Transient Expression of Hen Egg White Lysozyme (EWL) in Nicotiana benthamiana Influences Plant Pathogen Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. N. benthamiana Plant Preparation
2.3. Hen Egg White Lysozyme Gene Expression Vector Construction
2.4. In Silico Analysis of EWL Protein
2.5. Transient Expression of EWL in N. benthamiana
2.6. RT-qPCR
2.7. The Influence of EWL Protein on Viral Infection
2.8. GFP Florescence Imaging
2.9. Western Blot
3. Results
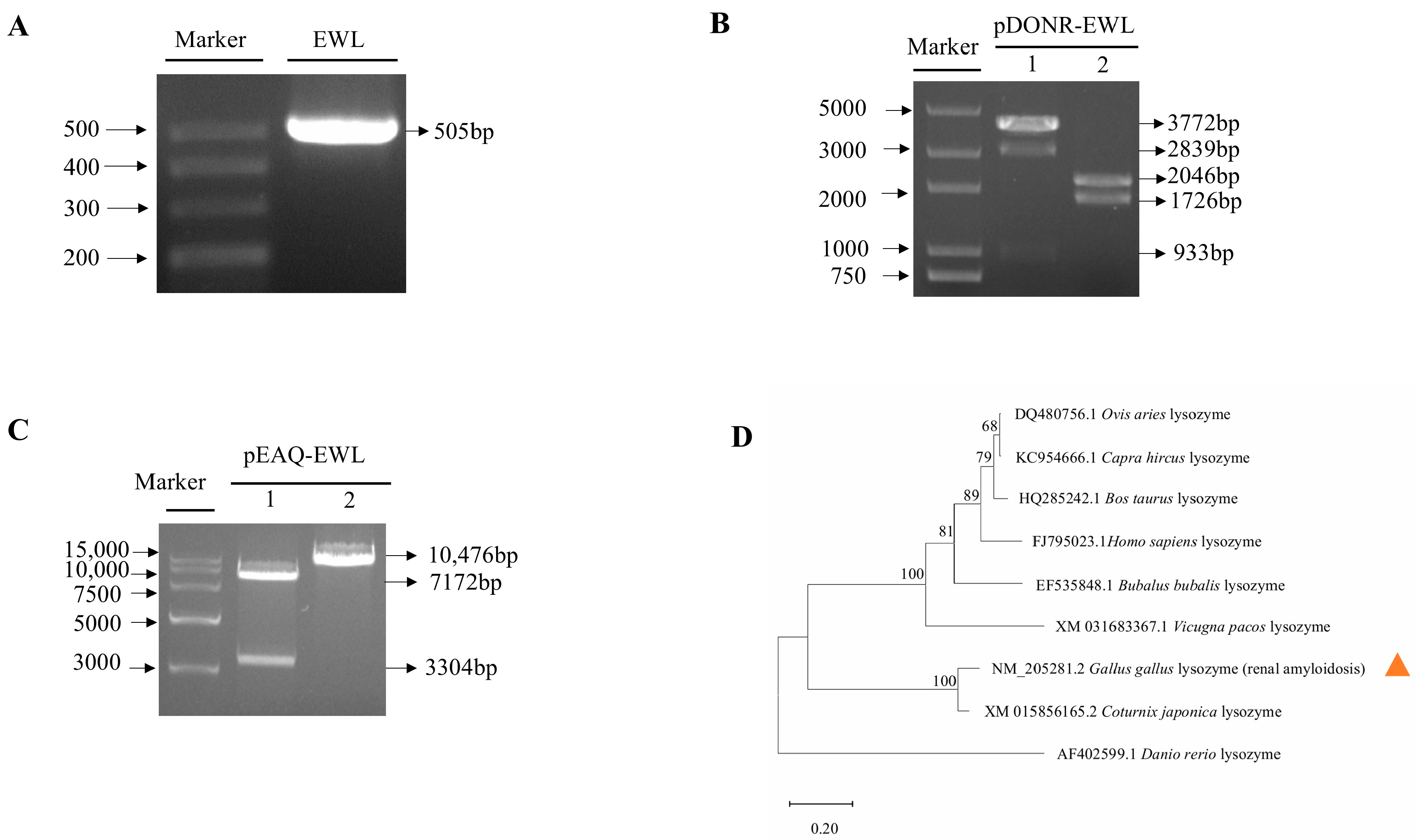
3.1. EWL Plant Expression Vector Construction
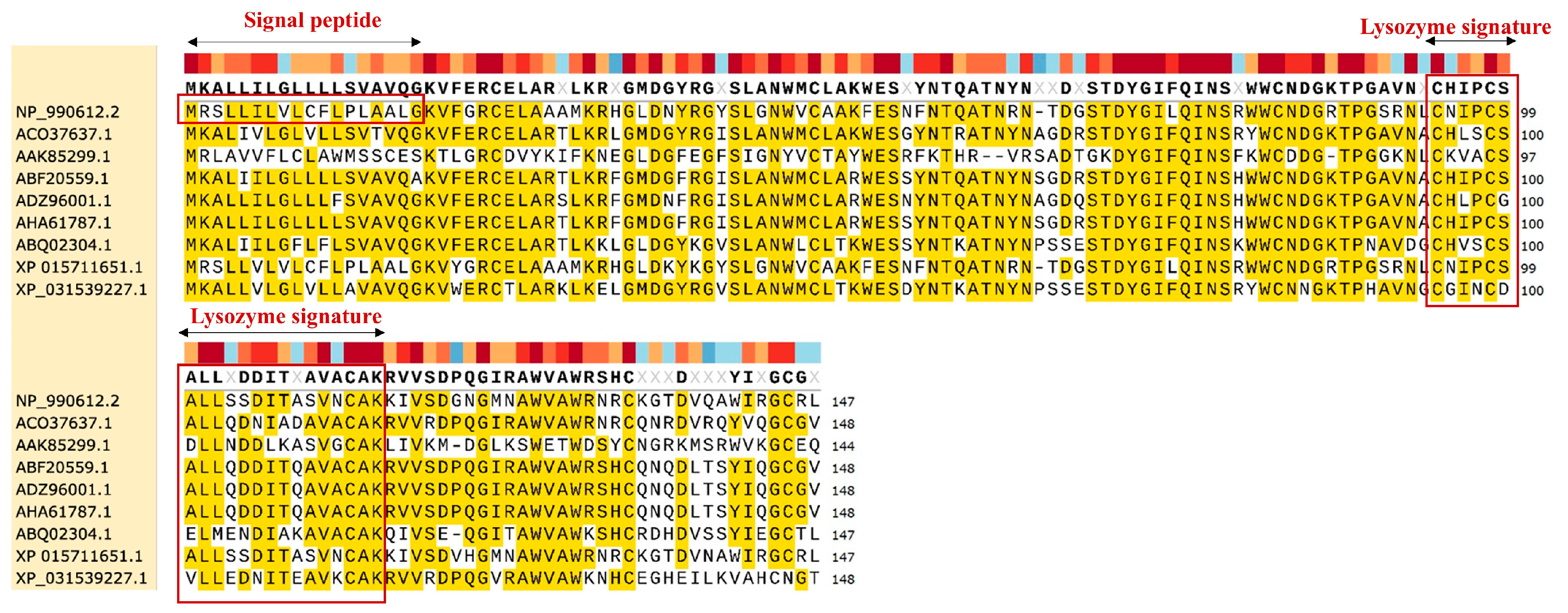
3.2. Conservation Analysis of EWL Lysozyme Among Different Species
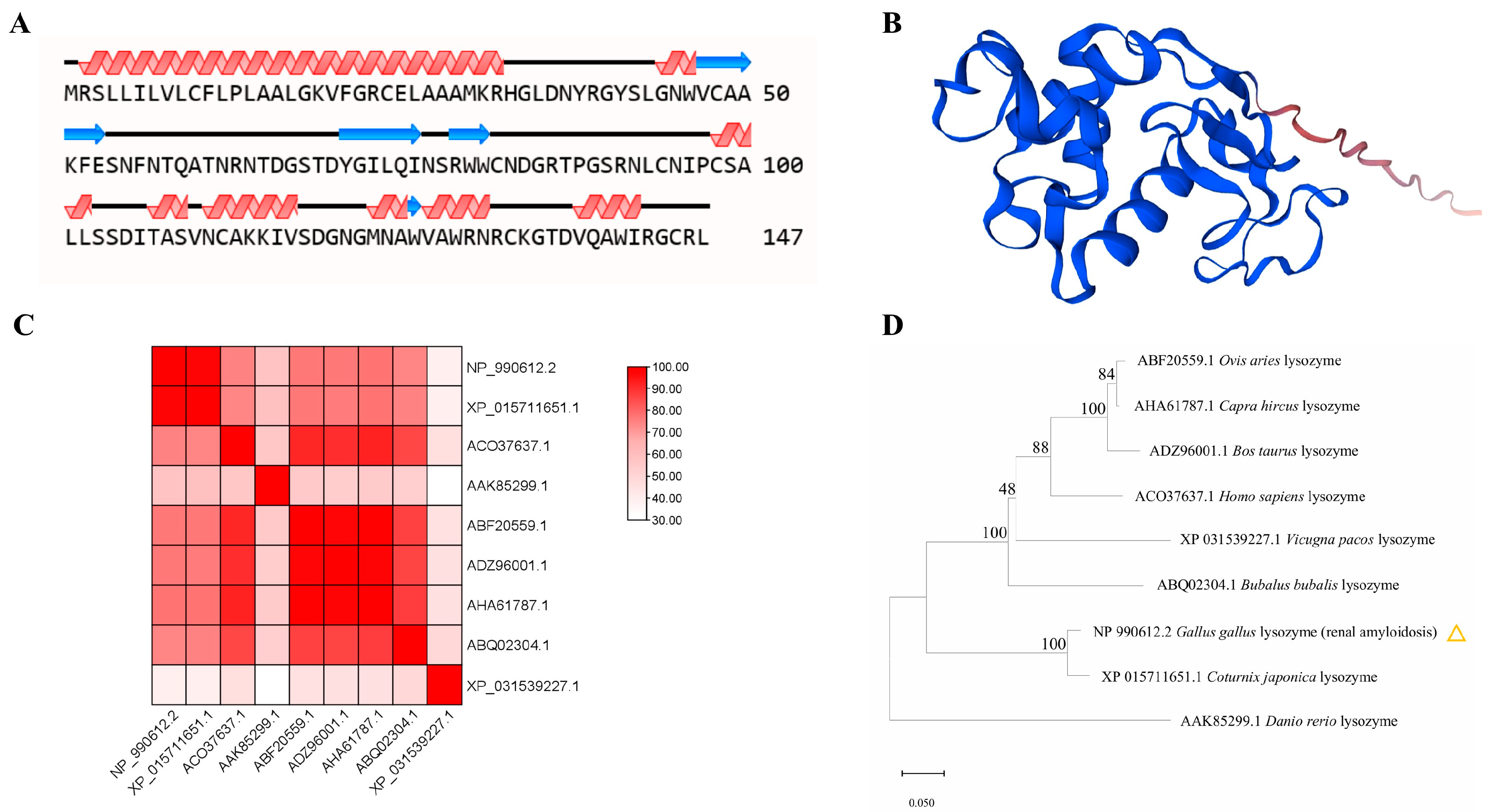
3.3. Hen Egg White Lysozyme Is Structurally Related to Coturnix japonica Lysozyme and Bubalus bubalis Lysozyme
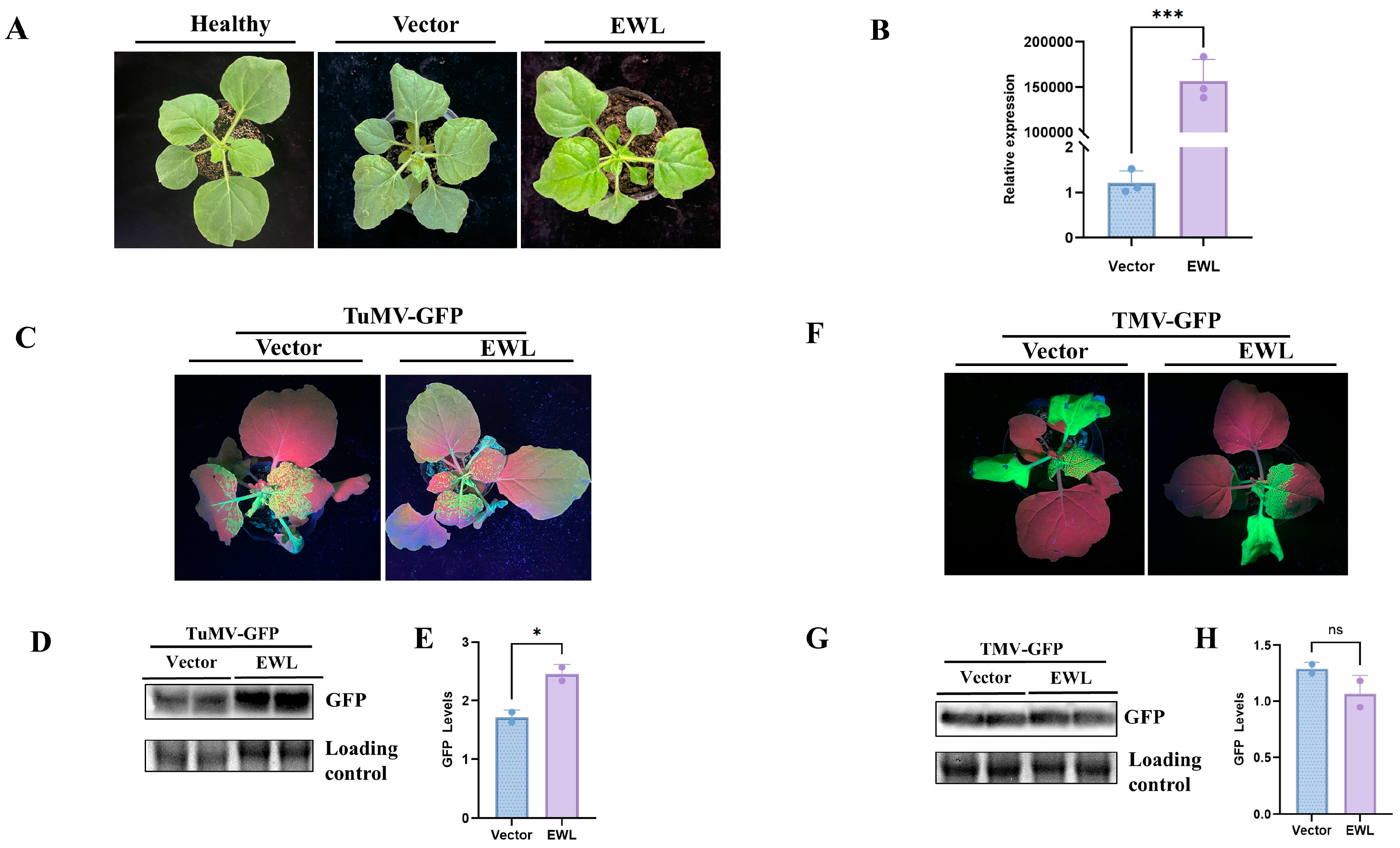
3.4. Effect of Transient Expression of EWL in N. benthamiana on Plant Viral Infection
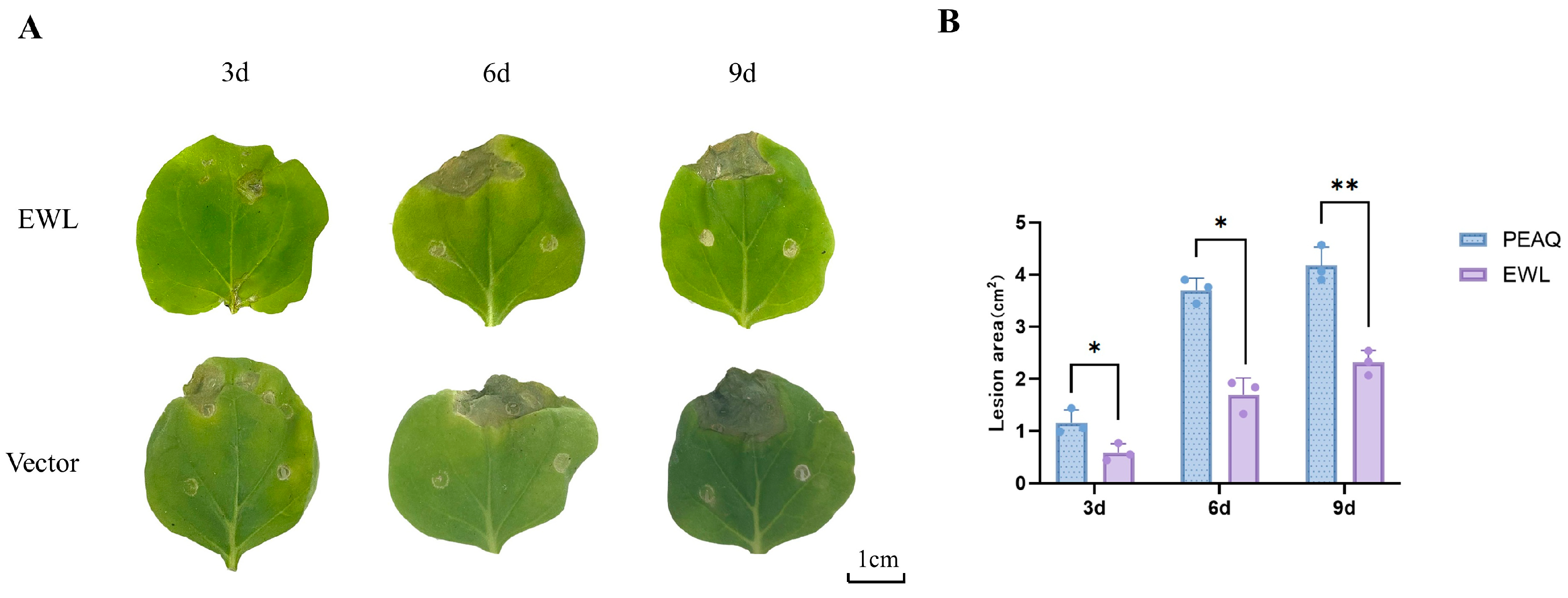
3.5. Effect of Transcient Expression of EWL in N. benthamiana on B. cinerea Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strynadka, N.C.; James, M.N. Lysozyme: A model enzyme in protein crystallography. EXS 1996, 75, 185–222. [Google Scholar] [PubMed]
- Callewaert, L.; Michiels, C.W. Lysozymes in the animal kingdom. J. Bio Sci. 2010, 35, 127–160. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cao, H.; Yan, J.; Li, C.; Li, F.; Li, Y.; Rao, Z. Effect of dietary supplementation with recombinant human lysozyme on growth performance, antioxidative characteristics, and intestinal health in broiler chickens. J. Anim. Sci. 2024, 102, skae121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Hu, F.; Wang, Y. Online preconcentration of lysozyme in hen egg white using responsive polymer coating in CE. J. Sep. Sci. 2021, 44, 3477–3488. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Liu, Y.; Jin, Y.; Ma, M. The antimicrobial spectrum of lysozyme broadened by reductive modification. Poult. Sci. 2018, 97, 3992–3999. [Google Scholar] [CrossRef]
- Bergamo, A.; Sava, G. Pharmacological modulation of host immunity with hen egg white lysozyme (HEWL)—A review. Molecules 2023, 28, 5027. [Google Scholar] [CrossRef]
- de Boeck, S.; Stockx, J. Egg white lysozyme is the major protein of the hen’s egg vitelline membrane. Int. J. Biochem. 1986, 18, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, B.; Ji, L.; Lv, X.; Feng, Y.; Li, L.A.; Wu, X. Dietary lysozyme improves growth performance and intestinal barrier function of weaned piglets. Anim. Nutr. 2023, 14, 249–258. [Google Scholar] [CrossRef]
- Xu, X.; Huang, P.; Cui, X.; Li, X.; Sun, J.; Ji, Q.; Liu, Y. Effects of Dietary coated lysozyme on the growth performance, antioxidant activity, immunity and gut health of weaned piglets. Antibiotics 2022, 11, 1470. [Google Scholar] [CrossRef]
- Mantzios, T.; Stylianaki, I.; Savvidou, S.; Dokou, S.; Papadopoulos, G.A.; Panitsidis, I.; Giannenas, I. Effects of dietary supplementation of essential oils, lysozyme, and vitamins’ blend on layer hen performance, viral vaccinal response, and egg quality characteristics. Vaccines 2024, 12, 147. [Google Scholar] [CrossRef]
