Validation of the Measurement of Beta-Hydroxybutyrate and Non-Esterified Fatty Acids in Bovine Saliva: A Pilot Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Analytical Methods
2.3. Analytical Validation
- (a).
- Imprecision. It was assessed by calculating the coefficient of variation (CV) from a batch of determinations. Two saliva samples, one with a high and another with a low concentration of the analyte, were analyzed six times in the same analytical batch for intra-assay CV calculation. For inter-assay CV, the two samples with different concentrations were analyzed six times on different days. Samples were aliquoted and stored frozen at −80 °C; a different aliquot was used each day in order to avoid any effect due to freezing–thawing cycles. CVs were calculated as × 100.
- (b).
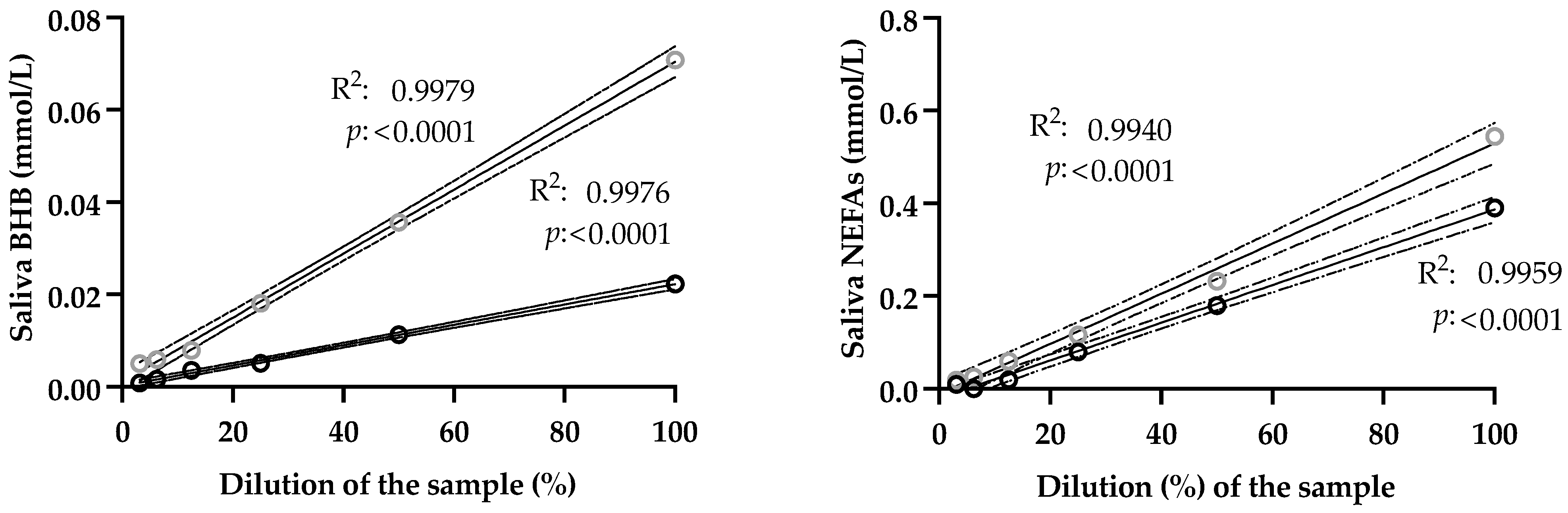
- Accuracy. It was indirectly estimated by linearity under dilution assays. Two samples of known analyte concentration were serially diluted with ultrapure water and then analyzed. Regression plots were constructed by facing observed vs. expected results in order to check whether slope was statistically different from zero.
- (c).
- Lower limit of detection (LLOD). It is defined as the lowest concentration capable of being detected by the assay. For its calculation, the assay diluent (ultrapure water) was analyzed 20 times in the same analytical batch. Then, the LLOD was estimated as .
- (d).
- Influence of dirtiness in the saliva sample. Saliva samples from the 73 adult cows were visually inspected and given a score depending on the presence of color and dirt in the sample, with a value of 0 being given when the sample showed no color or dirt and a value of 4 when the sample was cloudy, colored, and showed the presence of sediment, according to a previously published protocol [24]. Then, regression studies were performed in order to evaluate the effect of dirtiness on the assay results. In case of a significant effect, the most colored samples (scoring with 4) would be excluded for the rest of the assays.
- (e).
