Exploring the Ecological Impacts of Herbicides on Antibiotic Resistance Genes and Microbial Communities
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. ARG Profile
2.3. Taxonomic Assignment
2.4. Species Diversity Analysis
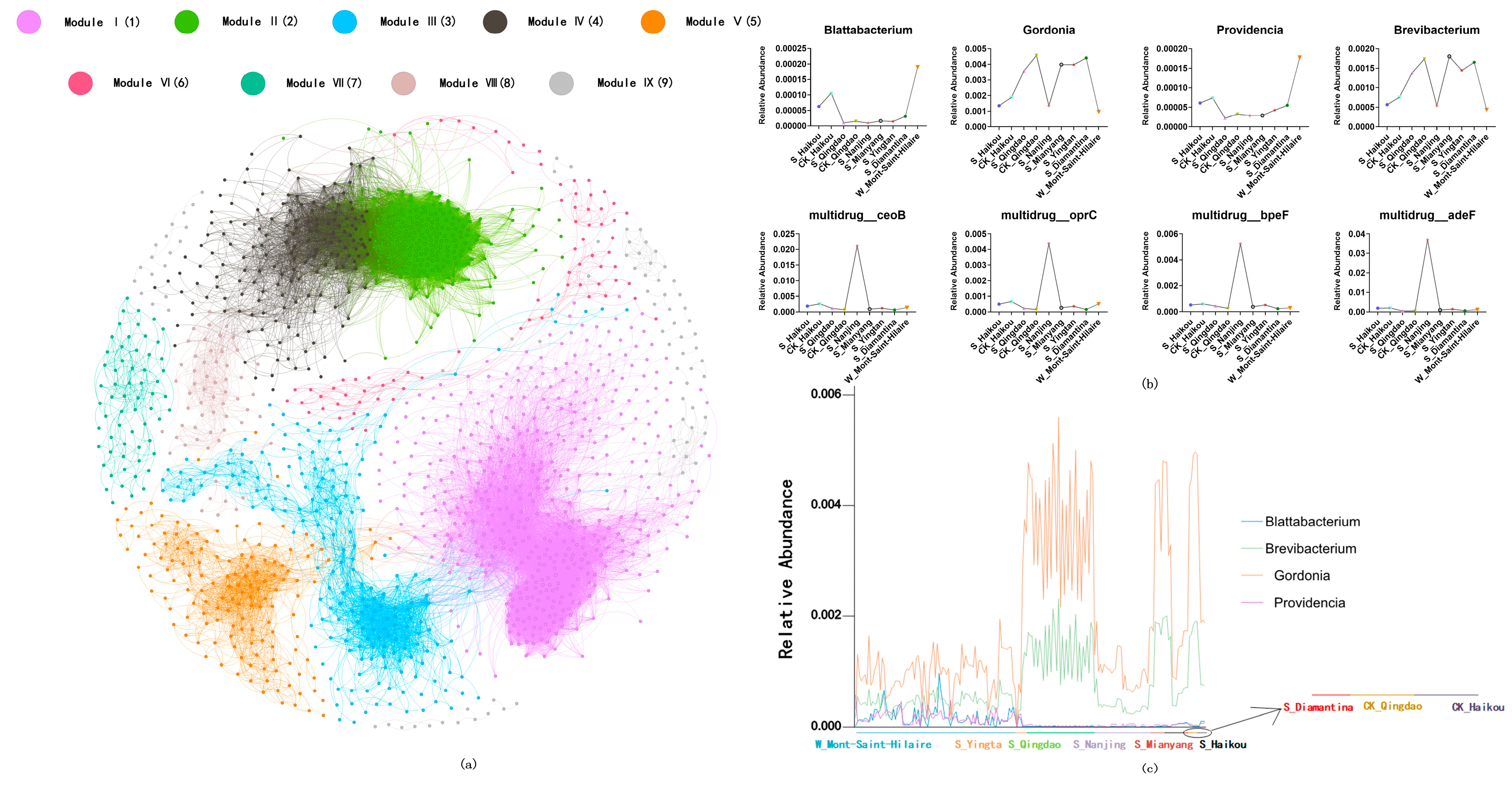
2.5. Network Analysis
2.6. Statistical Analys
3. Results
3.1. Impact of Herbicides on ARGs Abundance in Different Regions
3.2. Impact of Herbicides on the Distribution of ARGs in Different Regions
3.3. Impact of Herbicides on Microbial Communities in Different Regions
3.4. Correlation Analysis Between ARGs and Microbial Communities Across Different Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARGs | antibiotic resistance genes |
| MGEs | mobile genetic elements |
| HGT | horizontal gene transfer |
| PCoA | principal coordinates analysis |
References
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Qiu, D.; Ke, M.; Zhang, Q.; Zhang, F.; Lu, T.; Sun, L.; Qian, H. Response of Microbial Antibiotic Resistance to Pesticides: An Emerging Health Threat. Sci. Total Environ. 2022, 850, 158057. [Google Scholar] [CrossRef] [PubMed]
- Riesenfeld, C.S.; Goodman, R.M.; Handelsman, J. Uncultured Soil Bacteria Are a Reservoir of New Antibiotic Resistance Genes. Environ. Microbiol. 2004, 6, 981–989. [Google Scholar] [CrossRef]
- Hatosy, S.M.; Martiny, A.C. The Ocean as a Global Reservoir of Antibiotic Resistance Genes. Appl. Environ. Microbiol. 2015, 81, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, Y.; Liang, X.; Yu, K.; Zhang, T.; Li, X. Metagenomic Profiles of Antibiotic Resistance Genes (ARGs) between Human Impacted Estuary and Deep Ocean Sediments. Environ. Sci. Technol. 2013, 47, 12753–12760. [Google Scholar] [CrossRef] [PubMed]
- Segawa, T.; Takeuchi, N.; Rivera, A.; Yamada, A.; Yoshimura, Y.; Barcaza, G.; Shinbori, K.; Motoyama, H.; Kohshima, S.; Ushida, K. Distribution of Antibiotic Resistance Genes in Glacier Environments. Environ. Microbiol. Rep. 2013, 5, 127–134. [Google Scholar] [CrossRef]
- Rodríguez, M.M.; Power, P.; Sader, H.; Galleni, M.; Gutkind, G. Novel Chromosome-Encoded CTX-M-78 β-Lactamase from a Kluyvera Georgiana Clinical Isolate as a Putative Origin of CTX-M-25 Subgroup. Antimicrob. Agents Chemother. 2010, 54, 3070–3071. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Hooper, D.C. Phylogenetic Analysis of Chromosomally Determined Qnr and Related Proteins. Antimicrob. Agents Chemother. 2013, 57, 1930–1934. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic Resistance in the Environment: A Link to the Clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The Shared Antibiotic Resistome of Soil Bacteria and Human Pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.S.; Jones, D.L.; Zou, J. Antibiotics and Antibiotic Resistance Genes in Agricultural Soils: A Systematic Analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 847–864. [Google Scholar] [CrossRef]
