Potential Effect of Root Exudates from Ten Crops on Promoting Stress Tolerance in Alfalfa (Medicago sativa) Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Root Exudates
2.3. Seed Germination and Seedling Growth Conditions
2.4. Calculation of Germination Parameters and Allelopathy Index
2.5. Measurement of Physiological Indicators
2.6. Statistical Analysis
3. Results
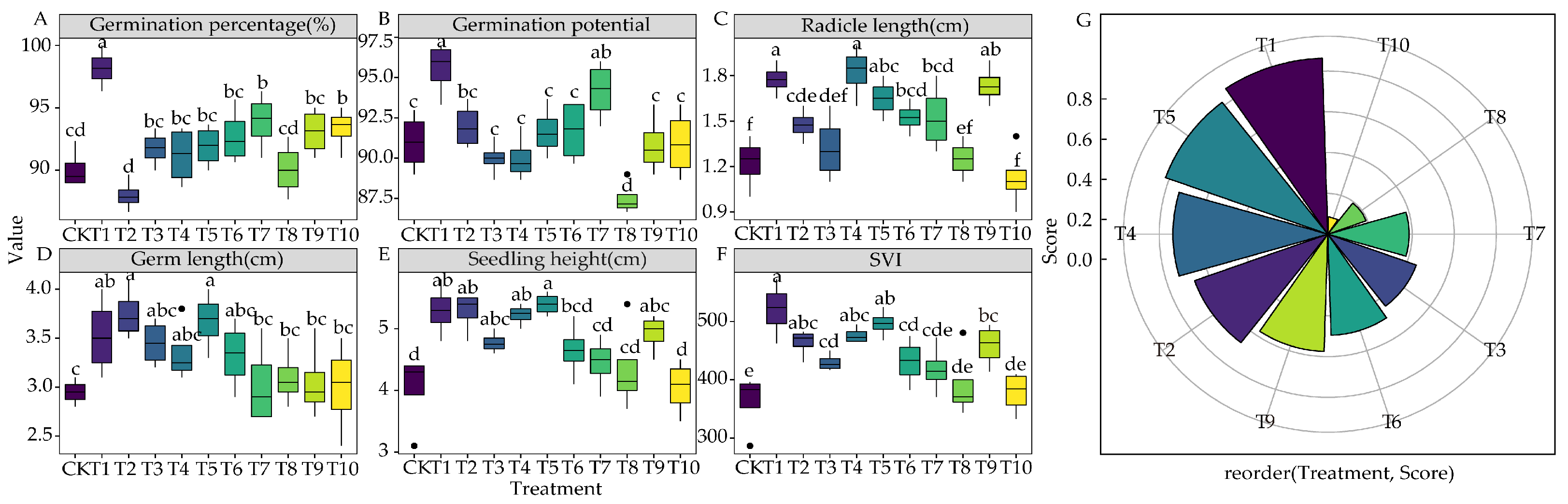
3.1. Effects of the Root Exudates from the 10 Plants on Seed Germination
3.2. Allelopathy of the Root Exudates of the 10 Plants
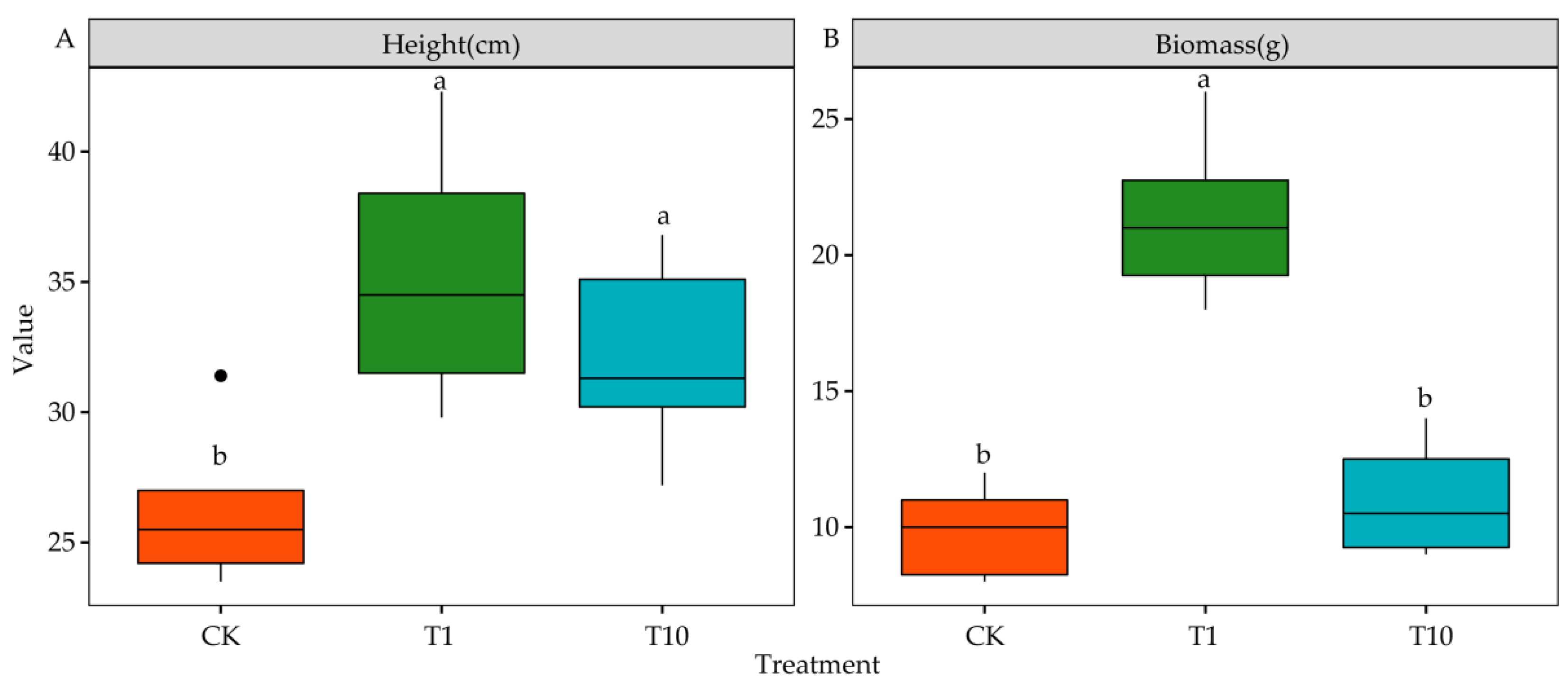
3.3. Effects of the Root Exudates on the Biomass and Height of Alfalfa Seedlings
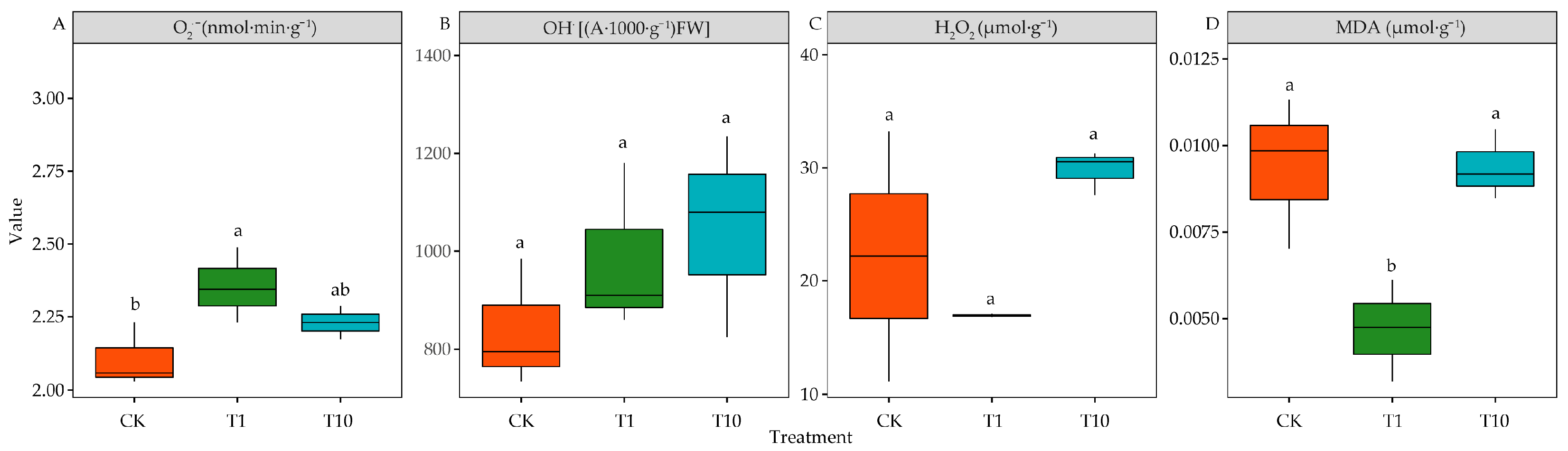
3.4. Effects of Root Exudates on ROS Production and Lipid Peroxidation of Alfalfa Seedlings
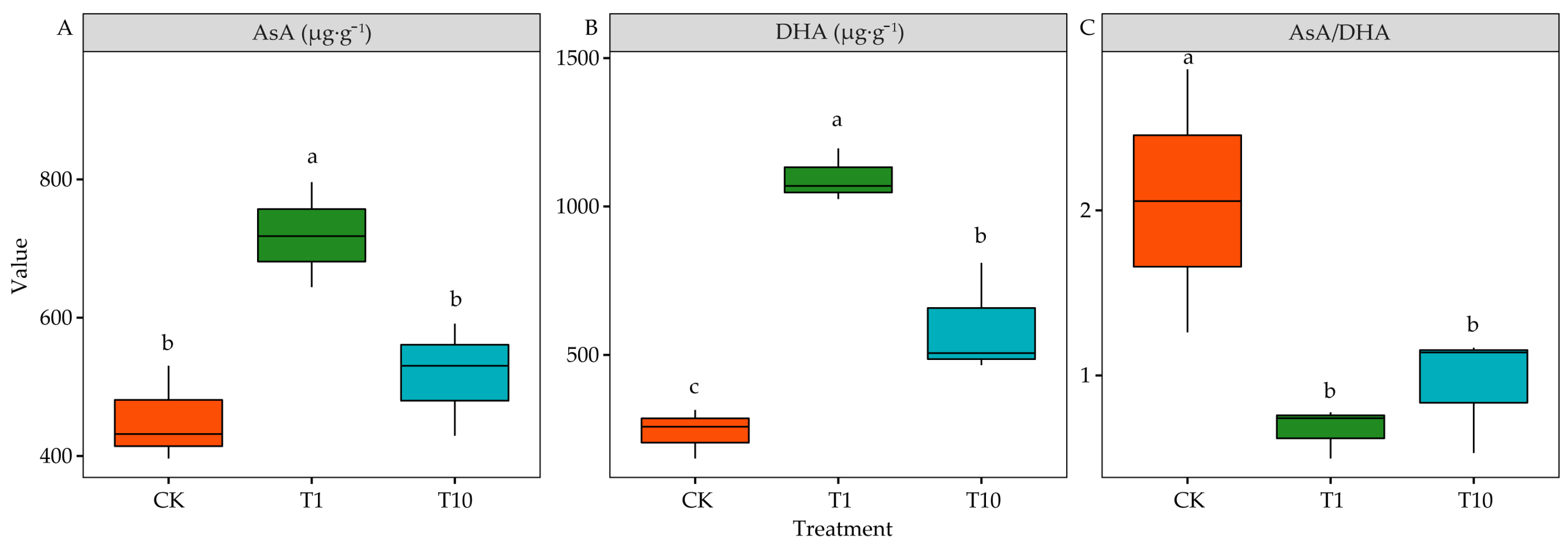
3.5. Effects of Root Exudates on Antioxidant of Alfalfa Seedling
3.6. Effects of the Root Exudates on the Antioxidant Enzyme Activities of Alfalfa Seedlings
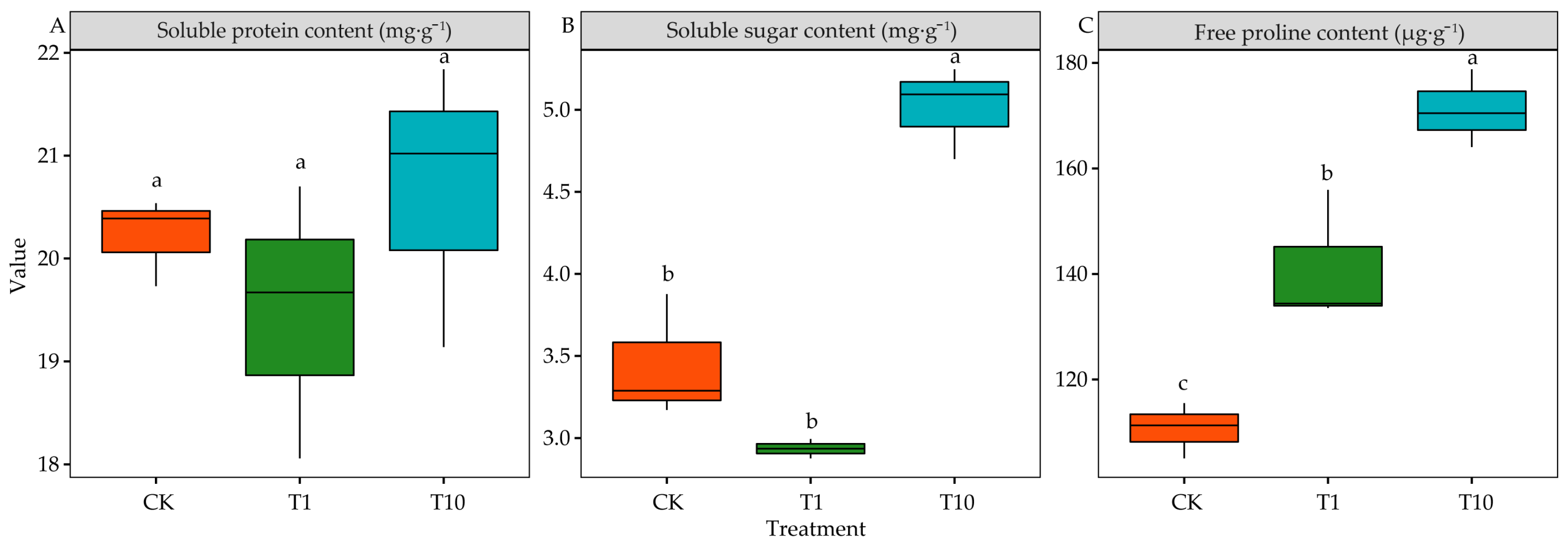
3.7. Effects of the Root Exudates on the Osmotic Adjustment Substance of Alfalfa Seedlings
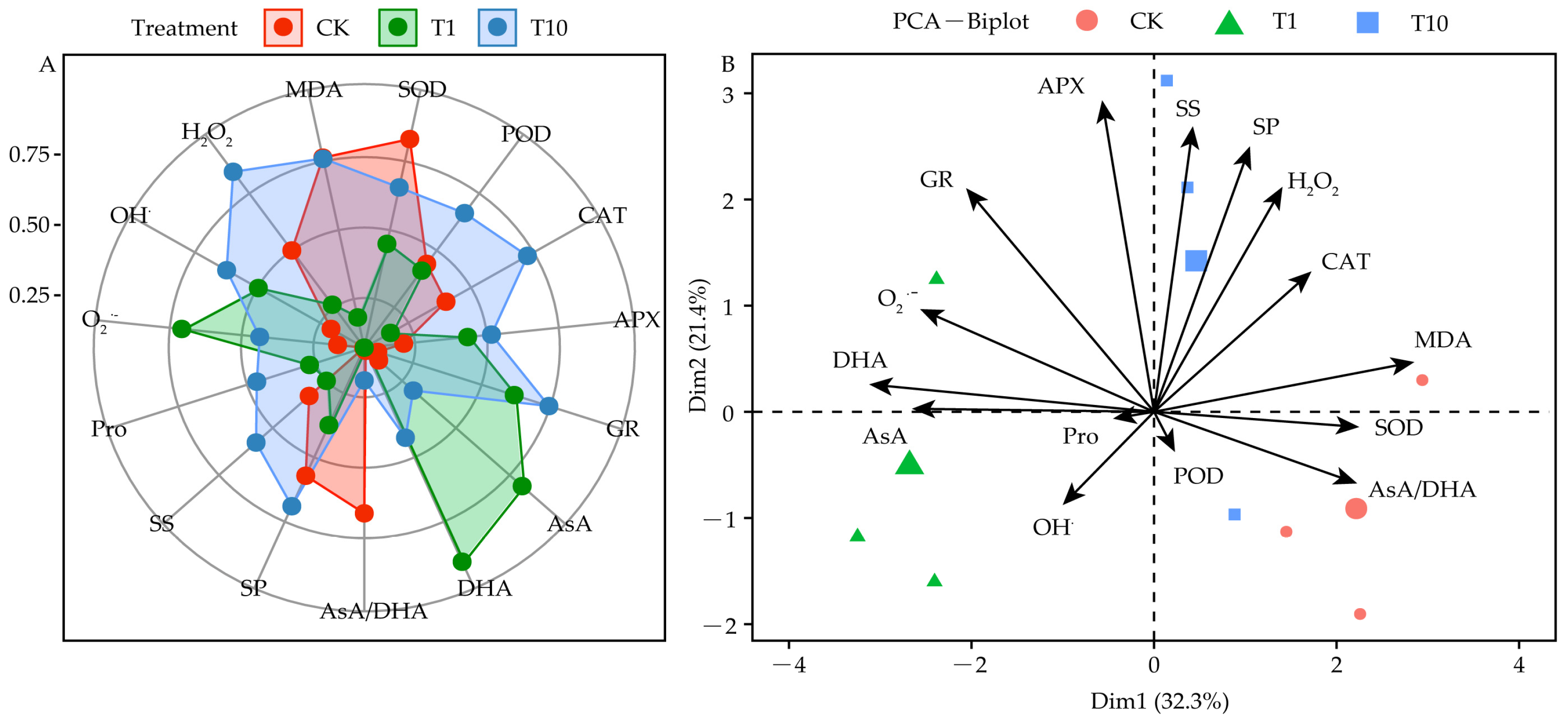
3.8. Responses of the Root Exudates in Promoting the Resistant Physiology of Alfalfa
4. Discussion
4.1. Strong and Weak Allelopathy and Its Effects on the Growth of Alfalfa
4.2. Alteration of Oxidative Damage in Alfalfa Under Strong and Weak Allelopathy
4.3. Alteration of the Antioxidant System and Osmotic Adjustment Substance in Alfalfa Under Strong and Weak Allelopathy
