossRef]
- Lianou, D.T.; Vasileiou, N.G.C.; Michael, C.K.; Valasi, I.; Mavrogianni, V.S.; Caroprese, M.; Fthenakis, G.C. Patterns of Reproductive Management in Sheep and Goat Farms in Greece. Animals 2022, 12, 3455. [Google Scholar] [CrossRef] [PubMed]
- Voulgarakis, N.; Gougoulis, D.; Psalla, D.; Papakonstantinou, G.; Katsoulis, K.; Angelidou-Tsifida, M.; Athanasiou, L.; Papatsiros, V.; Christodoulopoulos, G. Subacute Rumen Acidosis in Greek Dairy Sheep: Prevalence, Impact, and Colorimetry Management. Animals 2024, 14, 2061. [Google Scholar] [CrossRef]
- Tsekouras, N.; Christodoulopoulos, M.A.B.; Meletis, E.; Kousoulis, C.; Kostoulas, P.; Pantazis, V.; Papatsiros, V.G.; Dimoveli, K.; Gougoulis, D. Associations Between Non-Steroidal and Steroidal Anti-Inflammatory Drug Use, Welfare, and Milk Production in Dairy Sheep: A Multivariate Study. Animals 2025, 15, 1104. [Google Scholar] [CrossRef] [PubMed]
- Valasi, I.; Dymtsa, E.; Mamouzelos, E.; Amiridis, G.S. Induction of estrus and ovulation in seasonally anestrous Greek dairy sheep using intravaginal sponges or melatonin implants and a combination of eCG and PGF2α. Theriogenology 2012, 77, 80–88. [Google Scholar]
- Olivera-Muzante, J.; Gil, J.; Fierro, S.; Menchaca, A.; Rubianes, E. Alternatives to improve a prostaglandin-based protocol for timed artificial insemination in sheep. Theriogenology 2011, 76, 1501–1507. [Google Scholar] [CrossRef]
- Pinna, A.E.; Brandão, F.Z.; Cavalcanti, A.S.; Borges, A.M.; Souza, J.M.G.; Fonseca, J.F. Reproductive parameters of Santa Inês ewes submitted to short-term treatment with re-used progesterone devices. Arq. Bras. Med. Vet. Zootec. 2012, 64, 333–340. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Pallares, P.; Vazquez, M.I. Ultrasonographic imaging in small ruminant reproduction. Reprod. Domest. Anim. 2010, 45, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bayly, A.; Hankin, T.H.; Haugh, P. Eversion of the vagina in ewes. N. Z. J. Agric. 1936, 53, 19–27. [Google Scholar]
- Knottenbelt, D.C. Vaginal rupture associated with herniation of the abdominal viscera in pregnant ewes. Vet. Rec. 1988, 122, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Hosie, B.D. Prolapse and hernia. In Diseases of Sheep, 4th ed.; Aitken, I.D., Ed.; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Peter, A.T.; King, E.H. Management of Vaginal, Cervico-Vaginal, and Uterine Prolapse. In Bovine Reproduction; Hopper, R.M., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar]