- Glamočlija, U.; Mehić, M.; Šukalo, A.; Tanović Avdić, A.; Džananović Jaganjac, J. Lysozyme in the treatment of non-infectious sore throat. Bosn. J. Basic. Med. Sci. 2020, 20, 281–282. [Google Scholar] [CrossRef]
- Yuan, X.; Shi, W.; Jiang, J.; Li, Z.; Fu, P.; Yang, C.; Shi, D. Comparative metabolomics analysis of milk components between Italian Mediterranean buffaloes and Chinese Holstein cows based on LC-MS/MS technology. PLoS ONE 2022, 17, e0262878. [Google Scholar] [CrossRef] [PubMed]
- Larsen, I.S.; Jensen, B.A.H.; Bonazzi, E.; Choi, B.S.Y.; Kristensen, N.N.; Schmidt, E.G.W.; Marette, A. Fungal lysozyme leverages the gut microbiota to curb DSS-induced colitis. Gut Microbes 2021, 13, 1988836. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Jiang, Q.; Wu, D.; Hu, Y.; Chen, S.; Ding, T.; Chen, J. What is new in lysozyme research and its application in food industry? A review. Food Chem. 2019, 274, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Aminlari, L.; Hashemi, M.M.; Aminlari, M. Modified lysozymes as novel broad spectrum natural antimicrobial agents in foods. J. Food Sci. 2014, 79, R1077–R1090. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Tian, Y.; Yu, Y.; Gao, M.; Hu, G.; Zhang, Y. Generation of mastitis resistance in cows by targeting human lysozyme gene to β-casein locus using zinc-finger nucleases. Proc. Biol. Sci. 2014, 281, 20133368. [Google Scholar] [CrossRef]
