Presence of Coliforms and Reduced Water Quality in the Second Biggest Reservoir in São Paulo, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. The Reservoir and Sample Collection
2.2. Physicochemical Analysis
2.3. Bacterial Culture
2.4. Bacterial DNA Extraction, Amplification of the 16S rRNA Gene, Library Preparation and Sequencing
2.5. Sequencing Analysis—EzBioCloud and Qiime 2
2.6. Antibiotic Resistance
3. Results
3.1. Physicochemical Results
3.2. Microbiological Culture
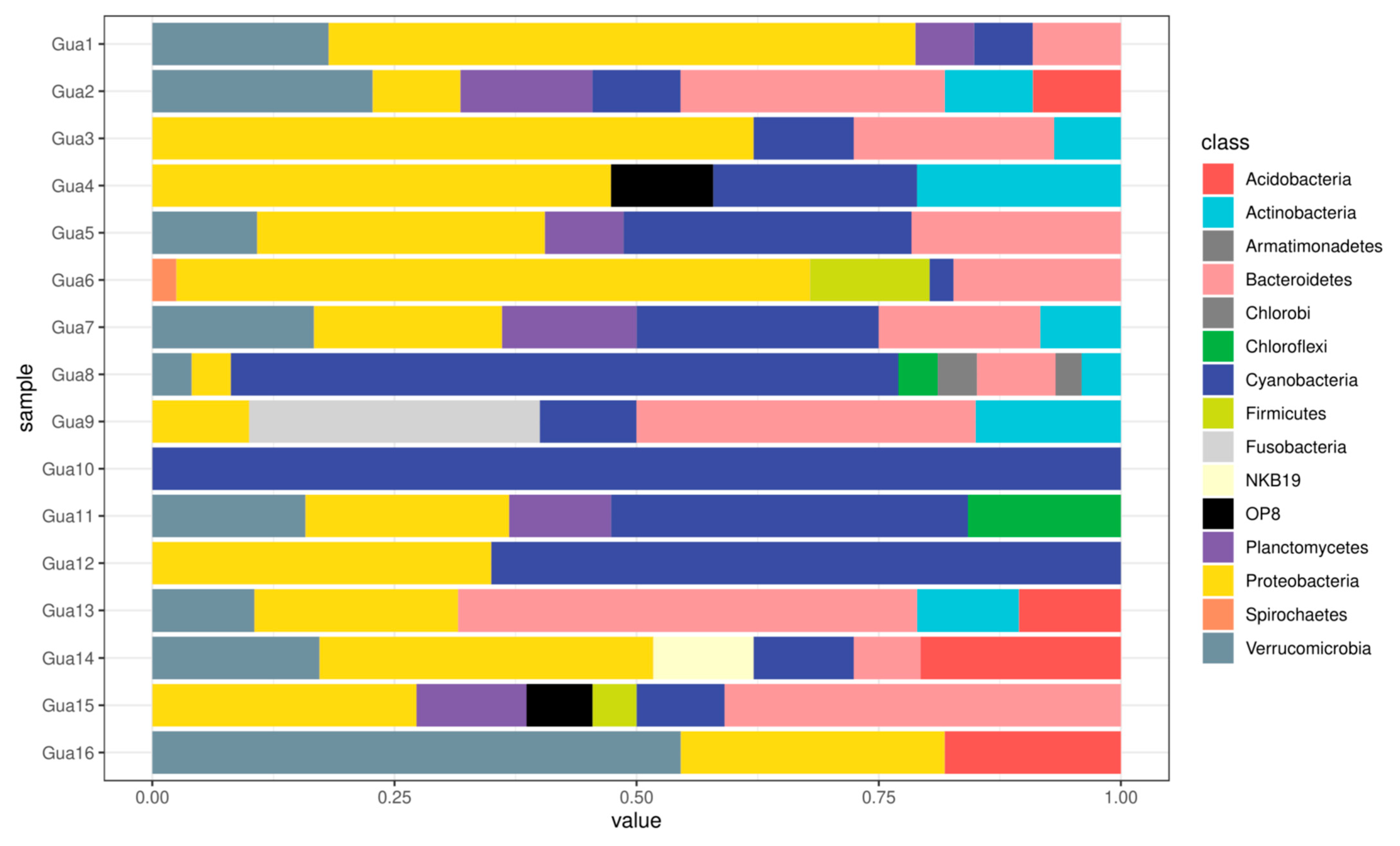
3.3. Sequencing Data
3.4. Phylogenetic Analysis
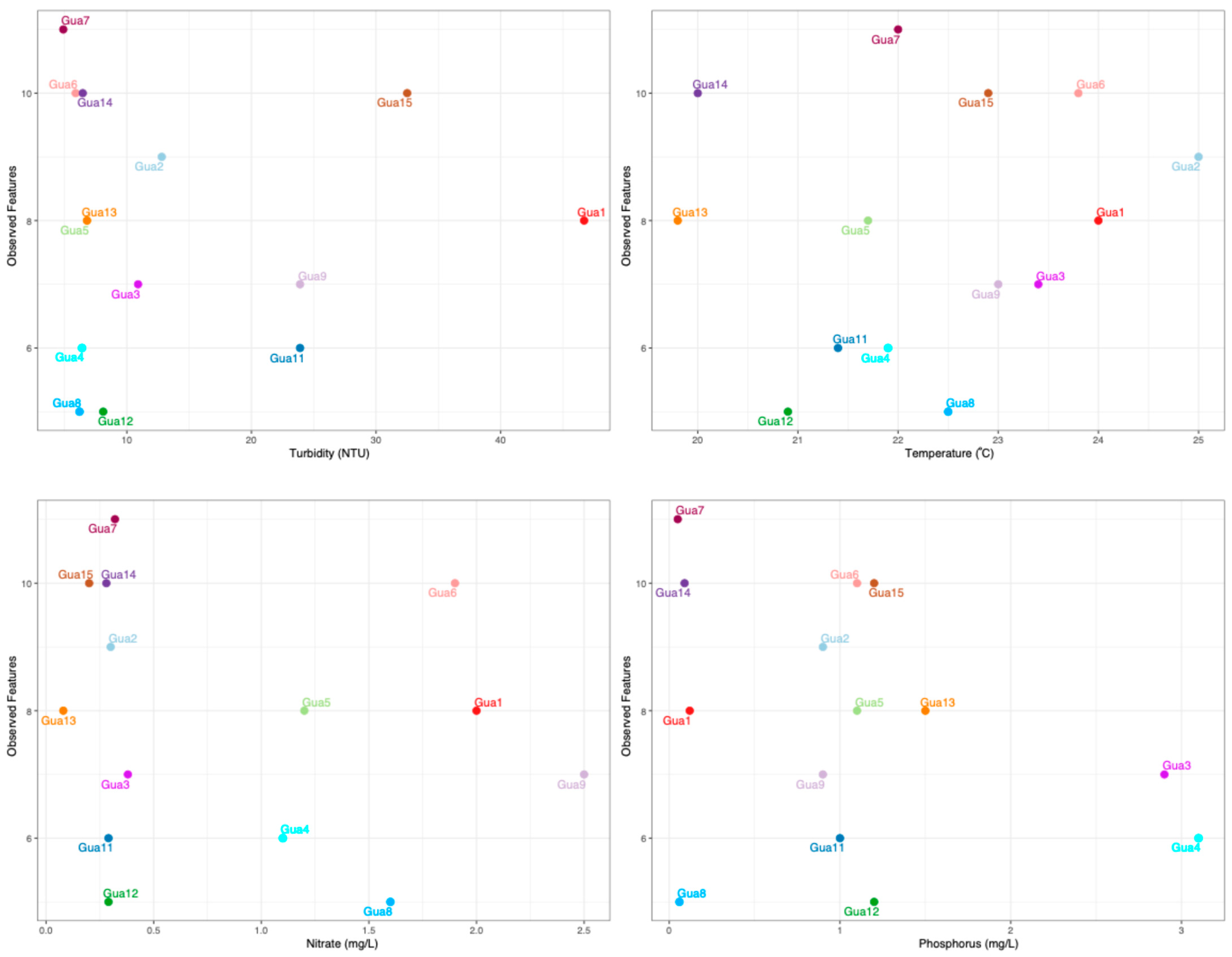
3.5. Interaction Between Physical–Chemical Characteristics and Bacteria—An Obesrvation
3.6. Antimicrobial Resistance
4. Discussion
4.1. Physicochemical
4.2. Bacteriome Diversity and Composition
4.3. Intereation Between Physicochemical Characteristics and Bacterial Composition
4.4. Antibiotic Resistance Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarapiranga, A.N. História da Guarapiranga. Available online: https://www.guarapiranga.org/historia (accessed on 17 June 2024).