- Sharma, A.; Kumar, P.; Singh, M.; Vasishta, N.K. Reproductive health status of north western Himalayan Gaddi sheep: An abattoir study. Open Vet. J. 2014, 4, 103–106. [Google Scholar] [CrossRef]
- Abdullah, F.F.J.; Abba, Y.; Adamu, L.; Tijjani, A.; Mohammed, K.; Osman, A.Y.; Haron, A.W. Management of grade I vaginal prolapse in a Friesian cross: A case report. IOSR J. Agric. Vet. Sci. 2014, 7, 74–77. [Google Scholar] [CrossRef]
- Kumar, A.; Saxena, A.; Anand, M.; Upmanyu, G. Genital prolapse in bovine and its management. Int. J. Sci. Environ. Technol. 2018, 7, 1435–1439. [Google Scholar]
- Scott, P.R. Sheep Medicine, 2nd ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Jesse, F.F.; Tukiran, N.A.; Hambali, I.U.; Lila, M.A.; Chung, E.L.; Abba, Y.; Bitrus, A.A.; Peter, I.D.; Norsidin, M.J. Review on Clinical Management involving Post-Partum Diseases in Ruminants. Adv. Anim. Vet. 2019, 7, 574–582. [Google Scholar] [CrossRef][Green Version]
- Christodoulopoulos, G. Vaginal prolapse in ewes: A critical review of the literature and the disease status in Greece. J. Hell. Vet. Med. Soc. 2023, 74, 5499–5504. [Google Scholar] [CrossRef]
- Brown, S.; Stafford, K.J.; Norris, G. A search for predictive biomarkers of ovine pre-partum vaginal prolapse. Res. Vet. Sci. 2021, 140, 251–258. [Google Scholar] [CrossRef]
- Voigt, K.; Najm, N.A.; Zablotski, Y.; Rieger, A.; Vassiliadis, P.; Steckeler, P.; Schabmeyer, S.; Balasopoulou, V.; Zerbe, H. Factors associated with ewe and lamb survival, and subsequent reproductive performance of sheep undergoing emergency caesarean section. Reprod. Domest. Anim. 2021, 56, 120–129. [Google Scholar] [CrossRef]
- Wahab, Y.A.; Olayinka, S.O.; Olofintuyi, O.K.; Raji, L.; Tijani, K.A. Correction of Vaginal Prolapse in a Pregnant EWE: A Case Report. Int. J. Environ. Agric. Biotechnol. 2019, 4, 1452–1455. [Google Scholar] [CrossRef]
- Jackson, R.; Hilson, R.; Roe, A.; Perkins, N.; Heuer, C.; West, D. Epidemiology of vaginal prolapse in mixed-age ewes in New Zealand. New Zealand Vet. J. 2014, 62, 328–337. [Google Scholar] [CrossRef]
- Brabant, O.; Laurence, M. Surgical correction of four cases of hydrometra in ewes. Vet. Rec. Case Rep. 2017, 5, e000555. [Google Scholar] [CrossRef]
- Maia, A.L.R.S.; Brandão, F.Z.; Souza-Fabjan, J.M.G.; Veiga, M.O.; Balaro, M.F.A.; Siqueira, L.G.B.; Facó, O.; Fonseca, J.F. Hydrometra in dairy goats: Ultrasonic variables and therapeutic protocols evaluated during the reproductive season. Anim. Reprod. Sci. 2018, 197, 203–211. [Google Scholar] [CrossRef]
