Efficacy of Different Irrigation Solutions on Bacterial Biofilm in Periprosthetic Joint Infections: A Systematic Review and Network Meta-Analysis †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Elegibility Criteria
2.4. Study Selection
2.5. Data Collection
2.6. Quality Assessment
2.7. Statistical Analyses
3. Results
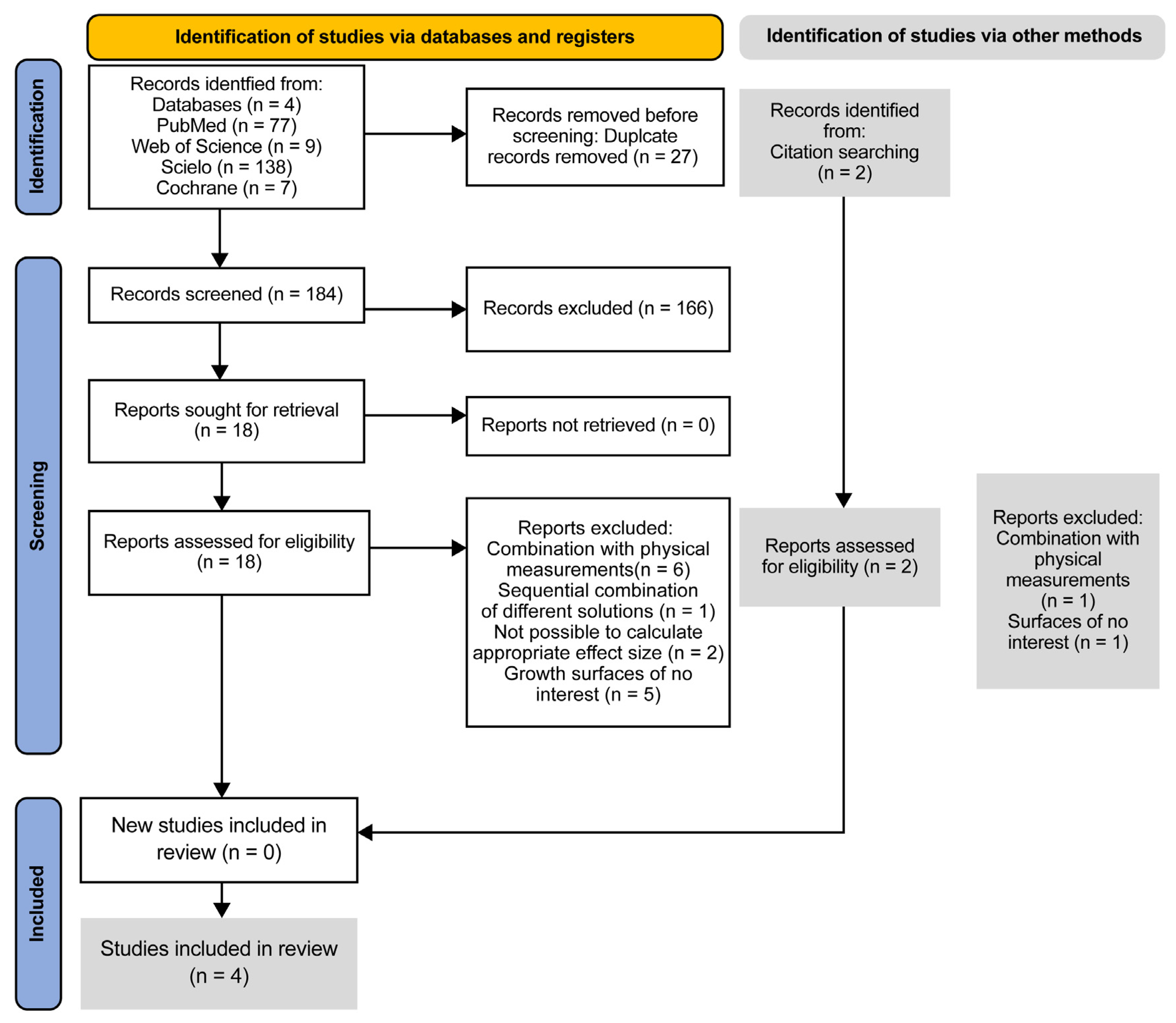
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Quality Assessment
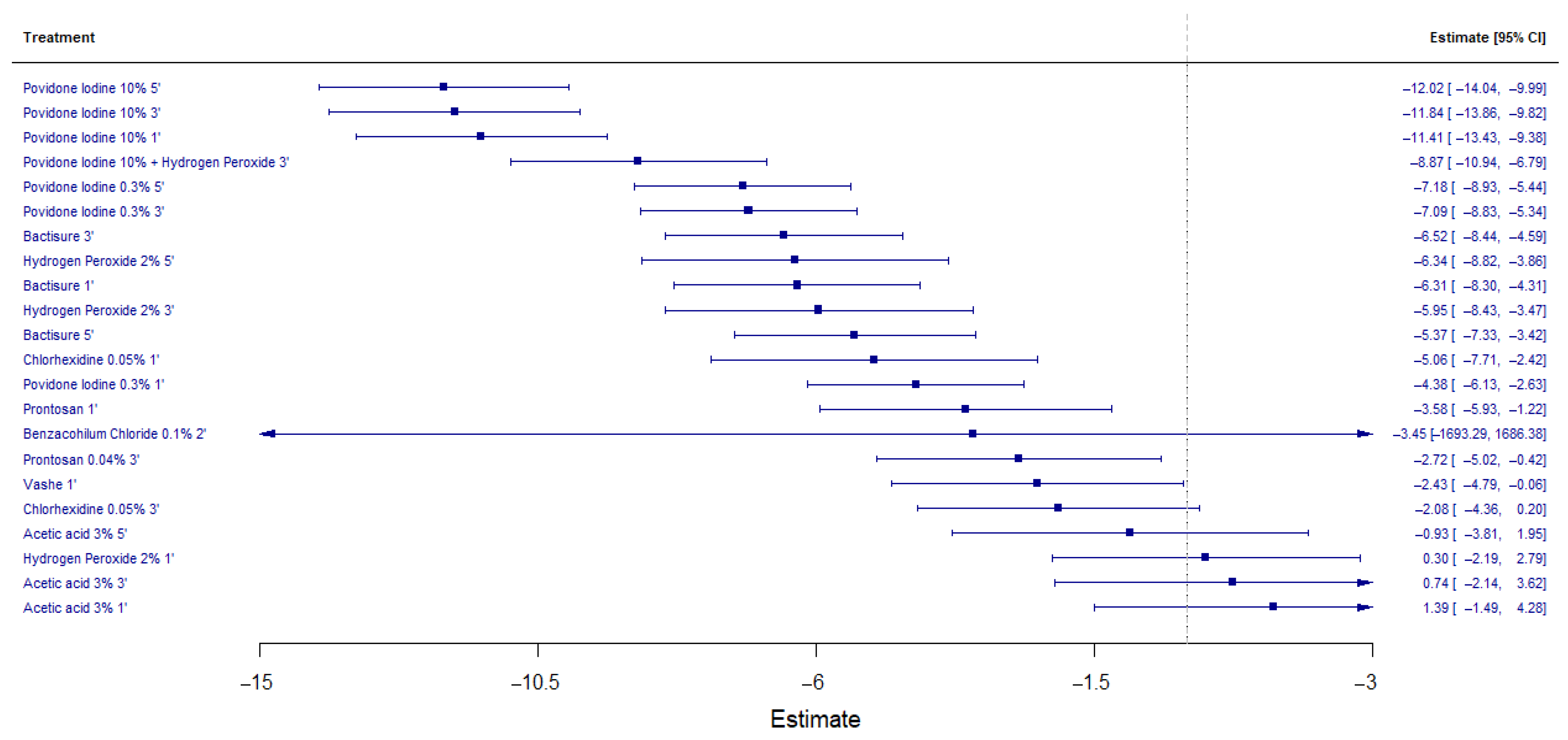
3.4. Antimicrobial Efficacy of Antiseptic Solutions
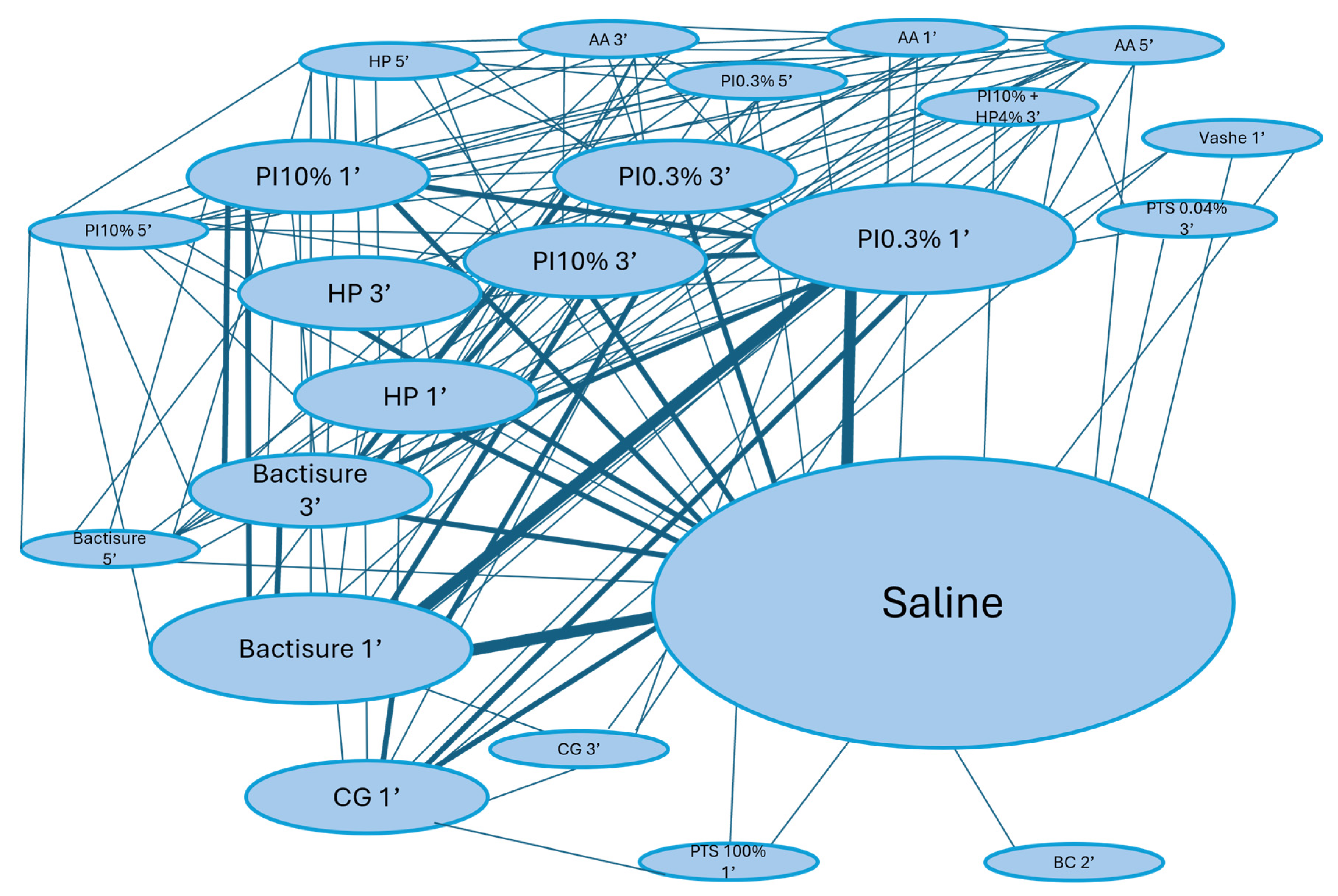
3.5. Ranking of Antiseptic Solutions
3.6. Heterogeneity and Consistency Analysis
3.7. Publication Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Multivariate Meta-Analysis Model (k = 157; Method: REML)
| Estim | Sqrt | Fixed | |
| tau^2 | 97.596 | 31.240 | no |
| rho | 0.5000 | yes |
| estimate | se | zval | pval | ci.lb | ci.ub | sig. | |
| AA31 | 13.917 | 14.720 | 0.9454 | 0.3444 | −14.934 | 42.768 | |
| AA33 | 0.7431 | 14.697 | 0.5056 | 0.6131 | −21.375 | 36.237 | |
| AA35 | −0.9278 | 14.687 | −0.6317 | 0.5276 | −38.064 | 19.509 | |