- Baltrusis, N. Environmental Rehabilitation and Public Health. The Guarapiranga Program; Ancona, A.L., Ed.; Saúde e Sociedade: São Paulo, Brazil, 2006; Volume 15, pp. 9–21. [Google Scholar] [CrossRef]
- Leal, P.R.; Moschini-Carlos, V.; López-Doval, J.C.; Cintra, J.P.; Yamamoto, J.K.; Bitencourt, M.D.; Santos, R.F.; Abreu, G.C.; Pompêo, M.L.M. Impact of copper sulfate application at an urban Brazilian reservoir: A geostatistical and ecotoxicological approach. Sci. Total Environ. 2018, 618, 621–634. [Google Scholar] [CrossRef]
- São Paulo (Estado) Secretaria do Meio Ambiente; Ambiental, C.d.E. Guarapiranga Caderno Ambiental; São Paulo (Estado) Secretaria do Meio Ambiente, Coordenadoria de Educação Ambiental: São Paulo, Brazil, 2008; p. 84. [Google Scholar]
- Borelli, E. A Bacia do Guarapiranga: Ocupação em Áreas de Mananciais e a Legislação Ambiental. Rev. Ciências SociaisPolítica Trab. 2006, 25, 189. Available online: https://periodicos.ufpb.br/ojs/index.php/politicaetrabalho/article/view/6745 (accessed on 10 October 2024).