- Paul, D.; Chakraborty, R.; Mandal, S.M. Biocides and Health-Care Agents Are More than Just Antibiotics: Inducing Cross to Co-Resistance in Microbes. Ecotoxicol. Environ. Saf. 2019, 174, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Bearson, B.L.; Douglass, C.H.; Duke, S.O.; Moorman, T.B.; Tranel, P.J. Effects of Glyphosate on Antibiotic Resistance in Soil Bacteria and Its Potential Significance: A Review. J. Environ. Qual. 2025, 54, 160–180. [Google Scholar] [CrossRef]
- Barbosa Da Costa, N.; Hébert, M.-P.; Fugère, V.; Terrat, Y.; Fussmann, G.F.; Gonzalez, A.; Shapiro, B.J. A Glyphosate-Based Herbicide Cross-Selects for Antibiotic Resistance Genes in Bacterioplankton Communities. mSystems 2022, 7, e01482-21. [Google Scholar] [CrossRef]
- Liao, H.; Li, X.; Yang, Q.; Bai, Y.; Cui, P.; Wen, C.; Liu, C.; Chen, Z.; Tang, J.; Che, J.; et al. Herbicide Selection Promotes Antibiotic Resistance in Soil Microbiomes. Mol. Biol. Evol. 2021, 38, 2337–2350. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Lu, T.; Zhang, K. Metagenomics Revealed the Mobility and Hosts of Antibiotic Resistance Genes in Typical Pesticide Wastewater Treatment Plants. Sci. Total Environ. 2022, 817, 153033. [Google Scholar] [CrossRef]
- Li, X.; Wen, C.; Liu, C.; Lu, S.; Xu, Z.; Yang, Q.; Chen, Z.; Liao, H.; Zhou, S. Herbicide Promotes the Conjugative Transfer of Multi-Resistance Genes by Facilitating Cellular Contact and Plasmid Transfer. J. Environ. Sci. 2022, 115, 363–373. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Wang, L.; Zhai, Z. Glyphosate Escalates Horizontal Transfer of Conjugative Plasmid Harboring Antibiotic Resistance Genes. Bioengineered 2021, 12, 63–69. [Google Scholar] [CrossRef]
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential Drivers of Antimicrobial Resistance across the World. Acc. Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef]
- Bakour, S.; Sankar, S.A.; Rathored, J.; Biagini, P.; Raoult, D.; Fournier, P.-E. Identification of Virulence Factors and Antibiotic Resistance Markers Using Bacterial Genomics. Future Microbiol. 2016, 11, 455–466. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, X.; Li, L.; Zhang, A.-N.; Jiang, X.-T.; Zhang, T. ARGs-OAP v3.0: Antibiotic-Resistance Gene Database Curation and Analysis Pipeline Optimization. Engineering 2023, 27, 234–241. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Han, L.; Liu, T.; Fang, K.; Li, X.; You, X.; Li, Y.; Wang, X.; Wang, J. Indigenous Functional Microbial Communities for the Preferential Degradation of Chloroacetamide Herbicide S-Enantiomers in Soil. J. Hazard. Mater. 2022, 423, 127135. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, H.; Liu, G.; Wei, Y.; Zhang, J.; Shi, H. Physiological and Metagenomic Strategies Uncover the Rhizosphere Bacterial Microbiome Succession Underlying Three Common Environmental Stresses in Cassava. J. Hazard. Mater. 2021, 411, 125143. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Harker, K.N.; Clayton, G.W.; Turkington, T.K.; Rice, W.A.; O’Donovan, J.T. Soil Microbial Biomass and Diversity after Herbicide Application. Can. J. Plant Sci. 2004, 84, 677–685. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural Soils, Pesticides and Microbial Diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef]