4.4. Difference of Physiological Stress Resistance Pathway in Alfalfa Between Strong and Weak Allelopathy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- O’Rourke, J.A.; Fu, F.L.; Bucciarelli, B.; Yang, S.S.; Samac, D.A.; Lamb, J.F.S.; Monteros, M.J.; Graham, M.A.; Gronwald, J.W.; Krom, N.; et al. The Medicago sativa gene index 1.2: A web-accessible gene expression atlas for investigating expression differences between Medicago sativa subspecies. BMC Genom. 2015, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.L.; Tang, B.L.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.H.; Liu, Y.B.; et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [PubMed]
- Fang, C.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar]
- Einhellig, F.A. Allelopathy-current status and future goals. In Allelopathy: Organisms, Processes, and Applications; Inderjit, A., Dakshini, K.M.M., Einhellig, F.A., Eds.; American Chemical Society Press: Washington, DC, USA, 1995; pp. 1–24. [Google Scholar]
- Zeng, R.S.; Mallik, A.U.; Luo, S.M. Allelopathy in Sustainable Agriculture and Forestry; Springer: New York, NY, USA, 2008. [Google Scholar]
- Zeng, R.S. Allelopathy-the solution is indirect. J. Chem. Ecol. 2014, 40, 515–516. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar]
- Cheema, Z.; Farooq, M.; Khaliq, A. (Eds.) Application of allelopathy in crop production: Success story from Pakistan. In Allelopathy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 113–143. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar]
- Zeng, R.S.; Luo, S.M.; Shi, Y.H.; Shi, M.B.; Tu, X.Y. Physiological and biochemical mechanism of allelopathy of secalonic acid F on higher plants. Agron. J. 2001, 93, 72–79. [Google Scholar]