- López-Doval, J.C.; Montagner, C.C.; de Alburquerque, A.F.; Moschini-Carlos, V.; Umbuzeiro, G.; Pompêo, M. Nutrients, emerging pollutants and pesticides in a tropical urban reservoir: Spatial distributions and risk assessment. Sci. Total Environ. 2017, 575, 1307–1324. [Google Scholar] [CrossRef]
- Richter, E.M.; Fornaro, A.; do Lago, C.L. Avaliação da composição química de águas do Sistema Guarapiranga: Estudo de caso nos anos de 2002 e 2003. Química Nova 2007, 30, 1147–1152. [Google Scholar] [CrossRef]
- Bueno, A.S.; Rodrigues, E.S.; Maruyama, L.S.; Castro, P.M.G. Evaluation of water quality in fee-fishing ponds located in Guarapiranga’s sub basin, Upper Tietê River basin, São Paulo State. Braz. J. Biol. 2020, 80, 319–329. [Google Scholar] [CrossRef]
- Paulo, G.d.E.d.S. Sabesp Amplia Produção de Água do Sistema Guarapiranga. Available online: https://www.saopaulo.sp.gov.br/spnoticias/ultimas-noticias/sabesp-amplia-producao-de-agua-do-sistema-guarapiranga-1/ (accessed on 17 June 2024).
- Llirós, M.; Inceoğlu, Ö.; García-Armisen, T.; Anzil, A.; Leporcq, B.; Pigneur, L.M.; Viroux, L.; Darchambeau, F.; Descy, J.P.; Servais, P. Bacterial community composition in three freshwater reservoirs of different alkalinity and trophic status. PLoS ONE 2014, 9, e116145. [Google Scholar] [CrossRef]
- Pinto, I.; Simões, M.; Gomes, I.B. An Overview of the Impact of Pharmaceuticals on Aquatic Microbial Communities. Antibiotics 2022, 11, 1700. [Google Scholar] [CrossRef]
- Grigorova, E.; Belkova, N.; Nemchenko, U.; Klimenko, E.; Pogodina, A.; Romanitsa, A.; Novikova, E.; Rychkova, L. Metasequencing of V3-V4 variable regions of 16S rRNA gene in opportunistic microbiota and gut biocenosis in obese adolescents. Bull. Exp. Biol. Med. 2021, 170, 321–325. [Google Scholar] [CrossRef]
- Francis, A.J.; Oribhabor, B.J. The public health implications of pathogens in polluted aquatic ecosystems: A review. Trop. Freshw. Biol. 2017, 25, 85–101. [Google Scholar] [CrossRef]
- Abia, A.L.K.; Baloyi, T.; Traore, A.N.; Potgieter, N. The African Wastewater Resistome: Identifying Knowledge Gaps to Inform Future Research Directions. Antibiotics 2023, 12, 805. [Google Scholar] [CrossRef]
- Fredriksen, S.; de Warle, S.; van Baarlen, P.; Boekhorst, J.; Wells, J.M. Resistome expansion in disease-associated human gut microbiomes. Microbiome 2023, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kim, Y.; Han, S.; Hur, H.G. Resistome Study in Aquatic Environments. J. Microbiol. Biotechnol. 2023, 33, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Bianco, K.; de Farias, B.O.; Gonçalves-Brito, A.S.; Alves do Nascimento, A.P.; Magaldi, M.; Montenegro, K.; Flores, C.; Oliveira, S.; Monteiro, M.A.; Spisso, B.F.; et al. Mobile resistome of microbial communities and antimicrobial residues from drinking water supply systems in Rio de Janeiro, Brazil. Sci. Rep. 2022, 12, 19050. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Franco, D.; Corbera-Rubio, F.; Cuesta-Sanz, M.; Pieterse, B.; de Ridder, D.; van Loosdrecht, M.C.M.; van Halem, D.; Laureni, M.; Weissbrodt, D.G. Microbiome, resistome and mobilome of chlorine-free drinking water treatment systems. Water Res. 2023, 235, 119905. [Google Scholar] [CrossRef]
- Vassallo, A.; Kett, S.; Purchase, D.; Marvasi, M. The Bacterial Urban Resistome: Recent Advances. Antibiotics 2022, 11, 512. [Google Scholar] [CrossRef]
- Hamed, Y.; Khelifi, F.; Houda, B.; Sâad, A.B.; Ncibi, K.; Hadji, R.; Melki, A.; Hamad, A. Phosphate mining pollution in southern Tunisia: Environmental, epidemiological, and socioeconomic investigation. Environ. Dev. Sustain. 2023, 25, 13619–13636. [Google Scholar] [CrossRef]
- Hamed, Y.; Ayadi, Y.; Hadji, R.; Ben Saad, A.; Gentilucci, M.; Elaloui, E. Environmental Radioactivity, Ecotoxicology (238U, 232Th and 40K) and Potentially Toxic Elements in Water and Sediments from North Africa Dams. Sustainability 2024, 16, 490. [Google Scholar] [CrossRef]
- Águas, A.N.d. Portal da Qualidade das Águas. Available online: https://portalpnqa.ana.gov.br/perguntas-frequentes.aspx (accessed on 19 June 2024).
- Cardoso-Silva, S.; López-Doval, J.C.; Moschini-Carlos, V.; Pompêo, M. Metals and limnological variables in an urban reservoir: Compartmentalization and identification of potential impacted areas. Environ. Monit. Assess. 2017, 190, 19. [Google Scholar] [CrossRef]
- Marcondes, M.A.; Nascimento, A.; Pessôa, R.; Victor, J.R.; Duarte, A.J.D.S.; Clissa, P.B.; Sanabani, S.S. Characterization of Bacterial Communities from the Surface and Adjacent Bottom Layers of Water in the Billings Reservoir. Life 2022, 12, 1280. [Google Scholar] [CrossRef]
- Instruments, H. Medidor Portátil Multiparâmetro para pH/EC/OD Óptico com Bluetooth. Available online: https://hannainst.com.br/produto/hi98494-10-medidor-portatil-multiparametro-para-ph-ec-od-optico-com-bluetooth-c-cabo-de-10-m/ (accessed on 10 October 2024).