- Kepler, R.M.; Epp Schmidt, D.J.; Yarwood, S.A.; Cavigelli, M.A.; Reddy, K.N.; Duke, S.O.; Bradley, C.A.; Williams, M.M.; Buyer, J.S.; Maul, J.E. Soil Microbial Communities in Diverse Agroecosystems Exposed to the Herbicide Glyphosate. Appl. Environ. Microbiol. 2020, 86, e01744-19. [Google Scholar] [CrossRef]
- Mendes, L.W.; Tsai, S.M.; Navarrete, A.A.; De Hollander, M.; Van Veen, J.A.; Kuramae, E.E. Soil-Borne Microbiome: Linking Diversity to Function. Microb. Ecol. 2015, 70, 255–265. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Acuña, J.J.; Huerta, N.; Bai, J.; Zhang, L.; Xiao, R.; Sadowsky, M.J. Multiple Antibiotic Resistance and Herbicide Catabolic Profiles of Bacteria Isolated from Lake Villarrica Surface Sediments (Chile). Environ. Pollut. 2024, 358, 124538. [Google Scholar] [CrossRef]
- Xu, X.; Zarecki, R.; Medina, S.; Ofaim, S.; Liu, X.; Chen, C.; Hu, S.; Brom, D.; Gat, D.; Porob, S.; et al. Modeling Microbial Communities from Atrazine Contaminated Soils Promotes the Development of Biostimulation Solutions. ISME J. 2019, 13, 494–508. [Google Scholar] [CrossRef]
- Florencia, F.M.; Carolina, T.; Enzo, B.; Leonardo, G. Effects of the Herbicide Glyphosate on Non-Target Plant Native Species from Chaco Forest (Argentina). Ecotoxicol. Environ. Saf. 2017, 144, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zabaloy, M.C.; Garland, J.L.; Gómez, M.A. An Integrated Approach to Evaluate the Impacts of the Herbicides Glyphosate, 2,4-D and Metsulfuron-Methyl on Soil Microbial Communities in the Pampas Region, Argentina. Appl. Soil Ecol. 2008, 40, 1–12. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, C.; Jin, M.; Qin, G.; Yu, Y.; Qiu, D.; Wang, Y.; Zhang, Z.; Zhang, Z.; Lu, T.; et al. Glyphosate Disorders Soil Enchytraeid Gut Microbiota and Increases Its Antibiotic Resistance Risk. J. Agric. Food Chem. 2024, 72, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Wicaksono, W.A.; Kusstatscher, P.; Erschen, S.; Reisenhofer-Graber, T.; Grube, M.; Cernava, T.; Berg, G. Antimicrobial-Specific Response from Resistance Gene Carriers Studied in a Natural, Highly Diverse Microbiome. Microbiome 2021, 9, 29. [Google Scholar] [CrossRef]
- Pehrsson, E.C.; Tsukayama, P.; Patel, S.; Mejía-Bautista, M.; Sosa-Soto, G.; Navarrete, K.M.; Calderon, M.; Cabrera, L.; Hoyos-Arango, W.; Bertoli, M.T.; et al. Interconnected Microbiomes and Resistomes in Low-Income Human Habitats. Nature 2016, 533, 212–216. [Google Scholar] [CrossRef]
- Bo, A.B.; Won, O.J.; Sin, H.T.; Lee, J.J.; Park, K.W. Mechanisms of Herbicide Resistance in Weeds. Korean J. Agric. Sci. 2017, 44. [Google Scholar] [CrossRef]