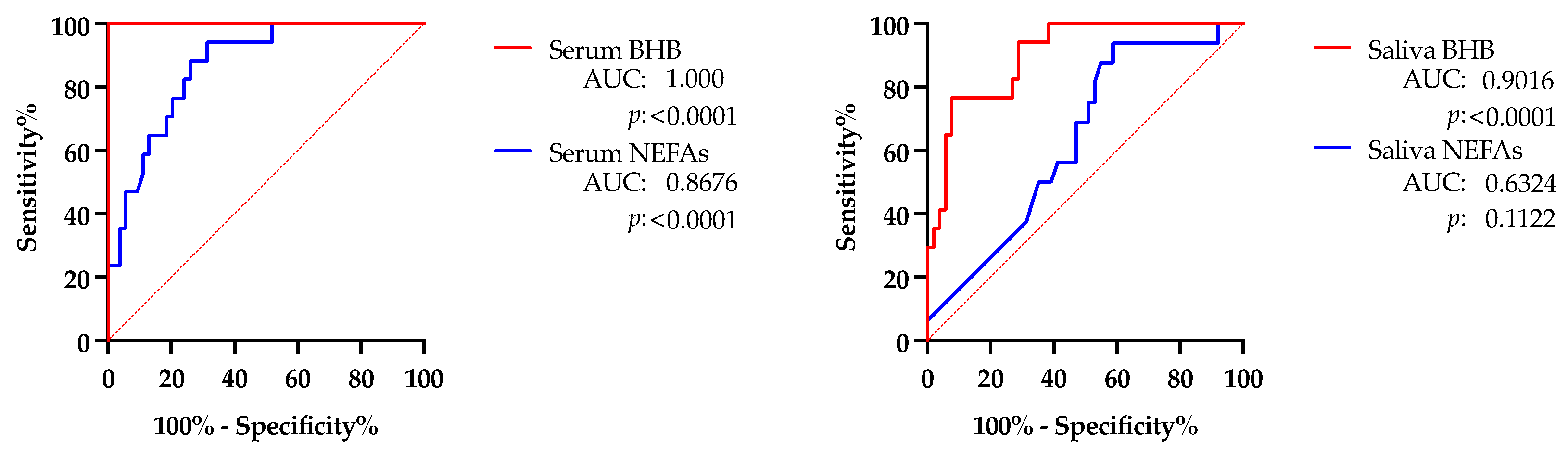
- Correlation between values in saliva vs. serum. In order to evaluate the possible correlations between salivary and serum levels, regression plots were constructed comparing the values of the different biomarkers obtained from paired saliva and serum samples from adult cows.
2.4. Changes in Physiological Conditions and Disease
2.4.1. Changes in Salivary Biomarkers in Adult Cows with Hyperketonemia or Metritis
- (a).
- The group of animals with neither clinical signs nor hyperketonemia (NCS-NH) was composed of 38 Holstein Friesian cows with a median age of 4.00 (Interquartile Range = 1.0) years old, median body condition score (BCS) of 2.9 (IQR = 0.3), median parity of 3.0 (IQR = 3.0), and median 26.0 (IQR = 59.5) days in milk (DIM). The animals did not show any evidence or sign of clinical disease at the time of sampling.
- (b).
- The cows without clinical signs but hyperketonemia (NCS-H) included 17 Holstein Friesian animals with a median age of 5.0 (IQR = 5.0) years old and a median BCS of 3.0 (IQR = 0.9). They had a median parity of 3.0 (IQR = 2.0) and a median of 14.3 (IQR = 8.1) DIM. The animals had no clinical signs but were all diagnosed with hyperketonemia since all of them had serum BHB levels > 1.2 mmol/L [12] using a GlucoMen LX β-Ketone Sensor (A. Menarini GmbH, Zürich, Switzerland), a method validated for bovine species [25].
- (c).
- The cows with clinical signs of metritis without hyperketonemia (CSM-NH) included 18 Holstein Friesian animals with a median age of 4.0 (IQR = 2.0) years old and median BCS of 2.8 (IQR = 0.6). They had a median parity of 3.0 (IQR = 3.0) and a median of 14.0 (IQR = 11.0) DIM. The diagnosis was based on visual inspection and clinical examination, according to previous research [26]. In brief, animals showed clinical signs compatible with metritis (enlarged uterus and fetid abnormal uterine discharge, associated with signs of systemic illness) within 21 days after parturition, and other potential complications such as lameness or ketosis were excluded.