- Muthu, M.R.B.; Se-Chul, C.A. Single-step purification of cauliflower lysozyme and its dual role against bacterial and fungal plant pathogens. Appl. Biochem. Biotechnol. 2015, 177, 556–566. [Google Scholar]
- Bergamo, A.; Sava, G. Lysozyme: A natural product with multiple and useful antiviral properties. Molecules 2024, 29, 652. [Google Scholar] [CrossRef]
- Cotton, S.; Grangeon, R.; Thivierge, K.; Mathieu, I.; Ide, C.; Wei, T.; Laliberté, J.F. Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. J. Virol. 2009, 83, 10460–10471. [Google Scholar] [CrossRef]
- Ye, J.; Song, J.; Gao, Y.; Lu, X.; Pei, W.; Li, F.; Yang, W. An automatic fluorescence phenotyping platform to evaluate dynamic infection process of Tobacco mosaic virus-green fluorescent protein in tobacco leaves. Front. Plant Sci. 2022, 13, 968855. [Google Scholar] [CrossRef]
- Yue, J.; Wei, Y.; Sun, Z.; Chen, Y.; Wei, X.; Wang, H.; Zhao, M. AlkB RNA demethylase homologues and N 6-methyladenosine are involved in Potyvirus infection. Mol. Plant Pathol. 2022, 23, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Buell, G.N.; Wickens, M.P.; Carbon, J.; Schimke, R.T. Isolation of recombinant plasmids bearing cDNA to hen ovomucoid and lysozyme mRNAs. J. Biol. Chem. 1979, 254, 9277–9283. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Schwede, T. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Degasperi, A.; Birtwistle, M.R.; Volinsky, N.; Rauch, J.; Kolch, W.; Kholodenko, B.N. Evaluating strategies to normalise biological replicates of Western blot data. PLoS ONE 2014, 9, e87293. [Google Scholar] [CrossRef]