- Smith, S.D.; Colgan, P.; Yang, F.; Rieke, E.L.; Soupir, M.L.; Moorman, T.B.; Allen, H.K.; Howe, A. Investigating the Dispersal of Antibiotic Resistance Associated Genes from Manure Application to Soil and Drainage Waters in Simulated Agricultural Farmland Systems. PLoS ONE 2019, 14, e0222470. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and Network Analysis Reveal Wide Distribution and Co-Occurrence of Environmental Antibiotic Resistance Genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef]
- Wright, G.D.; Sutherland, A.D. New Strategies for Combating Multidrug-Resistant Bacteria. Trends Mol. Med. 2007, 13, 260–267. [Google Scholar] [CrossRef]
- Cytryn, E. The Soil Resistome: The Anthropogenic, the Native, and the Unknown. Soil Biol. Biochem. 2013, 63, 18–23. [Google Scholar] [CrossRef]
- Mandler, M.D.; Baidin, V.; Lee, J.; Pahil, K.S.; Owens, T.W.; Kahne, D. Novobiocin Enhances Polymyxin Activity by Stimulating Lipopolysaccharide Transport. J. Am. Chem. Soc. 2018, 140, 6749–6753. [Google Scholar] [CrossRef]
- Eder, J.P.; Wheeler, C.A.; Teicher, B.A.; Schnipper, L.E. A Phase I Clinical Trial of Novobiocin, a Modulator of Alkylating Agent Cytotoxicity. Cancer Res. 1991, 51, 510–513. [Google Scholar] [PubMed]
- Vercellino, M.; Gómez, M.A. Denitrifying Capacity of Rhizobial Strains of Argentine Soils and Herbicide Sensitivity. Ann. Microbiol. 2013, 63, 1563–1570. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Qin, J.; Lu, N.; Cheng, G.; Wu, N.; Pan, Y.; Li, J.; Zhu, L.; Wang, X.; et al. Metagenome-Wide Analysis of Antibiotic Resistance Genes in a Large Cohort of Human Gut Microbiota. Nat. Commun. 2013, 4, 2151. [Google Scholar] [CrossRef] [PubMed]
- Cain, B.D.; Norton, P.J.; Eubanks, W.; Nick, H.S.; Allen, C.M. Amplification of the bacA Gene Confers Bacitracin Resistance to Escherichia Coli. J. Bacteriol. 1993, 175, 3784–3789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chalker, A.F.; Ingraham, K.A.; Lunsford, R.D.; Bryant, A.P.; Bryant, J.; Wallis, N.G.; Broskey, J.P.; Pearson, S.C.; Holmes, D.J. The bacA Gene, Which Determines Bacitracin Susceptibility in Streptococcus Pneumoniae and Staphylococcus Aureus, Is Also Required for Virulence. Microbiology 2000, 146, 1547–1553. [Google Scholar]
- Li, Q.; Na, G.; Zhang, L.; Lu, Z.; Gao, H.; Li, R.; Jin, S. Effects of Corresponding and Non-Corresponding Contaminants on the Fate of Sulfonamide and Quinolone Resistance Genes in the Laizhou Bay, China. Mar. Pollut. Bull. 2018, 128, 475–482. [Google Scholar] [CrossRef]
- Antunes, P.; Machado, J.; Sousa, J.C.; Peixe, L. Dissemination of Sulfonamide Resistance Genes (Sul1, Sul2, and Sul3) in Portuguese Salmonella Enterica Strains and Relation with Integrons. Antimicrob. Agents Chemother. 2005, 49, 836–839. [Google Scholar] [CrossRef]