- Yu, J.Q.; Ye, S.F.; Zhang, M.F.; Hu, W.H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. [Google Scholar]
- Politycka, B. Phenolics and the activities of phenylalanine ammonia-1yase, phenol-β-glucosyltransferase and β-glucosidase in cucumber roots as affected by phenolic allelochemicals. Acta Physiol. Plant. 1998, 20, 405–410. [Google Scholar] [CrossRef]
- Rama, D.S.; Prasad, M.N.V. Ferulic acid mediated changes in oxidative enzymes of maize seedlings: Implications in growth. Biol. Plant. 1996, 38, 387–395. [Google Scholar]
- Maqbool, N.; Wahid, A.; Farooq, M.; Cheema, Z.A. Allelopathy and abiotic stress interaction in crop plants. In Allelopathy; Cheema, Z., Farooq, M., Wahid, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 451–468. [Google Scholar]
- Zhang, X.Y.; Shi, S.L.; Li, X.L.; Li, C.N.; Zhang, C.M.; Yun, A.; Kang, W.J.; Yin, G.L. Effects of Autotoxicity on Alfalfa (Medicago sativa): Seed Germination, Oxidative Damage and Lipid Peroxidation of Seedlings. Agronomy 2021, 11, 1027. [Google Scholar] [CrossRef]
- Zhang, C.M.; Shi, S.L. Physiological and Proteomic Responses of Contrasting Alfalfa (Medicago sativa L.) Varieties to PEG-Induced Osmotic Stress. Front. Plant Sci. 2018, 9, 242. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 357–359. [Google Scholar]
- Williamson, G.B.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; ISTA Press: Basserdorf, Switzerland, 2012. [Google Scholar]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Willekens, H. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. Embo J. 1997, 16, 4806–4816. [Google Scholar]