- Yue, J.; Lu, Y.; Sun, Z.; Guo, Y.; San León, D.; Pasin, F.; Zhao, M. Methyltransferase-like (METTL) homologues participate in Nicotiana benthamiana antiviral responses. Plant Signal Behav. 2023, 18, 2214760. [Google Scholar] [CrossRef]
- Prager, E.M. Adaptive evolution of lysozyme: Changes in amino acid sequence, regulation of expression and gene number. EXS 1996, 75, 323–345. [Google Scholar]
- Imoto, T.; Ueda, T.; Tamura, T.; Isakari, Y.; Abe, Y.; Inoue, M.; Yamada, H. Lysozyme requires fluctuation of the active site for the manifestation of activity. Protein Eng. 1994, 7, 743–748. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.; Ma, Y.; Liu, L.; Wang, H.; Niu, X. Eco-friendly and non-toxic lysozyme immobilised on chitosan bacteriostatic agent for strawberry and fresh Tremella fuciformis preservation. Int. J. Food Sci. Technol. 2023, 58, 2985–2994. [Google Scholar] [CrossRef]
- El-Deep, M.H.; Amber, K.A.; Eid, Y.Z.; Alrashood, S.T.; Khan, H.A.; Sakr, M.S.; Dawood, M.A. The influence of dietary chicken egg lysozyme on the growth performance, blood health, and resistance against Escherichia coli in the growing rabbits’ cecum. Front. Vet. Sci. 2020, 7, 579576. [Google Scholar] [CrossRef]
- Trudel, J.; Potvin, C.; Asselin, A. Expression of active hen egg white lysozyme in transgenic tobacco. Plant Sci. 1992, 87, 55–62. [Google Scholar] [CrossRef]
- Serrano, C.; Arce-Johnson, P.; Torres, H.; Gebauer, M.; Gutierrez, M.; Moreno, M.; Holuigue, L. Expression of the chicken lysozyme gene in potato enhances resistance to infection by Erwinia Carotovora subsp. atroseptica. Am. J. Potato Res. 2000, 77, 191–199. [Google Scholar] [CrossRef]