- Sköld, O. Sulfonamide Resistance: Mechanisms and Trends. Drug Resist. Updates 2000, 3, 155–160. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Dai, Y.; Yu, Z.; Gu, W.; Li, T.; Li, X.; Li, X.; Wang, X.; Su, Z.; et al. Characterizing the Microbial Consortium L1 Capable of Efficiently Degrading Chlorimuron-Ethyl via Metagenome Combining 16S rDNA Sequencing. Front. Microbiol. 2022, 13, 912312. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Sandvang, D.; Andersen, S.R.; Seyfarth, A.M.; Porsbo, L.J.; Frimodt-Møller, N.; Heuer, O.E. Detection of Sul1, Sul2 and Sul3 in Sulphonamide Resistant Escherichia Coli Isolates Obtained from Healthy Humans, Pork and Pigs in Denmark. Int. J. Food Microbiol. 2006, 106, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.W.; Yanke, J.L.; Reuter, T.; Topp, E.; Read, R.R.; Selinger, B.L.; McAllister, T.A. Longitudinal Characterization of Antimicrobial Resistance Genes in Feces Shed from Cattle Fed Different Subtherapeutic Antibiotics. BMC Microbiol. 2011, 11, 19. [Google Scholar] [CrossRef]
- Wu, S.; Dalsgaard, A.; Hammerum, A.M.; Porsbo, L.J.; Jensen, L.B. Prevalence and Characterization of Plasmids Carrying Sulfonamide Resistance Genes among Escherichia Coli from Pigs, Pig Carcasses and Human. Acta Vet Scand. 2010, 52, 47. [Google Scholar] [CrossRef] [PubMed]
- Eustáquio, A.S.; Gust, B.; Galm, U.; Li, S.-M.; Chater, K.F.; Heide, L. Heterologous Expression of Novobiocin and Clorobiocin Biosynthetic Gene Clusters. Appl. Environ. Microbiol. 2005, 71, 2452–2459. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A Systematic Review of Antibiotics and Antibiotic Resistance Genes in Estuarine and Coastal Environments. Sci. Total Environ. 2021, 777, 146009. [Google Scholar] [CrossRef]
- Pérez, G.L.; Torremorell, A.; Mugni, H.; Rodríguez, P.; Vera, M.S.; Nascimento, M.D.; Allende, L.; Bustingorry, J.; Escaray, R.; Ferraro, M.; et al. Effects of the herbicide Roundup on freshwater microbial communities: A mesocosm study. Ecol. Appl. 2007, 17, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Saikkonen, K.; Helander, M. Glyphosate-Modulated Biosynthesis Driving Plant Defense and Species Interactions. Trends Plant Sci. 2021, 26, 312–323. [Google Scholar] [CrossRef]
- Ratcliff, A.W.; Busse, M.D.; Shestak, C.J. Changes in Microbial Community Structure Following Herbicide (Glyphosate) Additions to Forest Soils. Appl. Soil Ecol. 2006, 34, 114–124. [Google Scholar] [CrossRef]
- Thiour-Mauprivez, C.; Martin-Laurent, F.; Calvayrac, C.; Barthelmebs, L. Effects of Herbicide on Non-Target Microorganisms: Towards a New Class of Biomarkers? Sci. Total Environ. 2019, 684, 314–325. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Saikkonen, K. Glyphosate in Northern Ecosystems. Trends Plant Sci. 2012, 17, 569–574. [Google Scholar] [CrossRef]
- Tabassum, S.; Wang, Y.; Zhang, X.; Zhang, Z. Novel Mass Bio System (MBS) and Its Potential Application in Advanced Treatment of Coal Gasification Wastewater. RSC Adv. 2015, 5, 88692–88702. [Google Scholar] [CrossRef]
- Rose, M.T.; Cavagnaro, T.R.; Scanlan, C.A.; Rose, T.J.; Vancov, T.; Kimber, S.; Kennedy, I.R.; Kookana, R.S.; Van Zwieten, L. Impact of Herbicides on Soil Biology and Function. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2016; Volume 136, pp. 133–220. ISBN 978-0-12-804681-4. [Google Scholar]