- Sabila, N.m.; Setyaningrum, D. Coliform and Colifecal Analysis in Water Form Various Sources Using the MPN (Most Probable Numbers) Method: Analisis Coliform dan Colifecal pada Air dari Berbagai Sumber Menggunakan Metode MPN (Most Probable Numbers). J. Kim. Dan Rekayasa 2023, 3, 54–60. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Fonseca, T.A.; Pessôa, R.; Sanabani, S.S. Molecular Analysis of Bacterial Microbiota on Brazilian Currency Note Surfaces. Int. J. Environ. Res. Public Health 2015, 12, 13276–13288. [Google Scholar] [CrossRef]
- Pereira da Fonseca, T.A.; Pessôa, R.; Felix, A.C.; Sanabani, S.S. Diversity of Bacterial Communities on Four Frequently Used Surfaces in a Large Brazilian Teaching Hospital. Int. J. Environ. Res. Public Health 2016, 13, 152. [Google Scholar] [CrossRef]
- Pereira da Fonseca, T.A.; Pessôa, R.; Sanabani, S.S. Molecular investigation of bacterial communities: Data from two frequently used surfaces in the São Paulo Institute of Tropical Medicine. Data Brief 2016, 8, 399–403. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Tan, L.; Li, L.; Ashbolt, N.; Wang, X.; Cui, Y.; Zhu, X.; Xu, Y.; Yang, Y.; Mao, D.; Luo, Y. Arctic antibiotic resistance gene contamination, a result of anthropogenic activities and natural origin. Sci. Total Environ. 2018, 621, 1176–1184. [Google Scholar] [CrossRef]
- Semensatto, D.; Labuto, G.; Zorzal-Almeida, S.; McRae, D.V. Spatio-temporal changes in water quality in the Guarapiranga reservoir (São Paulo, Brazil): Insights from a long-term monitoring data series. Environ. Monit. Assess. 2021, 193, 380. [Google Scholar] [CrossRef] [PubMed]
- Conselho Nacional Do Meio Ambiente. Resolução CONAMA nº 357, de 17 de Março de 2005; FAO: Rome, Italy, 2005. [Google Scholar]
- Conselho Nacional Do Meio Ambiente. RESOLUÇÃO CONAMA nº 274, de 29 de Novembro de 2000; FAO: Rome, Italy, 2000. [Google Scholar]
- Fu, Q.; Zheng, B.; Zhao, X.; Wang, L.; Liu, C. Ammonia pollution characteristics of centralized drinking water sources in China. J. Environ. Sci. 2012, 24, 1739–1743. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z. Prediction of biochemical oxygen demand with genetic algorithm-based support vector regression. Water Qual. Res. J. 2023, 58, 87–98. [Google Scholar] [CrossRef]
- Rudaru, D.G.; Lucaciu, I.; Fulgheci, A.M. Correlation between BOD5 and COD–biodegradability indicator of wastewater. Rom. J. Ecol. Environ. Chem. 2022, 4, 80–86. [Google Scholar] [CrossRef]
- Asteris, P.G.; Alexakis, D.E.; Tsoukalas, M.Z.; Gamvroula, D.E.; Guney, D. Machine learning approach for rapid estimation of five-day biochemical oxygen demand in wastewater. Water 2022, 15, 103. [Google Scholar] [CrossRef]
- Fontana, L.; Albuquerque, A.L.S.; Brenner, M.; Bonotto, D.M.; Sabaris, T.P.P.; Pires, M.A.F.; Cotrim, M.E.B.; Bicudo, D.C. The eutrophication history of a tropical water supply reservoir in Brazil. J. Paleolimnol. 2014, 51, 29–43. [Google Scholar] [CrossRef]
- Andrade, M.R.M.d.; Salim, A.; Rossini-Penteado, D.; Costa, J.A.d.; Souza, A.A.d.; Saad, A.R.; Oliveira, A.M.d.S. Mapeamento de Uso da Terra Para Avaliação da Qualidade das Águas do Reservatório Guarapiranga. Geociências 2015, 34, 258–274. [Google Scholar]
- Li, Y.; Wang, F.; Feng, J.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xu, L.; Xie, S. Spatio-temporal variation and risk assessment of hydrochemical indices in a large diversion project of the Yellow River, northern China, from 2008 to 2017. Environ. Sci. Pollut. Res. 2020, 27, 28438–28448. [Google Scholar] [CrossRef]
- Marques, L.O.d.A.; Taffarello, D.; Calijuri, M.d.C.; Mendiondo, E.M.; Ferreira, M.d.S.; Cunha, D.G.F. Phosphorus and thermotolerant coliforms’ loads in Brazilian watersheds with limited data: Considerations on the integrated analysis of water quality and quantity. RBRH 2019, 24, e3. [Google Scholar] [CrossRef]