- Guo, W.; Li, G.; Wang, N.; Yang, C.; Peng, H.; Wang, M.; Liu, D. Hen egg white lysozyme (HEWL) Confers resistance to verticillium wilt in cotton by inhibiting the spread of fungus and generating ROS burst. Int. J. Mol. Sci. 2023, 24, 17164. [Google Scholar] [CrossRef]
- Li, J.; Wang, K.; Wang, N.; Gangqiang, L.; Ning, S.; Dehu, L. Codon optimization, constitutive expression and antimicrobial characterization of hen egg white lysozyme (HEWL) in Pichia pastoris. Afr. J. Biotechnol. 2012, 11, 11887–11893. [Google Scholar]
- Chen, T.T.; Tan, L.R.; Hu, N.; Dong, Z.Q.; Hu, Z.G.; Jiang, Y.M.; Lu, C. C-lysozyme contributes to antiviral immunity in Bombyx mori against nucleopolyhedrovirus infection. J. Insect Physiol. 2018, 108, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D. Insect lysozymes. EXS 1996, 75, 87–102. [Google Scholar]
- Nitta, K.; Sugai, S. The evolution of lysozyme and α-lactalbumin. Eur. J. Biochem. 1989, 182, 111–118. [Google Scholar] [CrossRef]
- Gohda, S.; Shimizu, A.; Ikeguchi, M.; Sugai, S. The superreactive disulfide bonds in alpha-lactalbumin and lysozyme. J. Protein Chem. 1995, 14, 731–737. [Google Scholar] [CrossRef]
- McKenzie, H.A. Alpha-Lactalbumins and lysozymes. EXS 1996, 75, 365–409. [Google Scholar]
- Shewale, J.G.; Sinha, S.K.; Brew, K. Evolution of alpha-lactalbumins. The complete amino acid sequence of the alpha-lactalbumin from a marsupial (Macropus rufogriseus) and corrections to regions of sequence in bovine and goat alpha-lactalbumins. J. Biol. Chem. 1984, 259, 4947–4956. [Google Scholar] [PubMed]