- Maia, A.L.R.S.; Silva, M.R.; Brandão, F.Z.; Souza-Fabjan, J.M.G.; Faria, L.S.; Côrtes, L.R.; Facó, O.; Fonseca, J.F. Epidemiological survey and risk factors associated with hydrometra in dairy goat herds. Small Rum. Res. 2019, 178, 79–84. [Google Scholar] [CrossRef]
- Mohammed, Z.A. A study of pathological abnormalities of genitalia in ewes in Duhok, Iraq. Iraqi J. Vet. Sci. 2021, 35, 421–427. [Google Scholar] [CrossRef]
- Smith, M.C.; Sherman, D.M. Goat Medicine, 3rd ed.; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Yotov, S.; Atanasova, I.; Ganeva, D.; Antonov, A.; Vassilev, N.A. Clinical case of hydrometra in a ewe following estrus synchronization. Bulg. J. Vet. Med. 2009, 12, 67–72. [Google Scholar]
- Rajashri, M.; Prasad, K.; Surabhi, K.R.; Reddy, M.R. Hydrometra in a Deccani ewe after oestrus synchronization and artificial insemination. Haryana Vet. 2017, 56, 113–114. [Google Scholar]
- Souza, J.M.G.; Maia, A.L.R.S.; Brandão, F.Z.; Vilela, C.G.; Oba, E.; Bruschi, J.H.; Fonseca, J.F. Hormonal treatment of dairy goats affected by hydrometra associated or not with ovarian follicular cyst. Small Rum. Res. 2013, 111, 104–109. [Google Scholar] [CrossRef]
- Balaro, M.F.A.; Cosentino, I.O.; Ribeiro, A.C.S.; Brandão, F.Z. Ultrasound Diagnosis in Small Ruminants: Occurrence and Description of Genital Pathologies. Vet. Sci. 2022, 9, 599. [Google Scholar] [CrossRef]
- Scott, P.R.; Gessert, M.E. Ultrasonographic examination of 12 ovine vaginal prolapses. Vet. J. 1998, 155, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, A.M.; Abass, N.A.; Badawi, M.E.; Ibrahim, M.T.; Elfadil, A.A.; Abdelghafar, R.M. Pseudopregnancy in goats: Sonographic prevalence and associated risk factors in Khartoum State, Sudan. Vet. World 2018, 11, 525–529. [Google Scholar] [CrossRef]
- Durrani, A.Z.; Kamal, N. Prevalence of genital tract problems in clinical cases of various species of animals. J. Anim. Plant. Sci. 2009, 19, 160–162. [Google Scholar]
- Venkatesh, S.M.; Kantharaj, K.; Hemalatha, S.; Patil, H.; Kumar, M.; Arjun, M.G. Management of estrual cervico-vaginal prolapse in a cross-bred Jersey cow: A case report. Int. J. Vet. Sci. Anim. Husb. 2023, 8, 188–190. [Google Scholar] [CrossRef]
- Cahyani, A.A.; Nurdin, M.A. Case Report Management of Vaginal Prolapse in Crossbreed Cattle (Simmental-Bali) in Mico Village, Palakka District, Bone Regency. J. Ris. Vet. Indones. 2024, 8. [Google Scholar] [CrossRef]
- Bhatti, M.S.; Ahmad, I.; Ahmad, N.; Lodhi, L.A.; Ahmad, M. Epidemiological survey of genital prolapse in buffaloes kept under different systems and serum micro mineral contents. Pak. Vet. J. 2006, 26, 197–200. [Google Scholar]