- Liu, Y.J.; Zhao, Z.G.; Si, J.; Di, C.; Han, J.; An, L. Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul. 2009, 59, 207–214. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib). Planta 1976, 130, 175–180. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Methods Enzymol. 1995, 2, 764–775. [Google Scholar]
- Havir, E.A.; Mchale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Murshed, R.; Lopezlauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate-glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Murshed, R.; Lopezlauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanumlycopersicon L. cv. Micro-Tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improve colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kong, C.H.; Zhao, H.; Xu, X.H.; Wang, P.; Gu, Y. Activity and allelopathy of soil of flavone O-glycosides from rice. J. Agric. Food Chem. 2007, 55, 6007–6012. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar]
- Ye, X.X.; Jia, J.N.; Ma, Y.Q.; Yu, A.N.; Dong, S. Effectiveness of ten commercial maize cultivars in inducing egyptian broomrape germination. Front. Agric. Sci. Eng. 2016, 3, 137–146. [Google Scholar]
- Macias, F.A.; Marin, D.; Oliveros-Bastidas, A.; Varela, R.M.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar]
- Inal, A.; Gunes, A.; Zhang, F.; Cakmak, I. Peanut/maize intercropping induced changes in rhizosphere and nutrient concentrations in shoots. Plant Physiol. Biochem. 2007, 45, 350–356. [Google Scholar] [PubMed]
- Dai, C.C.; Xie, H.; Wang, X.X.; Li, P.D.; Tan, X. Intercropping peanut with traditional Chinese medicinal plants improves soil microcosm environment and peanut production in subtropical China. Afr. J. Biotechnol. 2009, 8, 3739–3746. [Google Scholar]