- Soares, D.M.M.; Atum, S.V.F.; Bechara, E.J.H.; Setubal, J.C.; Stevani, C.V.; Freire, R.S. Exploring the Microbiota of the Guarapiranga Water Reservoir with Long-Read Sequencing Technology. Front. Mar. Sci. 2021, 8, 1–4. [Google Scholar] [CrossRef]
- Staley, C.; Gould, T.J.; Wang, P.; Phillips, J.; Cotner, J.B.; Sadowsky, M.J. Species sorting and seasonal dynamics primarily shape bacterial communities in the Upper Mississippi River. Sci. Total. Environ. 2015, 505, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Yun, J.; Hong, S.H.; Cho, K.S. Effects of water temperature and backwashing on bacterial population and community in a biological activated carbon process at a water treatment plant. Appl. Microbiol. Biotechnol. 2014, 98, 1417–1427. [Google Scholar] [CrossRef]
- Li, X.; Upadhyaya, G.; Yuen, W.; Brown, J.; Morgenroth, E.; Raskin, L. Changes in the structure and function of microbial communities in drinking water treatment bioreactors upon addition of phosphorus. Appl. Environ. Microbiol. 2010, 76, 7473–7481. [Google Scholar] [CrossRef]
- Li, Q.; Yu, S.; Li, L.; Liu, G.; Gu, Z.; Liu, M.; Liu, Z.; Ye, Y.; Xia, Q.; Ren, L. Microbial Communities Shaped by Treatment Processes in a Drinking Water Treatment Plant and Their Contribution and Threat to Drinking Water Safety. Front. Microbiol. 2017, 8, 2465. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Litchman, E. Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia 2016, 763, 357–369. [Google Scholar] [CrossRef]
- Du, C.; Wang, Q.; Li, Y.; Lyu, H.; Zhu, L.; Zheng, Z.; Wen, S.; Liu, G.; Guo, Y. Estimation of total phosphorus concentration using a water classification method in inland water. Int. J. Appl. Earth Obs. Geoinf. 2018, 71, 29–42. [Google Scholar] [CrossRef]
- Sharpley, A.N.; McDowell, R.W.; Kleinman, P.J. Phosphorus loss from land to water: Integrating agricultural and environmental management. Plant Soil 2001, 237, 287–307. [Google Scholar] [CrossRef]
- Xing, X.; Li, T.; Bi, Z.; Qi, P.; Li, Z.; Chen, Y.; Zhou, H.; Wang, H.; Xu, G.; Chen, C. Destruction of microbial stability in drinking water distribution systems by trace phosphorus polluted water source. Chemosphere 2021, 275, 130032. [Google Scholar] [CrossRef]
- Xie, J.; Xue, W.; Li, C.; Yan, Z.; Li, D.; Li, G.; Chen, X.; Chen, D. Water-soluble phosphorus contributes significantly to shaping the community structure of rhizospheric bacteria in rocky desertification areas. Sci. Rep. 2019, 9, 18408. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, M.; Zhu, Z.; Lu, L.; Ding, J.; Zhou, Q.; Gao, X.; Tang, N.; Li, S.; Li, X.; et al. The role of microorganisms in phosphorus cycling at river-lake confluences: Insights from a study on microbial community dynamics. Water Res. 2024, 268, 122556. [Google Scholar] [CrossRef]
- Godoy, R.G.; Marcondes, M.A.; Pessôa, R.; Nascimento, A.; Victor, J.R.; Duarte, A.J.D.S.; Clissa, P.B.; Sanabani, S.S. Bacterial community composition and potential pathogens along the Pinheiros River in the southeast of Brazil. Sci. Rep. 2020, 10, 9331. [Google Scholar] [CrossRef]
- Leng, Y.; Xiao, H.; Li, Z.; Wang, J. Tetracyclines, sulfonamides and quinolones and their corresponding resistance genes in coastal areas of Beibu Gulf, China. Sci. Total Environ. 2020, 714, 136899. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Liu, J.; Zhang, G.; Tong, Y.; Ma, N. Exploring the correlations between antibiotics and antibiotic resistance genes in the wastewater treatment plants of hospitals in Xinjiang, China. Environ. Sci. Pollut. Res. Int. 2016, 23, 15111–15121. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Le-Minh, N.; Khan, S.J.; Drewes, J.E.; Stuetz, R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010, 44, 4295–4323. [Google Scholar] [CrossRef] [PubMed]