- Rabbani, R.A.; Ahmad, I.; Lodhi, L.; Ahmad, N.; Muhammad, G. Prevalence of various reproductive disorders and economic losses caused by genital prolapse in buffaloes. Pak. Vet. J. 2010, 30, 44–48. [Google Scholar]
- Pande, N.; Agrawal, R.G.; Agrawal, R.; Shrivasatava, O.P.; Jain, S.K. Prevalence of periparturient reproductive disorders and calving pattern in buffaloes. Intas Polivet 2014, 15, 205–207. [Google Scholar]
- Hussien, M.; Abdel-Fattah, M.; Khalil, Y.; Sedeck, M. Evaluation of a modified surgical technique for correction of vaginal and uterine prolapse in bovine. Vet. Med. J. Giza 2011, 57, 115–131. [Google Scholar]
- Kuijlaars, M. The Occurrence of Vaginal Prolapse in Sheep and Cattle; Case Study; Ghent University, Faculty of Veterinary Medicine: Ghent, Belgium, 2011. [Google Scholar]
- Alam, M.A.; Bhuiyan, M.M.U.; Parvin, M.S.; Rahman, M.M.; Bari, F.Y. Prevalence of reproductive diseases and its associated risk factors in crossbred dairy cows. Res. Agric. Livest. Fish 2015, 1, 71–79. [Google Scholar] [CrossRef]
- Sarder, J.U.; Uddin, J.; Islam, H.; Islam, R. Prevalence of obstetrical disorders in dairy cows of northern Bangladesh. Asian J. Med. Biol. Res. 2015, 1, 216–221. [Google Scholar] [CrossRef][Green Version]
- Beredu, Y.; Biruk, A. Reproductive Disorders in Dairy Cattle; Retrospective Study in Asella Town, Central Ethiopia. Dairy Vet. Sci. J. 2019, 9, 555767. [Google Scholar]
- Dey, T.; Poddar, S.; Sultana, J.; Akter, S.; Surtadhar, B.C. Prevalence of Clinical Diseases and Disorders of Goat at Upazilla Veterinary Hospital, Pirojpur, Bangladesh. Turkish. J. Vet. Res. 2018, 2, 9–13. [Google Scholar]
- Sayeed, A.; Khatun, S.; Bari, S.; Dash, K.A.; Haldar, K.P.; Sarker, K.B. Prevalence of Gynecological Disorders of Goat and Pattern of Drug used at Chuadanga, Bangladesh. Agric. Sci. Dig. 2020, 40, 424–429. [Google Scholar] [CrossRef]
- Low, J.C.; Sutherland, H.K. A census of the prevalence of vaginal prolapse in sheep flocks in the Borders region of Scotland. Vet. Rec. 1987, 120, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Litherland, A.J.; Lambert, M.; Knight, T.W.; Cook, T.; McDougal, D. Incidence of bearings in ewes that had a bearing the preceding lambing. Proc. N. Z. Soc. Anim. Prod. 2000, 60, 44–46. [Google Scholar]
- Kloss, S.; Wehrend, A.; Failing, K.; Bostedt, H. Erhebungen zur Art und Häufigkeit mechanischer Geburtsstörungen beim Schaf unter besonderer Berücksichtigung des Prolapsus vaginae ante partum [Investigations about kind and frequency of mechanical dystocia in ewes with special regard to the vaginal prolapse ante partum]. Berl. Munch. Tierarztl. Wochenschr. 2002, 115, 247–251. [Google Scholar]