2.4.2. Changes in Salivary Biomarkers in Healthy Calves over Time
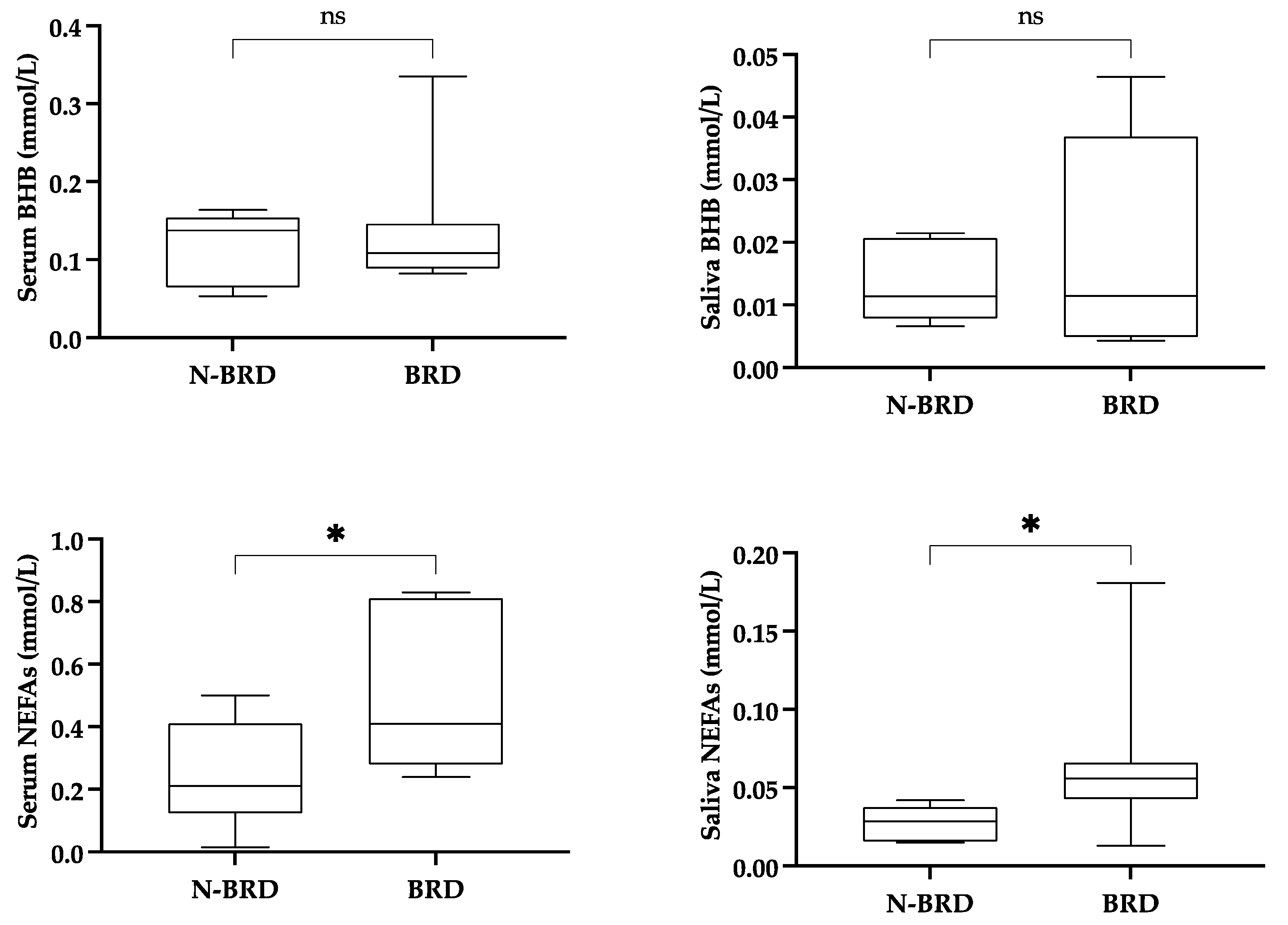
2.4.3. Changes in Salivary Biomarkers in Calves with Bovine Respiratory Disease
2.5. Statistical Analysis
3. Results
3.1. Analytical Validation
3.2. Changes in Adult Cows with Hyperketonemia or Metritis
3.3. Changes in Newborn Calves During the First Week of Life
3.4. Calves with or Without Clinical Manifestations of BRD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loiklung, C.; Sukon, P.; Thamrongyoswittayakul, C. Global Prevalence of Subclinical Ketosis in Dairy Cows: A Systematic Review and Meta-Analysis. Res. Vet. Sci. 2022, 144, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Raphael, W. Significance of Metabolic Stress, Lipid Mobilization, and Inflammation on Transition Cow Disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; McArt, J.A.; Overton, T.R.; Stokol, T.; Nydam, D.V. Using Nonesterified Fatty Acids and β-Hydroxybutyrate Concentrations During the Transition Period for Herd-Level Monitoring of Increased Risk of Disease and Decreased Reproductive and Milking Performance. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 387–412. [Google Scholar] [CrossRef]
- Mezzetti, M.; Cattaneo, L.; Passamonti, M.M.; Lopreiato, V.; Minuti, A.; Trevisi, E. The Transition Period Updated: A Review of the New Insights into the Adaptation of Dairy Cows to the New Lactation. Dairy 2021, 2, 617–636. [Google Scholar] [CrossRef]
- Ingvartsen, K.L. Feeding- and Management-Related Diseases in the Transition Cow. Anim. Feed Sci. Technol. 2006, 126, 175–213. [Google Scholar] [CrossRef]
- Lopreiato, V.; Mezzetti, M.; Cattaneo, L.; Ferronato, G.; Minuti, A.; Trevisi, E. Role of Nutraceuticals during the Transition Period of Dairy Cows: A Review. J. Anim. Sci. Biotechnol. 2020, 11, 96. [Google Scholar] [CrossRef]
- Fukao, T.; Mitchell, G.; Sass, J.O.; Hori, T.; Orii, K.; Aoyama, Y. Ketone Body Metabolism and Its Defects. J. Inherit. Metab. Dis. 2014, 37, 541–551. [Google Scholar] [CrossRef]
- Lisuzzo, A.; Bonelli, F.; Sgorbini, M.; Nocera, I.; Cento, G.; Mazzotta, E.; Turini, L.; Martini, M.; Salari, F.; Morgante, M.; et al. Differences of the Plasma Total Lipid Fraction from Pre-Foaling to Post-Foaling Period in Donkeys. Animals 2022, 12, 304. [Google Scholar] [CrossRef]
- Fiore, E.; Giambelluca, S.; Morgante, M.; Contiero, B.; Mazzotta, E.; Vecchio, D.; Vazzana, I.; Rossi, P.; Arfuso, F.; Piccione, G.; et al. Changes in Some Blood Parameters, Milk Composition and Yield of Buffaloes (Bubalus bubalis) during the Transition Period. Anim. Sci. J. 2017, 88, 2025–2032. [Google Scholar] [CrossRef]
- Ha, S.; Kang, S.; Jung, M.; Kim, S.B.; Hwang, S.; Lee, J.; Kim, D.; Choi, K.C.; Park, J. Changes in Haematological and Serum Biochemical Parameter Concentrations from the Day of Calving to Ketosis Onset in Holstein Dairy Cows during the Postpartum Period. Ir. Vet. J. 2025, 78, 8. [Google Scholar] [CrossRef]