- Shen, X.; Yang, F.; Xiao, C.W.; Zhou, Y. Increased contribution of root exudates to soil carbon input during grassland degradation. Soil Biol. Biochem. 2020, 146, 107817. [Google Scholar] [CrossRef]
- Guo, M.X.; Gong, Z.Q.; Miao, R.H.; Su, D.; Li, X.; Jia, C.; Zhuang, J. The influence of root exudates of maize and soybean on polycyclic aromatic hydrocarbons degradation and soil bacterial community structur. Ecol. Eng. 2017, 99, 22–30. [Google Scholar]
- Scheffknecht, S.; Mammerler, R.; Steinkellner, S.; Vierheilig, H. Root exudates of mycorrhizal tomato plants exhibit a different effect on microconidia germination of Fusarium oxysporum f. sp. lycopersici than root exudates from non-mycorrhizal tomato plants. Mycorrhiza 2006, 16, 365–370. [Google Scholar]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1, 4-benzoxazin-3(4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Zhang, J.; Boone, L.; Kocz, R.; Zhang, C.; Lynn, D.G. At the maize/Agrobacterium interface: Natural factors limiting host transformation. Chem. Biol. 2000, 7, 611–621. [Google Scholar]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar]
- Ding, J.; Sun, Y.; Xiao, C.L.; Yan, K.S.; Zhou, H.; Yu, J.Q. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J. Exp. Bot. 2007, 58, 3765–3773. [Google Scholar]
- Novo, E.; Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenes. Tissue Repair 2008, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Weismann, D.; Hartvigsen, K.; Lauer, N.; Bennett, K.L.; Scholl, H.P.; Charbel, I.P.; Cano, M.; Brandstätter, H.; Tsimikas, S.; Skerka, C.; et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 2011, 478, 76–81. [Google Scholar]