| BA102 | −34.528 | 8621.773 | −0.0040 | 0.9968 | −16,932.894 | 16,863.837 | |
| Bactisure1 | −63.063 | 10.168 | −62.024 | <0.0001 | −82.992 | −43.135 | *** |
| Bactisure3 | −65.177 | 0.9824 | −66.347 | <0.0001 | −84.431 | −45.923 | *** |
| Bactisure5 | −53.722 | 0.9967 | −53.900 | <0.0001 | −73.257 | −34.187 | *** |
| CHG0051 | −50.647 | 13.482 | −37.566 | 0.0002 | −77.072 | −24.222 | *** |
| CHG0053 | −20.837 | 11.635 | −17.909 | 0.0733 | −43.641 | 0.1967 | . |
| H2O21 | 0.3002 | 12.690 | 0.2366 | 0.8130 | −21.869 | 27.873 | |
| H2O23 | −59.514 | 12.666 | −46.988 | <0.0001 | −84.339 | −34.690 | *** |
| H2O25 | −63.402 | 12.661 | −50.077 | <0.0001 | −88.217 | −38.587 | *** |
| PhmbBtn3 | −27.161 | 11.730 | −23.154 | 0.0206 | −50.152 | −0.4169 | * |
| PI031 | −43.825 | 0.8929 | −49.082 | <0.0001 | −61.326 | −26.325 | *** |
| PI033 | −70.872 | 0.8910 | −79.540 | <0.0001 | −88.335 | −53.408 | *** |
| PI035 | −71.843 | 0.8916 | −80.578 | <0.0001 | −89.319 | −54.368 | *** |
| PI101 | −114.091 | 10.336 | −110.384 | <0.0001 | −134.349 | −93.833 | *** |