- Su, L.H.; Chen, H.L.; Chia, J.H.; Liu, S.Y.; Chu, C.; Wu, T.L.; Chiu, C.H. Distribution of a transposon-like element carrying bla(CMY-2) among Salmonella and other Enterobacteriaceae. J. Antimicrob. Chemother. 2006, 57, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Cuzon, G.; Truong, H.V.; Nordmann, P. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob. Agents Chemother. 2012, 56, 4753–4759. [Google Scholar] [CrossRef]
- Tristram, S.G.; Hawes, R.; Souprounov, J. Variation in selected regions of blaTEM genes and promoters in Haemophilus influenzae. J. Antimicrob. Chemother. 2005, 56, 481–484. [Google Scholar] [CrossRef]
- Poirel, L.; Ros, A.; Carricajo, A.; Berthelot, P.; Pozzetto, B.; Bernabeu, S.; Nordmann, P. Extremely drug-resistant Citrobacter freundii isolate producing NDM-1 and other carbapenemases identified in a patient returning from India. Antimicrob. Agents Chemother. 2011, 55, 447–448. [Google Scholar] [CrossRef]
- Pomba, C.; da Fonseca, J.D.; Baptista, B.C.; Correia, J.D.; Martínez-Martínez, L. Detection of the pandemic O25-ST131 human virulent Escherichia coli CTX-M-15-producing clone harboring the qnrB2 and aac(6’)-Ib-cr genes in a dog. Antimicrob. Agents Chemother. 2009, 53, 327–328. [Google Scholar] [CrossRef]
- Jahantigh, M.; Samadi, K.; Dizaji, R.E.; Salari, S. Antimicrobial resistance and prevalence of tetracycline resistance genes in Escherichia coli isolated from lesions of colibacillosis in broiler chickens in Sistan, Iran. BMC Vet. Res. 2020, 16, 267. [Google Scholar] [CrossRef]
- Suzuki, S.; Ogo, M.; Miller, T.W.; Shimizu, A.; Takada, H.; Siringan, M.A. Who possesses drug resistance genes in the aquatic environment? Sulfamethoxazole (SMX) resistance genes among the bacterial community in water environment of Metro-Manila, Philippines. Front. Microbiol. 2013, 4, 102. [Google Scholar] [CrossRef]
- Harrower, J.; McNaughtan, M.; Hunter, C.; Hough, R.; Zhang, Z.; Helwig, K. Chemical Fate and Partitioning Behavior of Antibiotics in the Aquatic Environment-A Review. Environ. Toxicol. Chem. 2021, 40, 3275–3298. [Google Scholar] [CrossRef]
- Park, S.; Choi, K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 2008, 17, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Reinhold, D. Biosolid-borne tetracyclines and sulfonamides in plants. Environ. Sci. Pollut. Res. Int. 2013, 20, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Shihomatsu, H.; Martins, E.; Cotrim, M.; Lebre, D.; Ortiz, N.; Pires, F. Guarapiranga Reservoir—Pharmaceuticals and Historical Urban Occupation in a Water Source. J. Geosci. Environ. Prot. 2017, 5, 1–17. [Google Scholar] [CrossRef]





| Genes | Resistance | Genes | Resistance |
|---|---|---|---|
| aacC2 | Aminoglycosides | int1 | Various antibiotics (presence of integrons) |
| aacC3 | Aminoglycosides | qepA | Fluoroquinolones |
| aacC4 | Aminoglycosides | qnrA | Fluoroquinolones |
| AmpC | Beta-lactams | qnrB | Fluoroquinolones |
| blaCMY | Beta-lactams | qnrD | Fluoroquinolones |
| blaDHA | Beta-lactams | qnrS | Fluoroquinolones |
| blaGes | Beta-lactams | Sul1 | Sulfonamides |
| blaKPC | Beta-lactams * | Sul2 | Sulfonamides |
| blaNDM | Beta-lactams * | Sul3 | Sulfonamides |
| blaOxa-1 | Beta-lactams | ermC | Macrolides (erythromycin) |
| blaOxa-10 | Beta-lactams | tetA | Tetracyclines |
| blaOxa-2 | Beta-lactams | tetB | Tetracyclines |
| blaSHV | Beta-lactams | tetC | Tetracyclines |
| blaTEM | Beta-lactams | tetD | Tetracyclines |
| blaVIM | Beta-lactams * | tetM | Tetracyclines |
| Samples | Genus/Species |
|---|---|
| Gua1 | Salmonella spp., Shiguella spp. and Escherichia coli |