- Ennen, S.; Kloss, S.; Scheiner-Bobis, G.; Failing, K.; Wehrend, A. Histological, hormonal and biomolecular analysis of the pathogenesis of ovine Prolapsus vaginae ante partum. Theriogenology 2011, 75, 212–219. [Google Scholar] [CrossRef]
- Alves, M.B.R.; Benesi, F.J.; Gregory, L.; Della Libera, A.M.; Sucupira, M.C.A.; Pogliani, F.C.; Gomes, V. Uterine and vaginal prolapse in ewes. Pesq. Vet. 2013, 33, 171–176. [Google Scholar] [CrossRef]
- Hassaneen Ahmed, S.A. Complete Cervico-vaginal Prolapse (CVP) in a Ewe. SVU-Int. J. Vet. Sci. 2018, 1, 50–59. [Google Scholar] [CrossRef][Green Version]
- Yılmaz, B.; Boğa Kuru, B.; Kuru, M. Some reproductive and gynecological characteristics of Morkaraman ewes. J. Adv. Vetbio Sci. Tech. 2022, 7, 274–282. [Google Scholar] [CrossRef]
- Scott, P.R.; Sargison, N.D.; Penny, C.D.; Strachan, W.D. The use of combined xylazine and lignocaine epidural injection in ewes with vaginal or uterine prolapses. Theriogenology 1995, 43, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T. Vaginal, Cervical, and Uterine Prolapse. In Bovine Reproduction; Hopper, R.M., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2014. [Google Scholar]
- Rynkevic, R.; Martins, P.; Andre, A.; Parente, M.; Mascarenhas, T.; Almeida, H.; Fernandes, A.A. The effect of consecutive pregnancies on the ovine pelvic soft tissues: Link between biomechanical and histological components. Ann. Anat. 2019, 222, 166–172. [Google Scholar] [CrossRef]
- Bodinga, H.A.; Oviawe, E.I.; Buhari, S.; Idris, B.; Kabir, I.; Adamu, U.; Abubakar, A.; Yakubu, A.S.; Abubakar, N.K. Management of dystocia due to faulty fetal disposition complicated with vaginal prolapse in a 2-year-old Yankasa ewe. J. Anim. Sci. Vet. Med. 2020, 5, 56–61. [Google Scholar]
- Kumar, P.; Dayal, S.; Tiwari, R.; Sengupta, D.; Barari, S.K.; Dey, A.K. Vaginal prolapse in peri-partum primiparous murrah buffalo complicated into endometritis and cystitis: A case report. Buffalo Bull. 2015, 34, 153–159. [Google Scholar]
- Hasan, T.; Azizunnesa Parvez, M.A.; Paul, P.; Akter, S.; Faruk, M.O.; Hossain, D. Correction and management of vaginal prolapse in a cow by Buhner’s technique. Res. J. Vet. Pract. 2017, 5, 1–4. [Google Scholar]