- Hu, L.F.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Sytykiewicz, H. Expression patterns of genes involved in ascorbateglutathione cycle in aphid-infested maize (Zea mays L.) Seedlings. Int. J. Mol. Sci. 2016, 17, 268. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Terzi, R.; Kalaycıoglu, E.; Demiralay, M.; Saglam, A.; Kadioglu, A. Exogenous ascorbic acid mitigates accumulation of abscisic acid, proline and polyamine under osmotic stress in maize leaves. Acta Physiol. Plant. 2015, 37, 43. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Breusegem, F.V. ROS signaling: The new wave? Trends in Plant Science 2011, 16, 300–309. [Google Scholar] [PubMed]
- Kayihan, C.; Eyidogan, F.; Afsar, N.; Oktem, H.A.; Yucel, M. Cu/Zn superoxide dismutase activity and respective gene expression during cold acclimation and freezing stress in barley cultivars. Biol. Plant. 2012, 56, 693–698. [Google Scholar] [CrossRef]
- Liang, W.J.; Ma, X.L.; Wan, P. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar]
- Pell, E.J.; Dann, M.S. Multiple stress and plant senescence. In Integrated Response of Plant to Stress; Mooney, H.A., Winner, W.E., Pell, E.J., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 189–284. [Google Scholar]