- Li, T.; Liu, R.; Wang, Q.; Rao, J.; Liu, Y.; Dai, Z.; Gooneratne, R.; Wang, J.; Xie, Q.; Zhang, X. A Review of the Influence of Environmental Pollutants (Microplastics, Pesticides, Antibiotics, Air Pollutants, Viruses, Bacteria) on Animal Viruses. J. Hazard. Mater. 2024, 468, 133831. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jahn, M.T.; Sun, M.; Friman, V.-P.; Balcazar, J.L.; Wang, J.; Shi, Y.; Gong, X.; Hu, F.; Zhu, Y.-G. Organochlorine Contamination Enriches Virus-Encoded Metabolism and Pesticide Degradation Associated Auxiliary Genes in Soil Microbiomes. ISME J. 2022, 16, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Tang, Z.; Mo, G.; Zhang, B.; Ling, X.; Qiu, Z. Taxonomic and Functional Changes in Wheat Rhizosphere Microbiome Caused by Imidazoline-Based Herbicide and Genetic Modification. Environ. Res. 2024, 262, 119726. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Tang, L.; Heenan, M.; Xu, Z. Effects of Nitrification Inhibitor and Herbicides on Nitrification, Nitrite and Nitrate Consumptions and Nitrous Oxide Emission in an Australian Sugarcane Soil. Biol. Fertil. Soils 2018, 54, 697–706. [Google Scholar] [CrossRef]
- Ding, H.; Zou, Y.; Zheng, X.; Zhang, Y.; Yu, J.; Chen, D. Inhibitory Effects of Different Types and Doses of Herbicides on Soil Nitrification Potentials. Water Air Soil Pollut. 2019, 230, 198. [Google Scholar] [CrossRef]
- Carlisle, S.M.; Trevors, J.T. Effect of the Herbicide Glyphosate on Nitrification, Denitrification, and Acetylene Reduction in Soil. Water Air Soil Pollut. 1986, 29. [Google Scholar] [CrossRef]
- Yasemi, M.; Jalali, A.; Asadzadeh, M.; Komijani, M. Organophosphate Pesticides and Their Potential in the Change of Microbial Population and Frequency of Antibiotic Resistance Genes in Aquatic Environments. Chemosphere 2025, 376, 144296. [Google Scholar] [CrossRef]
- Hu, A.; Ju, F.; Hou, L.; Li, J.; Yang, X.; Wang, H.; Mulla, S.I.; Sun, Q.; Bürgmann, H.; Yu, C. Strong Impact of Anthropogenic Contamination on the Co-occurrence Patterns of a Riverine Microbial Community. Environ. Microbiol. 2017, 19, 4993–5009. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Mu, C.-L.; Luo, Z.; Zhu, W.-Y. Bromochloromethane, a Methane Analogue, Affects the Microbiota and Metabolic Profiles of the Rat Gastrointestinal Tract. Appl. Environ. Microbiol. 2016, 82, 778–787. [Google Scholar] [CrossRef]
- Zhong, S.; Feng, J.; Kong, J.; Huang, Y.; Chen, X.; Zhang, S. Differences in Bacterial Co-Occurrence Networks and Ecological Niches at the Surface Sediments and Bottom Seawater in the Haima Cold Seep. Microorganisms 2023, 11, 3001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, S.; Gong, Y.; Wang, Y.; Wei, Q.; Zhu, Y.; Liu, L.; Wu, R.; Du, S. Does the Herbicide Napropamide Exhibit Enantioselective Effects across Genus Plasmid Transfer from Escherichia Coli to Bacillus Subtilis? J. Hazard. Mater. 2025, 484, 136704. [Google Scholar] [CrossRef]