- Steinbiss, H.H.; Davidson, A. Transient gene expression of chimeric genes in cells and tissues of crops. Subcell. Biochem. 1991, 17, 143–166. [Google Scholar] [PubMed]
| Parameter | EWL |
|---|---|
| Molecular formula | C705H1116N214O204S12 |
| Molecular weight | 16,238.65 |
| Aliphatic coefficient | 81.70 |
| Instability index | 19.86 |
| Theoretical isoelectric point | 9.36 |
| Grand average of hydropathicity | −0.150 |
| Total number of positively charged residues (Arg+Lys) | 18 |
| Total number of negatively charged residues (Asp+Glu) | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Wang, H.; Jia, C.; Chen, G.; Zhao, M. Transient Expression of Hen Egg White Lysozyme (EWL) in Nicotiana benthamiana Influences Plant Pathogen Infection. Life 2025, 15, 642. https://doi.org/10.3390/life15040642
Meng Z, Wang H, Jia C, Chen G, Zhao M. Transient Expression of Hen Egg White Lysozyme (EWL) in Nicotiana benthamiana Influences Plant Pathogen Infection. Life. 2025; 15(4):642. https://doi.org/10.3390/life15040642
Chicago/Turabian StyleMeng, Zhuo, Haijuan Wang, Chongyi Jia, Guihua Chen, and Mingmin Zhao. 2025. "Transient Expression of Hen Egg White Lysozyme (EWL) in Nicotiana benthamiana Influences Plant Pathogen Infection" Life 15, no. 4: 642. https://doi.org/10.3390/life15040642
APA StyleMeng, Z., Wang, H., Jia, C., Chen, G., & Zhao, M. (2025). Transient Expression of Hen Egg White Lysozyme (EWL) in Nicotiana benthamiana Influences Plant Pathogen Infection. Life, 15(4), 642. https://doi.org/10.3390/life15040642