- Sheeba, A.; Singh, V.P.; Sricastava, P.K. Differential physiologicaland biochemical responses of two cyanobacteria Nostoc muscorum and Phormidium foveolarum against oxyfluorfen and UV-B radiation. Ecotoxicol. Environ. Saf. 2011, 74, 1981–1993. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Hakeem, K.; Chandna, R.; Ahmad, P. Role of glutathione reductase in plant abiotic stress. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 149–158. [Google Scholar]
- Seo, J.S.; Lee, K.W.; Rhee, J.S.; Hwang, D.S.; Lee, M.; Park, H.G.; Ahn, I.Y.; Lee, J.S. Environmental stressors (salinity, heavy metals, H2O2) modulate expression of glutathione reductase (GR) gene from the intertidal copepod Tigriopus japonicus. Aquat. Toxicol. 2006, 80, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Torres-Franklin, M.L.; Contour-Ansel, D.; Zuily-Fodil, Y.; Pham-Thi, A.T. Molecular cloning of glutathione reductase cDNAs and analysis of GR gene expression in cowpea and common bean leaves during recovery from moderate drought stress. J. Plant Physiol. 2008, 165, 514–521. [Google Scholar] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar]
- Pallavi, S.; Bhushan, J.A.; Shanker, D.R.; Mohammad, P. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar]
- Molinari, H.B.C.; Marur, C.J.; Daros, E.; Freitas, M.K.; Portela, J.F.R.; Filhob, J.C.B.; Pereirac, L.F.P.; Vieiraa, L.G.E. Evaluation of the stress inducible production of proline in transgenic sugarcane (Saccharum spp.): Osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol. Plant. 2010, 130, 218–229. [Google Scholar] [CrossRef]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar]
- Balestrini, R.; Ott, T.; Guther, M.; Bonfante, P.; Udvardi, M.K.; Tullio, M.C.D. Ascorbate oxidase: The unexpected involvement of a “wasteful enzyme” in the symbioses with nitrogen-fi xing bacteria and arbuscular mycorrhizal fungi. Plant Physiol. Biochem. 2012, 59, 71–79. [Google Scholar]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef]