- Jung, C.M.; Crocker, F.H.; Eberly, J.O.; Indest, K.J. Horizontal Gene Transfer (HGT) as a Mechanism of Disseminating RDX-Degrading Activity among Actinomycete Bacteria: Genetic Transfer of RDX Degradation. J. Appl. Microbiol. 2011, 110, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.; Xi, B.; Cao, L.; Huang, C. Mechanisms and Influencing Factors of Horizontal Gene Transfer in Composting System: A Review. Sci. Total Environ. 2024, 955, 177017. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Feng, K.; Wang, Z.; Zhang, Y.; Yang, M.; Zhu, Y.-G.; Virta, M.P.J.; Deng, Y. High-Throughput Single-Cell Technology Reveals the Contribution of Horizontal Gene Transfer to Typical Antibiotic Resistance Gene Dissemination in Wastewater Treatment Plants. Environ. Sci. Technol. 2021, 55, 11824–11834. [Google Scholar] [CrossRef]
- Shi, X.; Xia, Y.; Wei, W.; Ni, B.-J. Accelerated Spread of Antibiotic Resistance Genes (ARGs) Induced by Non-Antibiotic Conditions: Roles and Mechanisms. Water Res. 2022, 224, 119060. [Google Scholar] [CrossRef]
- Wolfe, Z.M.; Scharf, M.E. Differential Microbial Responses to Antibiotic Treatments by Insecticide-Resistant and Susceptible Cockroach Strains (Blattella germanica L.). Sci. Rep. 2021, 11, 24196. [Google Scholar] [CrossRef]
- Kong, X.; Jin, D.; Tai, X.; Yu, H.; Duan, G.; Yan, X.; Pan, J.; Song, J.; Deng, Y. Bioremediation of Dibutyl Phthalate in a Simulated Agricultural Ecosystem by Gordonia sp. Strain QH-11 and the Microbial Ecological Effects in Soil. Sci. Total Environ. 2019, 667, 691–700. [Google Scholar] [CrossRef]
- ElBestawy, E. Comparison among the Efficiency of Different Bioremediation Technologies of Atrazine–Contaminated Soils. J. Bioremed. Biodeg. 2014, 5, 237. [Google Scholar] [CrossRef]
- Liu, Z.; Han, L.; Zhang, X.; Chen, S.; Wang, X.; Fang, H. Core Bacteria Carrying the Genes Associated with the Degradation of Atrazine in Different Soils. Environ. Int. 2023, 181, 108303. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding Human Health Risks Caused by Antibiotic Resistant Bacteria (ARB) and Antibiotic Resistance Genes (ARG) in Water Environments: Current Knowledge and Questions to Be Answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and Fate of Antibiotics, Antibiotic Resistant Genes (ARGs) and Antibiotic Resistant Bacteria (ARB) in Municipal Wastewater Treatment Plant: An Overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Mejia, M.P.; Rojas, C.A.; Curd, E.; Renshaw, M.A.; Edalati, K.; Shih, B.; Vincent, N.; Lin, M.; Nguyen, P.H.; Wayne, R.; et al. Soil Microbial Community Composition and Tolerance to Contaminants in an Urban Brownfield Site. Microb. Ecol. 2023, 85, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, J.M.; Morton, J.T.; Tripathi, A.; Hansen, S.; Gaffney, M.; Carpenter, C.; Weldon, K.C.; Shah, R.; Parampil, A.; Fidgett, A.L.; et al. Metabolome-Informed Microbiome Analysis Refines Metadata Classifications and Reveals Unexpected Medication Transfer in Captive Cheetahs. mSystems 2020, 5, e00635-19. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, S.; Puglisi, E.; Papadopoulou, E.S.; Pertile, G.; Suciu, N.; Pappolla, R.A.; Tourna, M.; Karas, P.A.; Papadimitriou, F.; Kasiotakis, A.; et al. Blame It on the Metabolite: 3,5-Dichloroaniline Rather than the Parent Compound Is Responsible for the Decreasing Diversity and Function of Soil Microorganisms. Appl. Environ. Microbiol. 2018, 84, e01536-18. [Google Scholar] [CrossRef]
- Overbeek, W.; Lucotte, M.; D’Astous-Pagé, J.; Jeanne, T.; Pin, C.; Moingt, M.; Hogue, R. Impacts of Cropping Systems on Glyphosate and Aminomethylphosphonic Acid Contents and Microbial Community in Field Crop Soils in Quebec (Canada). Agronomy 2024, 14, 686. [Google Scholar] [CrossRef]
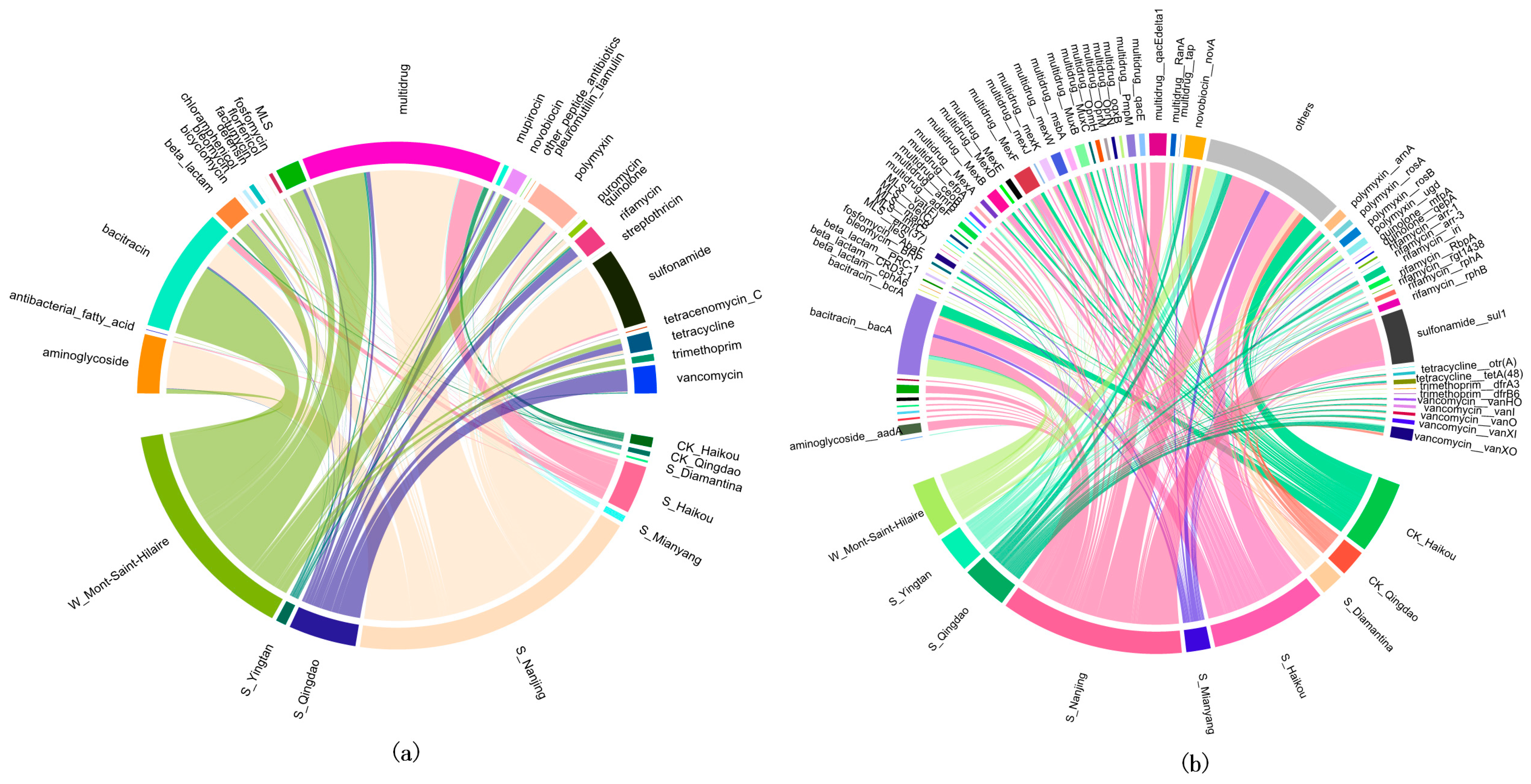
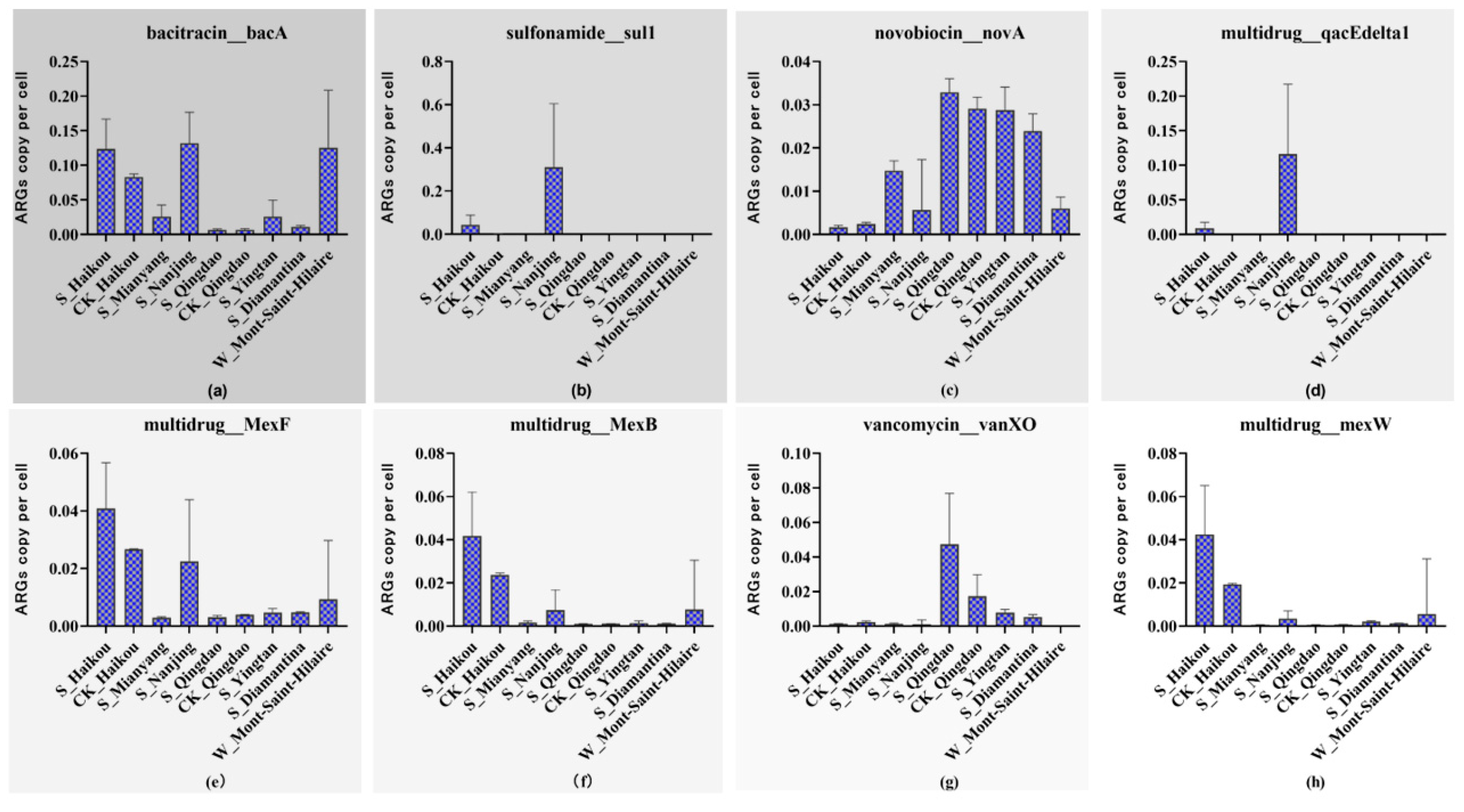
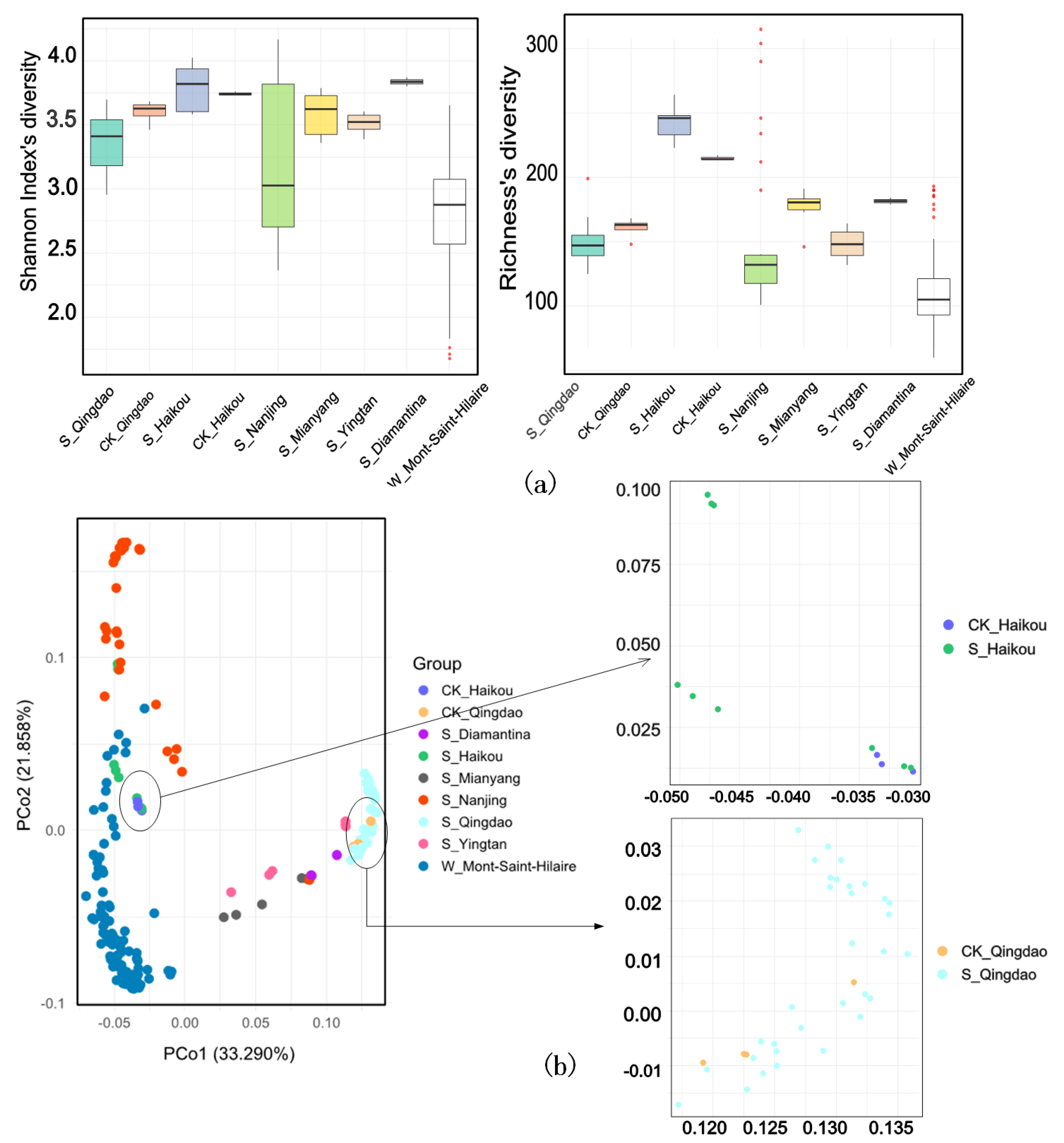
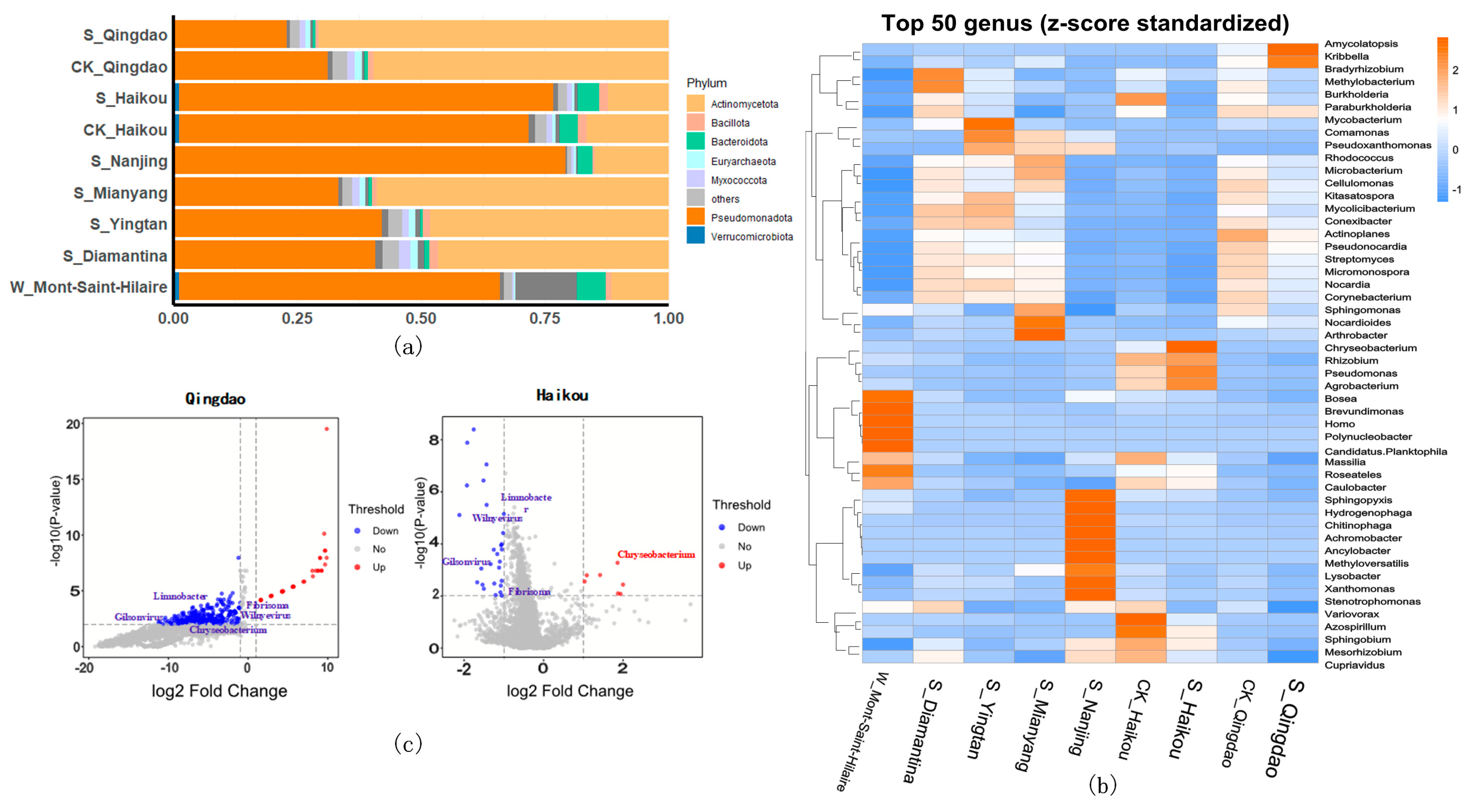
| Group | Geographic Coordinates | Number of Samples | Sample Type | Type of Herbicide | Herbicide Contamination | Control Group? |
|---|---|---|---|---|---|---|
| S_Qingdao | 36°4′33.4″ N, 120°24′30.7″ E | 32 | Soil | Chloroacetamide herbicides | Yes | No |
| CK_Qingdao | 36°4′33.4″ N, 120°24′30.7″ E | 4 | Soil | \ | No | Yes |
| S_Haikou | 20°2′48.7″ N, 110°11′44.4″ E | 9 | Soil | Organophosphorous herbicides | Yes | No |
| CK_Haikou | 20°2′48.7″ N, 110°11′44.4″ E | 3 | Soil | \ | No | Yes |
| S_Nanjing | 32°3′41.6″ N, 118°47′29.6″ E | 35 | Soil | Phenoxyalkanoic acid herbicides/Sulfonylurea herbicides/Systemic herbicides | Yes | No |
| S_Mianyang | 31°27′45.8″ N, 104°44′46.8″ E | 6 | Soil | Systemic herbicides | Yes | No |
| S_Yingtan | 28°20′37.8″ N, 116°55′45.5″ E | 6 | Soil | Systemic herbicides | Yes | No |
| S_Diamantina | 18°11′12.9″ S, 43°32′14.6″ W | 2 | Soil | Triazine herbicides | Yes | No |
| W_Mont-Saint-Hilaire | 45°32′ N, 73°09′ W | 88 | Water | Organophosphorous herbicides | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, Y.; Lu, J.; Zhu, B.; Li, A.-D. Exploring the Ecological Impacts of Herbicides on Antibiotic Resistance Genes and Microbial Communities. Life 2025, 15, 547. https://doi.org/10.3390/life15040547
Zhao Y, Wang Y, Lu J, Zhu B, Li A-D. Exploring the Ecological Impacts of Herbicides on Antibiotic Resistance Genes and Microbial Communities. Life. 2025; 15(4):547. https://doi.org/10.3390/life15040547
Chicago/Turabian StyleZhao, Yunfei, Yixiao Wang, Jie Lu, Baoli Zhu, and An-Dong Li. 2025. "Exploring the Ecological Impacts of Herbicides on Antibiotic Resistance Genes and Microbial Communities" Life 15, no. 4: 547. https://doi.org/10.3390/life15040547
APA StyleZhao, Y., Wang, Y., Lu, J., Zhu, B., & Li, A.-D. (2025). Exploring the Ecological Impacts of Herbicides on Antibiotic Resistance Genes and Microbial Communities. Life, 15(4), 547. https://doi.org/10.3390/life15040547