| Gua2 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua3 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua4 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua5 | Salmonella spp., Pseudomonas spp., Shiguella spp. and Escherichi coli |
| Gua6 | Escherichia coli e Salmonella spp. |
| Gua7 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua8 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua9 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua10 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua11 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua12 | Salmonella spp., Shiguella spp. and Escherichia coli |
| Gua13 | Salmonella spp., Shiguella spp. and Escherichia coli |
| Gua14 | Salmonella spp., Shiguella spp. and Escherichia coli |
| Gua15 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Gua16 | Salmonella spp., Shiguella spp. and Escherichia coli |
| Gua17 | Salmonella spp., Shiguella spp., Klebsiella spp. and Eschericha coli |
| Sample (Bottom) | Depth (Meter) | Turbidity (NTU) | Water Temperature (°C) | DO (mg/L) | pH | Ammonia (mg/L) | Nitrate (mg/L) | Phosphorus (mg/L) | CFU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| Gua1 | 1.40 | 0.39 | 108.08 | 30.00 | 21.83 | 0.96 | 5.10 | 1.01 | 7.05 | 0.37 | 6.28 | 0.28 | 1.93 | 0.69 | 11.66 | 0.50 | 30,750.00 | 10,734.48 |
| Gua2 | 2.83 | 0.43 | 334.75 | 208.48 | 21.75 | 0.51 | 6.60 | 2.26 | 6.60 | 0.11 | 4.20 | 0.35 | 0.20 | 0.04 | 2.21 | 0.26 | 9725.00 | 4712.28 |
| Gua3 | 1.50 | 0.25 | 44.08 | 14.64 | 21.60 | 0.66 | 6.80 | 1.18 | 7.13 | 0.08 | 5.75 | 1.21 | 0.62 | 0.04 | 2.02 | 0.32 | 61,175.00 | 18,529.63 |
| Gua4 | 1.23 | 0.20 | 97.98 | 7.28 | 21.60 | 0.56 | 6.63 | 4.53 | 6.90 | 0.07 | 5.50 | 0.19 | 1.05 | 0.06 | 3.05 | 0.05 | 72,000.00 | 13,335.42 |
| Gua5 | 1.15 | 0.06 | 144.61 | 46.18 | 21.33 | 0.64 | 5.25 | 1.64 | 6.95 | 0.03 | 5.43 | 0.22 | 3.65 | 0.81 | 4.37 | 2.40 | 576,000.00 | 107,063.84 |
| Gua6 | 1.90 | 0.09 | 215.85 | 96.87 | 21.35 | 0.61 | 2.00 | 0.60 | 6.00 | 0.07 | 4.85 | 0.39 | 2.07 | 0.27 | 6.33 | 0.25 | 696,500.00 | 107,698.27 |
| Gua7 | 4.13 | 0.56 | 147.60 | 26.27 | 21.05 | 0.53 | 7.18 | 1.31 | 6.68 | 0.27 | 3.70 | 0.11 | 0.89 | 0.04 | 0.85 | 0.56 | 23,500.00 | 3662.88 |
| Gua8 | 2.25 | 0.55 | 198.00 | 29.45 | 20.55 | 0.68 | 5.80 | 1.43 | 6.78 | 0.13 | 1.70 | 0.39 | 0.90 | 0.25 | 0.67 | 0.53 | 1,792,500.00 | 1,171,589.91 |
| Gua9 | 2.58 | 0.63 | 117.28 | 42.60 | 21.08 | 0.63 | 4.45 | 0.36 | 6.78 | 0.23 | 4.70 | 0.32 | 2.58 | 0.23 | 3.72 | 2.29 | 62,500.00 | 8490.19 |
| Gua10 | 2.68 | 0.48 | 189.15 | 55.76 | 21.25 | 0.75 | 4.73 | 0.17 | 6.38 | 0.22 | 3.88 | 0.13 | 0.42 | 0.14 | 1.97 | 0.30 | 689,250.00 | 182,758.21 |
| Gua11 | 4.70 | 0.56 | 141.68 | 41.01 | 20.53 | 0.50 | 4.43 | 0.37 | 6.90 | 0.18 | 5.18 | 0.29 | 0.34 | 0.02 | 1.74 | 0.33 | 178,000.00 | 4546.06 |
| Gua12 | 2.00 | 0.38 | 187.65 | 68.13 | 20.05 | 0.53 | 4.58 | 0.67 | 7.48 | 0.51 | 4.58 | 0.23 | 0.34 | 0.02 | 6.96 | 1.18 | 202,500.00 | 23,935.68 |
| Gua13 | 6.63 | 0.84 | 92.45 | 24.20 | 20.33 | 0.34 | 5.38 | 0.22 | 7.20 | 0.14 | 4.08 | 0.09 | 0.10 | 0.00 | 1.97 | 0.05 | 223,750.00 | 14,343.26 |
| Gua14 | 7.93 | 1.52 | 268.93 | 178.84 | 21.08 | 0.55 | 6.45 | 1.39 | 7.10 | 0.11 | 4.13 | 0.06 | 0.29 | 0.04 | 1.84 | 0.48 | 228,250.00 | 12,769.59 |
| Gua15 | 1.38 | 0.14 | 240.15 | 78.80 | 20.68 | 0.49 | 3.03 | 1.14 | 7.10 | 0.07 | 4.33 | 0.12 | 0.25 | 0.01 | 1.59 | 0.16 | 276,250.00 | 11,433.69 |
| Gua16 | 2.20 | 0.47 | 94.43 | 33.51 | 20.18 | 0.42 | 4.29 | 0.51 | 7.15 | 0.06 | 3.88 | 0.27 | 0.21 | 0.03 | 1.34 | 0.19 | 62,800.00 | 46,121.29 |
| Gua17 | 1.70 | 0.60 | 123.00 | 8.96 | 19.20 | 0.49 | 3.90 | 0.91 | 6.77 | 0.19 | 3.97 | 0.09 | 1.17 | 0.18 | 1.58 | 0.23 | 254,666.67 | 8666.67 |