- Hosie, B.D.; Low, J.C.; Bradley, H.K.; Robb, J. Nutritional factors associated with vaginal prolapse in ewes. Vet. Rec. 1991, 128, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Litherland, A.J.; Knight, T.W.; Lambert, M.G.; Cook, T.G.; McDougal, D.B.O.; Day, A. Calcium balance in mid and late pregnancy and vaginal prolapse. Proc. N. Z. Soc. Anim. Prod. 2007, 67, 61–67. [Google Scholar]
- Akhtar, M.S.; Lodhi, L.A.; Ahmad, I.; Qureshi, Z.I.; Muhammad, G. Serum trace mineral variations in Nili-Ravi buffaloes suffering with prepartum vaginal prolapse in two different agro-ecological zones of Punjab, Pakistan. Theriogenology 2012, 77, 1328–1333. [Google Scholar] [CrossRef]
- Allott, B.S.; Dittmer, K.E.; Kenyon, A.G.; Elder, P.A. Preliminary investigation of the effect of treating sheep during pregnancy with a vitamin A, D, E formulation on the incidence of vaginal prolapse. N. Z. Vet. J. 2020, 68, 193–197. [Google Scholar] [CrossRef]
- Nischitha, B.M.; Shilpa, B.S.; Pramod, K.S.; Roddannanavar, M.; Vijay, S.S.; Siddiq, E.; Akbar, A. Successful therapeutic management of cervico vaginal prolapse using Minchev’s technique in Jersey cow: A case study. J. Pharm. Innov. 2023, 12, 1874–1875. [Google Scholar]
- Akambaram, S.; Gupta, C. Genital Prolapse and Its Management in Cows. In Periparturient Diseases of Cattle; Rana, T., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2024. [Google Scholar]
- Assad, I.N.; Khatun, A.; Bhat, G.R.; Sakeena, S.; Naikoo, M.; Malik, A.A.; Malik, F.A. Management of Recurrent Cervicovaginal Prolapse in a Pregnant Crossbred Jersey Cow. Indian J. Anim. Reprod. 2024, 45, 70–72. [Google Scholar] [CrossRef]
- McLean, J.W. Vaginal prolapse in sheep. Part III: The effect of topography on incidence. N. Z. Vet. J. 1957, 4, 93–97. [Google Scholar] [CrossRef]
- Christodoulopoulos, G. Vaginal prolapse in ewes. Vet. Rec. Case Rep. 2022, 10, e375. [Google Scholar] [CrossRef]
- Dawson, L.J.; Peter, A.T. An update on vaginal and uterine eversions in cattle. Clin. Theriogenology 2012, 4, 115–131. [Google Scholar]
- Mushonga, B.; Twiyizeyimna, S.; Habarugira, G.; Kandiwa, E.; Chinyoka, S.; Samkange, A.; Bishi, A. Study of Incidence of Gross Urogenital Lesions and Abnormalities on Does Slaughtered at Nyagatare Slaughterhouse, Eastern Province, Rwanda. J. Vet. Med. 2017, 2017, 7564019. [Google Scholar] [CrossRef]
- Garba, I.; Dawuda, P.M.; Ate, I.U.; Abenga, J.N. Genital Tract Morphopathology of Red Sokoto and West African Dwarf Does in Makurdi. Open J. Vet. Med. 2019, 9, 21–44. [Google Scholar] [CrossRef]
- Kumar, P.; Dholpuria, S.; Purohit, G.N. Hysteroscopic and ultrasonographic evaluation of goat (Capra hircus) uterus. J. Entomol. Zool. Stud. 2020, 8, 1108–1112. [Google Scholar]