- Raboisson, D.; Mounié, M.; Maigné, E. Diseases, Reproductive Performance, and Changes in Milk Production Associated with Subclinical Ketosis in Dairy Cows: A Meta-Analysis and Review. J. Dairy Sci. 2014, 97, 7547–7563. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; McArt, J.A.A. Hyperketonemia. Vet. Clin. N. Am. Food Anim. Pract. 2023, 39, 307–324. [Google Scholar] [CrossRef]
- Melendez, P.; Serrano, M.V. Update on Ketosis in Dairy Cattle with Major Emphasis on Subclinical Ketosis and Abdominal Adiposity. Vet. Med. Sci. 2024, 10, e1525. [Google Scholar] [CrossRef]
- Yu, K.; Canalias, F.; Solà-Oriol, D.; Arroyo, L.; Pato, R.; Saco, Y.; Terré, M.; Bassols, A. Age-Related Serum Biochemical Reference Intervals Established for Unweaned Calves and Piglets in the Post-Weaning Period. Front. Vet. Sci. 2019, 6, 123. [Google Scholar] [CrossRef]
- Bellato, A.; Tondo, A.; Dellepiane, L.; Dondo, A.; Mannelli, A.; Bergagna, S. Estimates of Dairy Herd Health Indicators of Mastitis, Ketosis, Inter-Calving Interval, and Fresh Cow Replacement in the Piedmont Region, Italy. Prev. Vet. Med. 2023, 212, 105834. [Google Scholar] [CrossRef] [PubMed]
- Zwald, N.R.; Weigel, K.A.; Chang, Y.M.; Welper, R.D.; Clay, J.S. Genetic Selection for Health Traits Using Producer-Recorded Data. I. Incidence Rates, Heritability Estimates, and Sire Breeding Values. J. Dairy Sci. 2004, 87, 4287–4294. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and Diagnosing Postpartum Clinical Endometritis and Its Impact on Reproductive Performance in Dairy Cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J. Review: Relationships between Metabolism and Neutrophil Function in Dairy Cows in the Peripartum Period. Animal 2020, 14, s44–s54. [Google Scholar] [CrossRef]
- Ster, C.; Loiselle, M.-C.; Lacasse, P. Effect of Postcalving Serum Nonesterified Fatty Acids Concentration on the Functionality of Bovine Immune Cells. J. Dairy Sci. 2012, 95, 708–717. [Google Scholar] [CrossRef]
- Santo Tomás, H.; Barreto, M.; Vázquez, B.; Villoria, P.; Teixeira, R.; Solé, M. Bovine Respiratory Disease Complex: Prevalence of the Main Respiratory Viruses Involved in Pneumonia in Spain. J. Anim. Sci. Res. 2023, 7, 1–5. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Martínez-Subiela, S.; López-Jornet, P.; Lamy, E. Saliva in Health and Disease; Tvarijonaviciute, A., Martínez-Subiela, S., López-Jornet, P., Lamy, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-37680-2. [Google Scholar]
- Botía, M.; López-Arjona, M.; Escribano, D.; Contreras-Aguilar, M.D.; Vallejo-Mateo, P.J.; Cerón, J.J.; Martínez-Subiela, S. Measurement of Haptoglobin in Saliva of Cows: Validation of an Assay and a Pilot Study of Its Potential Application. Res. Vet. Sci. 2023, 158, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Vallejo-Mateo, P.J.; Lamy, E.; Escribano, D.; Cerón, J.J.; Tecles, F.; Rubio, C.P. Changes in Saliva Analytes in Dairy Cows during