| Number | Varieties | Latin Name |
|---|---|---|
| T1 | Maize | Zea mays L. |
| T2 | Wheat | Triticum aestivum L. |
| T3 | Wheatgrass | Agropyron cristatum L. Gaertn |
| T4 | Oats | Avena sativa L. |
| T5 | Red clover | Trifolium pratense L. |
| T6 | Tall Fescue | Festuca elata Keng ex E. Alexeev |
| T7 | White clover | Trrifolium repens L. |
| T8 | Perennial ryegrass | Lolium perenne L. |
| T9 | Smooth brome grass | Bromus inermis Leyss. |
| T10 | Soybean | Glycine max (Linn.) Merr. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Shi, S.; Li, X.; Li, C.; Li, Q. Potential Effect of Root Exudates from Ten Crops on Promoting Stress Tolerance in Alfalfa (Medicago sativa) Seedlings. Life 2025, 15, 600. https://doi.org/10.3390/life15040600
Zhang X, Shi S, Li X, Li C, Li Q. Potential Effect of Root Exudates from Ten Crops on Promoting Stress Tolerance in Alfalfa (Medicago sativa) Seedlings. Life. 2025; 15(4):600. https://doi.org/10.3390/life15040600
Chicago/Turabian StyleZhang, Xiaoyan, Shangli Shi, Xiaolong Li, Changning Li, and Qian Li. 2025. "Potential Effect of Root Exudates from Ten Crops on Promoting Stress Tolerance in Alfalfa (Medicago sativa) Seedlings" Life 15, no. 4: 600. https://doi.org/10.3390/life15040600
APA StyleZhang, X., Shi, S., Li, X., Li, C., & Li, Q. (2025). Potential Effect of Root Exudates from Ten Crops on Promoting Stress Tolerance in Alfalfa (Medicago sativa) Seedlings. Life, 15(4), 600. https://doi.org/10.3390/life15040600