| Sample (Surface) | BOD (mg/L) | Turbidity (NTU) | Water temperature (°C) | DO (mg/L) | pH | Ammonia (mg/L) | Nitrate (mg/L) | Phosphorus (mg/L) | CFU | |||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |
| Gua1 | 7.18 | 0.45 | 23.23 | 9.33 | 22.88 | 0.53 | 6.43 | 1.16 | 7.43 | 0.31 | 5.25 | 0.23 | 1.42 | 0.52 | 0.27 | 0.06 | 27,850.00 | 9727.41 |
| Gua2 | 6.25 | 0.19 | 14.62 | 4.45 | 22.65 | 0.92 | 6.88 | 1.15 | 6.85 | 0.23 | 4.23 | 0.30 | 0.20 | 0.07 | 0.79 | 0.21 | 7725.00 | 3942.16 |
| Gua3 | 8.20 | 0.37 | 12.88 | 6.14 | 21.80 | 0.94 | 9.10 | 1.91 | 6.93 | 0.21 | 4.68 | 1.05 | 0.46 | 0.06 | 1.72 | 0.49 | 52,075.00 | 15,370.61 |
| Gua4 | 8.10 | 0.18 | 12.12 | 5.29 | 21.78 | 0.78 | 9.98 | 3.28 | 6.53 | 0.22 | 5.03 | 0.11 | 0.90 | 0.07 | 2.85 | 0.09 | 44,000.00 | 3341.66 |
| Gua5 | 6.75 | 0.19 | 14.38 | 5.54 | 21.45 | 0.70 | 7.10 | 1.12 | 6.95 | 0.10 | 4.93 | 0.35 | 0.94 | 0.15 | 2.02 | 1.08 | 556,500.00 | 103,628.74 |
| Gua6 | 8.88 | 0.21 | 4.51 | 0.53 | 22.20 | 1.02 | 4.90 | 1.08 | 6.15 | 0.12 | 4.40 | 0.40 | 1.43 | 0.25 | 1.14 | 0.22 | 565,000.00 | 82,209.08 |
| Gua7 | 4.98 | 0.41 | 16.40 | 6.94 | 21.55 | 0.80 | 8.90 | 1.53 | 6.40 | 0.19 | 3.25 | 0.23 | 0.30 | 0.02 | 0.78 | 0.71 | 18,500.00 | 1707.83 |
| Gua8 | 5.60 | 0.21 | 10.84 | 5.46 | 22.10 | 0.96 | 7.65 | 1.38 | 7.13 | 0.05 | 3.04 | 0.27 | 1.46 | 0.30 | 0.65 | 0.56 | 127,975.00 | 88,544.34 |
| Gua9 | 5.90 | 0.25 | 15.87 | 5.88 | 22.18 | 0.73 | 6.60 | 1.14 | 6.98 | 0.05 | 2.20 | 0.13 | 1.30 | 0.42 | 0.91 | 0.34 | 44,750.00 | 9953.01 |
| Gua10 | 6.35 | 0.21 | 15.00 | 7.42 | 21.98 | 0.78 | 10.38 | 2.06 | 6.55 | 0.35 | 2.20 | 0.21 | 0.64 | 0.12 | 1.07 | 0.14 | 440,000.00 | 125,233.12 |
| Gua11 | 6.08 | 0.42 | 16.14 | 5.72 | 21.10 | 0.50 | 6.93 | 1.81 | 7.15 | 0.22 | 4.75 | 0.17 | 0.25 | 0.02 | 1.07 | 0.14 | 162,000.00 | 4546.06 |
| Gua12 | 7.08 | 0.28 | 11.96 | 6.78 | 21.33 | 0.25 | 6.20 | 0.53 | 7.48 | 0.23 | 4.10 | 0.29 | 0.25 | 0.02 | 1.76 | 0.29 | 127,000.00 | 38,594.04 |
| Gua13 | 8.05 | 0.30 | 13.00 | 5.16 | 21.40 | 0.54 | 9.65 | 1.09 | 7.60 | 0.14 | 3.95 | 0.06 | 0.08 | 0.00 | 1.60 | 0.10 | 206,750.00 | 12,202.29 |
| Gua14 | 6.15 | 0.31 | 12.04 | 5.27 | 21.33 | 0.47 | 12.60 | 0.65 | 7.23 | 0.11 | 3.80 | 0.14 | 0.29 | 0.01 | 0.42 | 0.34 | 191,500.00 | 4272.00 |
| Gua15 | 11.13 | 0.77 | 19.39 | 7.02 | 21.68 | 0.48 | 4.33 | 1.74 | 7.18 | 0.11 | 4.20 | 0.24 | 0.19 | 0.00 | 1.23 | 0.08 | 236,250.00 | 5543.39 |
| Gua16 | 8.03 | 0.18 | 13.93 | 5.13 | 20.75 | 0.38 | 5.68 | 0.87 | 7.48 | 0.11 | 3.58 | 0.28 | 0.18 | 0.03 | 1.06 | 0.06 | 55,250.00 | 41,825.78 |
| Gua17 | 8.30 | 0.21 | 15.48 | 11.39 | 21.17 | 1.30 | 6.50 | 2.40 | 7.37 | 0.09 | 3.93 | 0.09 | 1.07 | 0.12 | 1.25 | 0.46 | 233,333.33 | 14,529.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, A.; Fernandes, L.A.; de Biagi, C.A.O., Jr.; Marcondes, M.A.; Sanabani, S.S. Presence of Coliforms and Reduced Water Quality in the Second Biggest Reservoir in São Paulo, Brazil. Life 2025, 15, 729. https://doi.org/10.3390/life15050729
Nascimento A, Fernandes LA, de Biagi CAO Jr., Marcondes MA, Sanabani SS. Presence of Coliforms and Reduced Water Quality in the Second Biggest Reservoir in São Paulo, Brazil. Life. 2025; 15(5):729. https://doi.org/10.3390/life15050729
Chicago/Turabian StyleNascimento, Andrezza, Lorena A. Fernandes, Carlos A. O. de Biagi, Jr., Marta A. Marcondes, and Sabri Saeed Sanabani. 2025. "Presence of Coliforms and Reduced Water Quality in the Second Biggest Reservoir in São Paulo, Brazil" Life 15, no. 5: 729. https://doi.org/10.3390/life15050729
APA StyleNascimento, A., Fernandes, L. A., de Biagi, C. A. O., Jr., Marcondes, M. A., & Sanabani, S. S. (2025). Presence of Coliforms and Reduced Water Quality in the Second Biggest Reservoir in São Paulo, Brazil. Life, 15(5), 729. https://doi.org/10.3390/life15050729