- Begum, K.; Azizunnessa, A.M. A clinical survey on the prevalence and therapeutics of reproductive disorders in goats in Chattogram, Bangladesh. Vet. Sci. Res. Rev. 2023, 9, 114–120. [Google Scholar] [CrossRef]
- Abou-Rawash, A.A.; Elsawak, A.; Abdo, W. Abattoir survey of acquired reproductive abnormalities in ewes in Egypt. Kafrelsheikh Vet. Med. J. 2008, 6, 70–112. [Google Scholar] [CrossRef]
- Dawood, K.E. Pathological abnormalities of the reproductive tracts of ewes in Basra, Iraq. Vet. Rec. 2010, 166, 205. [Google Scholar] [CrossRef]
- Palmieri, C.; Schiavi, E.D.; Della, S.L. Congenital and acquired pathology of ovary and tubular genital organs in ewes: A review. Theriogenology 2011, 75, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.; Vashistha, N.; Sharma, A.; Sharma, R.; Singh, M.; Kumar, P. Histopathological Study of Naturally Occurring Pathological Conditions of Uterus Affecting Reproduction in Small Ruminants. Indian J. Vet. Sci. Biotechnol. 2015, 11, 19–22. [Google Scholar]
- Silva, R.M.; Macêdo, J.T.; Lacerda, M.D.; Azevedo, J.P.; Júnior, J.A.; Cerqueira, R.B.; Pedroso, P.M. Lesions of the sheep reproductive system found in a slaughterhouse in the state of Bahia, Brazil. Pesq. Vet. 2020, 40, 955–962. [Google Scholar] [CrossRef]
- Kouamo, J.; Obama, S.F.F.; Sassa, M.S. Prevalence and Risk Factors of Genital Diseases of Goats and Ewes in the far North Region of Cameroon. PSM Vet. Res. 2019, 4, 62–73. [Google Scholar]
- Maia, A.L.R.S.; Brandão, F.Z.; Souza-Fabjan, J.M.G.; Veiga, M.O.; Balaro, M.F.A.; Facó, O.; Fonseca, J. Transrectal ultrasound evaluation in tropical dairy goats: An indispensable tool for the diagnosis of reproductive disorders. Trop. Anim. Health Prod. 2018, 50, 787–792. [Google Scholar] [CrossRef]
- Shatsky, K.O.; Dyulger, G.P.; Akchurina, I.V. Clinical and epidemiological characteristics of the of false pregnancy in Zaanen goats. BIO Web Conf. 2024, 139, 09001. [Google Scholar] [CrossRef]
- Acritopoulou, S.; Haresign, W. Response of ewes to a single injection of an analogue of PGF-2 alpha given at different stages of the oestrous cycle. J. Reprod. Fertil. 1980, 58, 219–221. [Google Scholar] [CrossRef]
- Viñoles, C.; Forsberg, M.; Banchero, G.; Rubianes, E. Effect of long-term and short-term progestagen treatment on follicular development and pregnancy rate in cyclic ewes. Theriogenology 2001, 55, 993–1004. [Google Scholar] [CrossRef]
| Parameter | Mean ± SD/Frequency (%) |
|---|---|
| Adult body weight (kg) | 70.0 ± 4.1 |