Peripartum: A Pilot Study. Animals 2021, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Hevia, M.L.; Escribano, D.; Lamy, E.; Tecles, F.; Cerón, J.J. Effect of Food Contamination and Collection Material in the Measurement of Biomarkers in Saliva of Horses. Res. Vet. Sci. 2020, 129, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Iwersen, M.; Klein-Jöbstl, D.; Pichler, M.; Roland, L.; Fidlschuster, B.; Schwendenwein, I.; Drillich, M. Comparison of 2 Electronic Cowside Tests to Detect Subclinical Ketosis in Dairy Cows and the Influence of the Temperature and Type of Blood Sample on the Test Results. J. Dairy Sci. 2013, 96, 7719–7730. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Mateo, P.J.; Contreras-Aguilar, M.D.; Muñoz-Prieto, A.; Botia, M.; Tvarijonaviciute, A.; Rubio, C.P.; Zelvyte, R.; Cerón, J.J.; Franco-Martínez, L. Saliva as a Potential Source of Biomarkers in Cows with Metritis: A Pilot Study. Vet. Sci. 2024, 11, 446. [Google Scholar] [CrossRef]
- McGuirk, S.M.; Peek, S.F. Timely Diagnosis of Dairy Calf Respiratory Disease Using a Standardized Scoring System. Anim. Health Res. Rev. 2014, 15, 145–147. [Google Scholar] [CrossRef]
- Katz, J.B.; Ridpath, J.F.; Bolin, S.R. Presumptive Diagnostic Differentiation of Hog Cholera Virus from Bovine Viral Diarrhea and Border Disease Viruses by Using a CDNA Nested-Amplification Approach. J. Clin. Microbiol. 1993, 31, 565–568. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Brechtbühl, K.; Stäuber, N. Rapid Characterization of New Pestivirus Strains by Direct Sequencing of PCR-Amplified CDNA from the 5? Noncoding Region. Arch. Virol. 1994, 139, 217–229. [Google Scholar] [CrossRef]
- Hakhverdyan, M.; Hägglund, S.; Larsen, L.-E.; Belák, S. Evaluation of a Single-Tube Fluorogenic RT-PCR Assay for Detection of Bovine Respiratory Syncytial Virus in Clinical Samples. J. Virol. Methods 2005, 123, 195–202. [Google Scholar] [CrossRef]
- Wang, J.; O’Keefe, J.; Orr, D.; Loth, L.; Banks, M.; Wakeley, P.; West, D.; Card, R.; Ibata, G.; Van Maanen, K.; et al. Validation of a Real-Time PCR Assay for the Detection of Bovine Herpesvirus 1 in Bovine Semen. J. Virol. Methods 2007, 144, 103–108. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Vallejo-Mateo, P.J.; Lamy, E.; Cerón, J.J.; Rubio, C.P. Changes in Salivary Analytes in Cows Due to the in Vitro Presence of Feed. BMC Vet. Res. 2022, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Larsson, B.; Olivecrona, G.; Ericson, T. Lipids in Human Saliva. Arch. Oral Biol. 1996, 41, 105–110. [Google Scholar] [CrossRef]
- Mattes, R.D. Accumulating Evidence Supports a Taste Component for Free Fatty Acids in Humans. Physiol. Behav. 2011, 104, 624–631. [Google Scholar] [CrossRef]
- Schmitz, R.; Schnabel, K.; Frahm, J.; von Soosten, D.; Meyer, U.; Hüther, L.; Spiekers, H.; Rehage, J.; Sauerwein, H.; Dänicke, S. Effects of Energy Supply from Roughage and Concentrates and the Occurrence of Subclinical Ketosis on Blood Chemistry and Liver Health in Lactating Dairy Cows during Early Lactation. Dairy 2021, 2, 25–39. [Google Scholar] [CrossRef]
- Guliński, P. Ketone Bodies—Causes and Effects of Their Increased Presence in Cows’ Body Fluids: A Review. Vet. World 2021, 14, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Overton, M.W. Hyperketonemia in Early Lactation Dairy Cattle: A Deterministic Estimate of Component and Total Cost per Case. J. Dairy Sci. 2015, 98, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of Subclinical Ketosis in Early Lactation Dairy Cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef]
- Tufarelli, V.; Puvača, N.; Glamočić, D.; Pugliese, G.; Colonna, M.A. The Most Important Metabolic Diseases in Dairy Cattle during the Transition Period. Animals 2024, 14, 816. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk Factors for Postpartum Uterine Diseases in Dairy Cows. J. Dairy Sci. 2010, 93, 5764–5771. [Google Scholar] [CrossRef]
- Chapinal, N.; Carson, M.; Duffield, T.F.; Capel, M.; Godden, S.; Overton, M.; Santos, J.E.P.; LeBlanc, S.J. The Association of Serum Metabolites with Clinical Disease during the Transition Period. J. Dairy Sci. 2011, 94, 4897–4903. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, Y.; Xiao, J.; Qian, B.; You, L.; Zhang, Y.; Yu, S.; Zong, X.; Cao, S. Serum Adipokines Play Different Roles in Type I and II Ketosis. Asian-Australas. J. Anim. Sci. 2020, 33, 1930–1939. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, B.; Shu, S.; Zhang, H.; Xu, C.; Wu, L.; Xia, C. Critical Thresholds of Liver Function Parameters for Ketosis Prediction in Dairy Cows Using Receiver Operating Characteristic (ROC) Analysis. Vet. Q. 2015, 35, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Nicola, I.; Chupin, H.; Roy, J.-P.; Buczinski, S.; Fauteux, V.; Picard-Hagen, N.; Cue, R.; Dubuc, J. Association between Prepartum Nonesterified Fatty Acid Serum Concentrations and Postpartum Diseases in Dairy Cows. J. Dairy Sci. 2022, 105, 9098–9106. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.M.; Huntimer, L.; Falkenberg, S.; Henningson, J.; Lechtenberg, K.; Halbur, T. An Inactivated Influenza D Virus Vaccine Partially Protects Cattle from Respiratory Disease Caused by Homologous Challenge. Vet. Microbiol. 2017, 199, 47–53. [Google Scholar] [CrossRef]
- Wolfger, B.; Schwartzkopf-Genswein, K.S.; Barkema, H.W.; Pajor, E.A.; Levy, M.; Orsel, K. Feeding Behavior as an Early Predictor of Bovine Respiratory Disease in North American Feedlot Systems1. J. Anim. Sci. 2015, 93, 377–385. [Google Scholar] [CrossRef]
- Pardon, B.; Buczinski, S. Bovine Respiratory Disease Diagnosis. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 425–444. [Google Scholar] [CrossRef]
- Alkheraif, A.A.; Topliff, C.L.; Reddy, J.; Massilamany, C.; Donis, R.O.; Meyers, G.; Eskridge, K.M.; Kelling, C.L. Type 2 BVDV Npro Suppresses IFN-1 Pathway Signaling in Bovine Cells and Augments BRSV Replication. Virology 2017, 507, 123–134. [Google Scholar] [CrossRef]