| Annual milk yield (kg) | 420 ± 42 |
| Total reproductive females | 97,215 |
| Hoggets | 14,494 (14.9%) |
| Ewes (2nd parity) | 30.30% |
| Ewes (3rd parity) | 30.60% |
| Ewes (4th parity) | 24.10% |
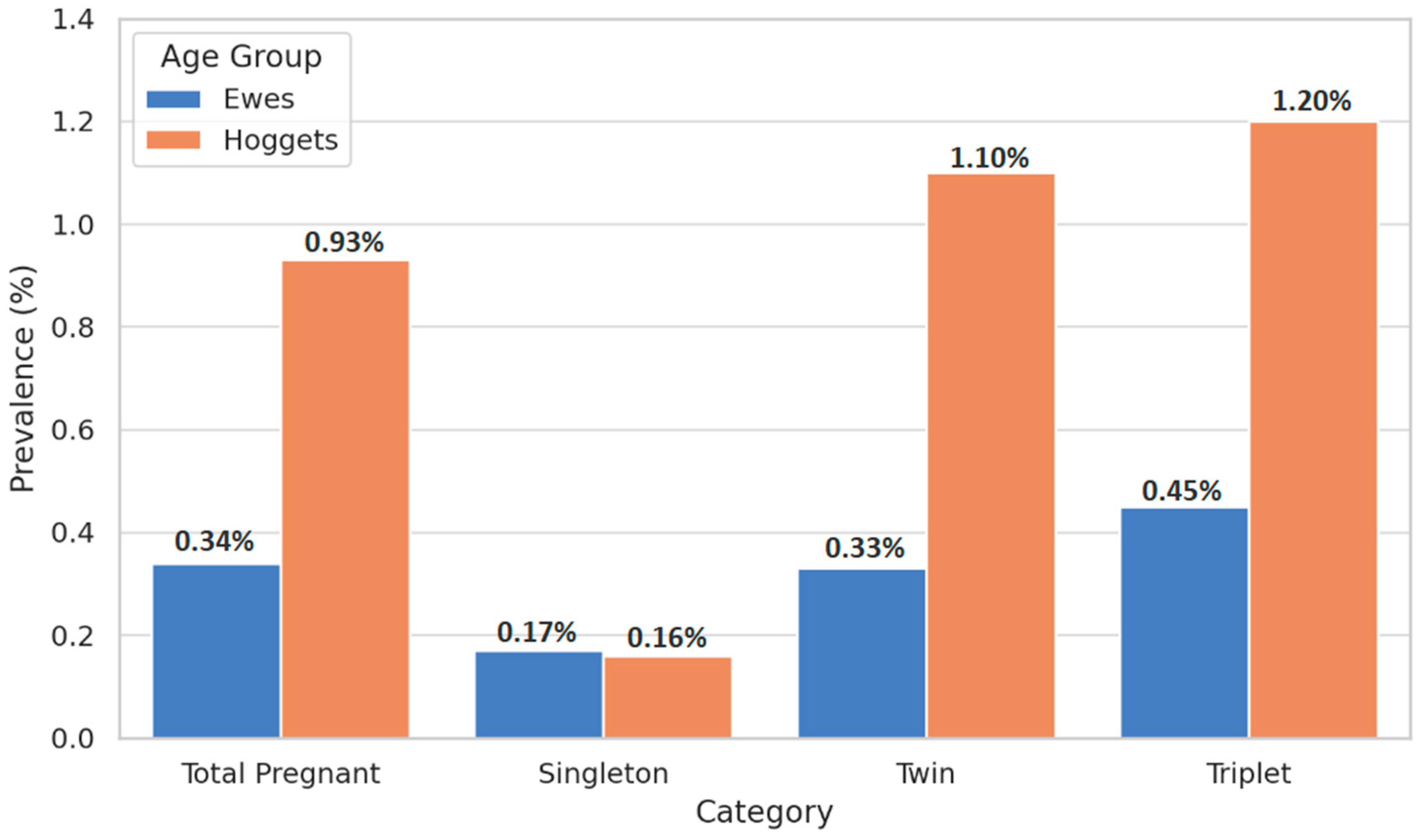
| Category | Hoggets (n, %) | Vaginal Prolapse in Hoggets (n, %) | Ewes (n, %) | Vaginal Prolapse in Ewes (n, %) |
|---|---|---|---|---|
| Total Pregnant Animals | 14,494 (100%) | 135 (0.93 ‡a) | 71,445 (100%) | 243 (0.34 §a) |
| Singleton Pregnancies | 3154 (21.76%) | 5 (0.16 ‡b) | 14,245 (19.94%) | 24 (0.17 §b) |
| Twin Pregnancies | 6275 (43.29%) | 69 (1.10 ‡c) | 31,740 (44.43%) | 105 (0.33 §a) |
| Triplet Pregnancies | 5065 (34.95%) | 61 (1.20 ‡c) | 25,460 (35.64%) | 114 (0.45 §c) |
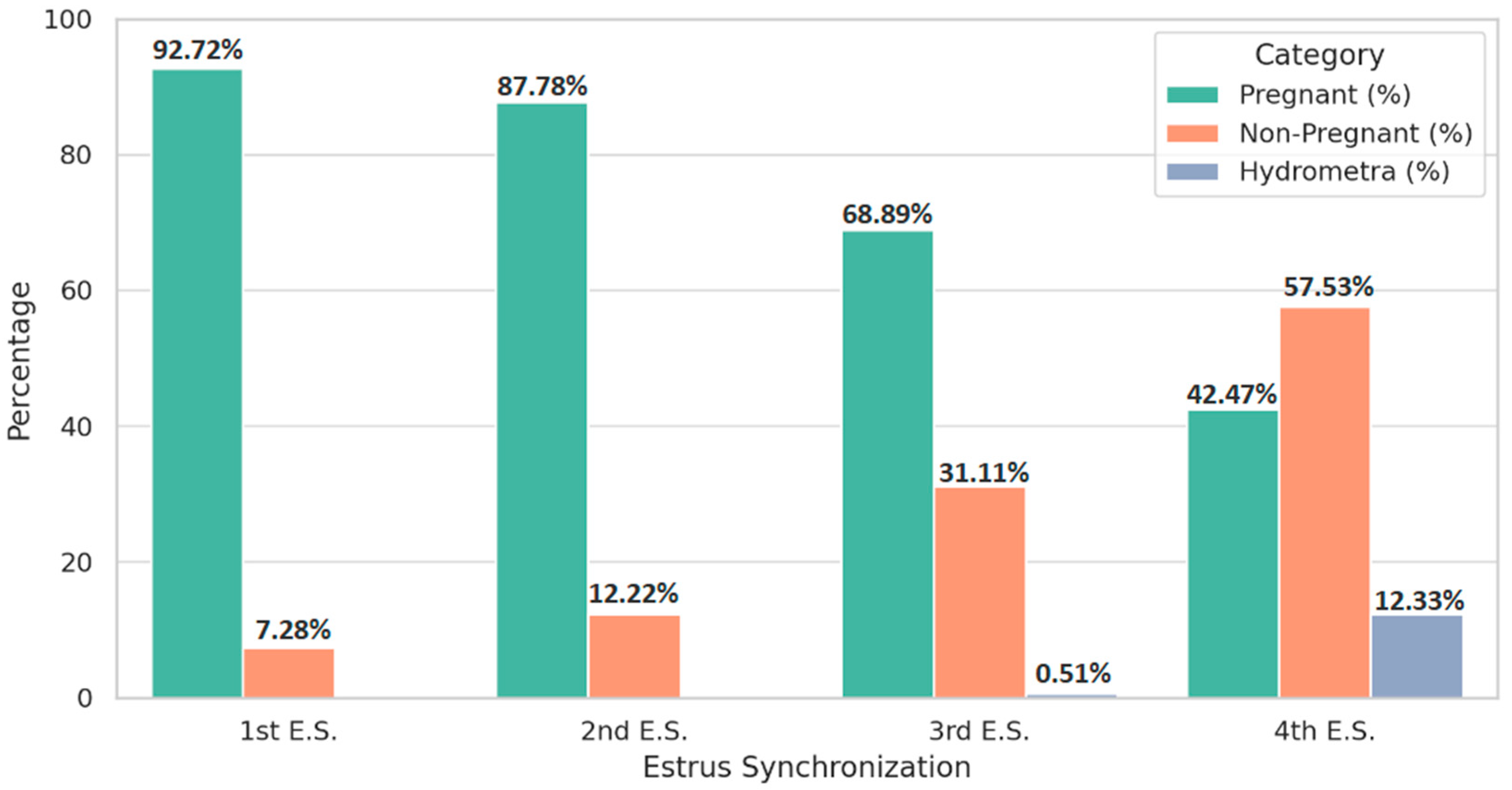
| Category | 1st E.S. (n, %) | 2nd E.S. (n, %) | 3rd E.S. (n, %) | 4th E.S. (n, %) |
|---|---|---|---|---|
| Treated | 80,250 (100%) | 5542 (100%) | 585 (100%) | 146 (100%) |
| Pregnant | 74,408 (92.72 a) | 4865 (87.78 b) | 403 (68.89 c) | 62 (42.47 d) |
| Non-Pregnant † | 5842 (7.28 a) | 677 (12.22 b) | 182 (31.11 c) | 84 (57.53 d) |
| Hydrometra | 0 (0%) | 0 (0%) | 3 (0.51 a) | 18 (12.33 b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsekouras, N.; Tsakmakidis, I.; Gougoulis, D.; Christodoulopoulos, M.A.B.; Kousoulis, C.; Papakonstantinou, G.I.; Papatsiros, V.G.; Christodoulopoulos, G. Flaws in Estrus Synchronization Protocols Increase Vaginal Prolapse and Hydrometra Risk in Sheep. Life 2025, 15, 795. https://doi.org/10.3390/life15050795
Tsekouras N, Tsakmakidis I, Gougoulis D, Christodoulopoulos MAB, Kousoulis C, Papakonstantinou GI, Papatsiros VG, Christodoulopoulos G. Flaws in Estrus Synchronization Protocols Increase Vaginal Prolapse and Hydrometra Risk in Sheep. Life. 2025; 15(5):795. https://doi.org/10.3390/life15050795
Chicago/Turabian StyleTsekouras, Nikolaos, Ioannis Tsakmakidis, Dimitrios Gougoulis, Mathis A. B. Christodoulopoulos, Christos Kousoulis, Georgios I. Papakonstantinou, Vasileios G. Papatsiros, and Georgios Christodoulopoulos. 2025. "Flaws in Estrus Synchronization Protocols Increase Vaginal Prolapse and Hydrometra Risk in Sheep" Life 15, no. 5: 795. https://doi.org/10.3390/life15050795
APA StyleTsekouras, N., Tsakmakidis, I., Gougoulis, D., Christodoulopoulos, M. A. B., Kousoulis, C., Papakonstantinou, G. I., Papatsiros, V. G., & Christodoulopoulos, G. (2025). Flaws in Estrus Synchronization Protocols Increase Vaginal Prolapse and Hydrometra Risk in Sheep. Life, 15(5), 795. https://doi.org/10.3390/life15050795