- Pardon, B.; Callens, J.; Maris, J.; Allais, L.; Van Praet, W.; Deprez, P.; Ribbens, S. Pathogen-Specific Risk Factors in Acute Outbreaks of Respiratory Disease in Calves. J. Dairy Sci. 2020, 103, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Richeson, J.T. Behavior Assessment and Applications for BRD Diagnosis: Beef. Anim. Health Res. Rev. 2020, 21, 192–195. [Google Scholar] [CrossRef]
- White, B.J.; Renter, D.G. Bayesian Estimation of the Performance of Using Clinical Observations and Harvest Lung Lesions for Diagnosing Bovine Respiratory Disease in Post-Weaned Beef Calves. J. Vet. Diagn. Investig. 2009, 21, 446–453. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect Size, Confidence Interval and Statistical Significance: A Practical Guide for Biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Kotsampasi, B.; Karatzia, M.A.; Tsiokos, D.; Chadio, S. Nutritional Strategies to Alleviate Stress and Improve Welfare in Dairy Ruminants. Animals 2024, 14, 2573. [Google Scholar] [CrossRef] [PubMed]




| Mean | SD | CV | ||
|---|---|---|---|---|
| BHB | ||||
| Intra-assay | High | 0.0784 | 0.0021 | 2.71 |
| Low | 0.0238 | 0.0016 | 6.55 | |
| Inter-assay | High | 0.0749 | 0.0040 | 5.32 |
| Low | 0.0228 | 0.0011 | 4.69 | |
| NEFAs | ||||
| Intra-assay | High | 0.400 | 0.006 | 1.58 |
| Low | 0.058 | 0.004 | 7.00 | |
| Inter-assay | High | 0.408 | 0.023 | 5.67 |
| Low | 0.066 | 0.009 | 13.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, C.P.; Rigueira, L.; Miranda, M.; Vallejo, P.J.; Semitiel, J.; del Olmo, D.; Contreras-Aguilar, M.D.; Silva, F.G.; Lamy, E.; De la Fe, C.; et al. Validation of the Measurement of Beta-Hydroxybutyrate and Non-Esterified Fatty Acids in Bovine Saliva: A Pilot Report. Life 2025, 15, 854. https://doi.org/10.3390/life15060854
Rubio CP, Rigueira L, Miranda M, Vallejo PJ, Semitiel J, del Olmo D, Contreras-Aguilar MD, Silva FG, Lamy E, De la Fe C, et al. Validation of the Measurement of Beta-Hydroxybutyrate and Non-Esterified Fatty Acids in Bovine Saliva: A Pilot Report. Life. 2025; 15(6):854. https://doi.org/10.3390/life15060854
Chicago/Turabian StyleRubio, Camila P., Lucas Rigueira, Marta Miranda, Pedro Javier Vallejo, Jesús Semitiel, David del Olmo, María D. Contreras-Aguilar, Flávio G. Silva, Elsa Lamy, Christian De la Fe, and et al. 2025. "Validation of the Measurement of Beta-Hydroxybutyrate and Non-Esterified Fatty Acids in Bovine Saliva: A Pilot Report" Life 15, no. 6: 854. https://doi.org/10.3390/life15060854
APA StyleRubio, C. P., Rigueira, L., Miranda, M., Vallejo, P. J., Semitiel, J., del Olmo, D., Contreras-Aguilar, M. D., Silva, F. G., Lamy, E., De la Fe, C., Cerón, J. J., & Tecles, F. (2025). Validation of the Measurement of Beta-Hydroxybutyrate and Non-Esterified Fatty Acids in Bovine Saliva: A Pilot Report. Life, 15(6), 854. https://doi.org/10.3390/life15060854