| PI103 | −118.400 | 10.331 | −114.602 | <0.0001 | −138.649 | −98.150 | *** |
| PI105 | −120.152 | 10.336 | −116.252 | <0.0001 | −140.409 | −99.895 | *** |
| PI10PO3 | −88.694 | 10.586 | −83.781 | <0.0001 | −109.443 | −67.945 | *** |
| Prontosan1 | −35.762 | 12.018 | −29.757 | 0.0029 | −59.317 | −12.208 | ** |
| Vashe1 | −24.253 | 12.044 | −20.136 | 0.0441 | −47.859 | −0.0646 | * |
| Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’. | |||||||
References
- Rezapoor, M.; Parvizi, J. Prevention of Periprosthetic Joint Infection. J. Arthroplast. 2015, 30, 902–907. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. 2018, 100, 1455–1460. [Google Scholar] [CrossRef]
- Karachalios, T.; Komnos, G.A. Management Strategies for Prosthetic Joint Infection: Long-Term Infection Control Rates, Overall Survival Rates, Functional and Quality of Life Outcomes. EFORT Open Rev. 2021, 6, 727–734. [Google Scholar] [CrossRef]
- Parvizi, J.; Adeli, B.; Zmistowski, B.; Restrepo, C.; Greenwald, A.S. Management of Periprosthetic Joint Infection: The Current Knowledge: AAOS Exhibit Selection. J. Bone Jt. Surg. 2012, 94, e104. [Google Scholar] [CrossRef]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic Penetration into Bone and Joints: An Updated Review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Abdo, Z.E.; Springer, B.D.; Chen, A.F. Pursuit of the Ideal Antiseptic Irrigation Solution in the Management of Periprosthetic Joint Infections. J. Bone Jt. Infect. 2021, 6, 189–198. [Google Scholar] [CrossRef]
- Anderson, J.T.; Barnes, A.J.; Stambough, J.B. The Role of Antiseptic Irrigation Solutions and Topical Antibiotics in Total Joint Arthroplasty. J. Surg. Orthop. Adv. 2021, 30, 226–230. [Google Scholar]
- Wood, T.; Ekhtiari, S.; Mundi, R.; Citak, M.; Sancheti, P.K.; Guerra-Farfan, E.; Schemitsch, E.; Bhandari, M. The Effect of Irrigation Fluid on Periprosthetic Joint Infection in Total Hip and Knee Arthroplasty: A Systematic Review and Meta-Analysis. Cureus 2020, 12, e7813. [Google Scholar] [CrossRef]
- Schwechter, E.M.; Folk, D.; Varshney, A.K.; Fries, B.C.; Kim, S.J.; Hirsh, D.M. Optimal Irrigation and Debridement of Infected Joint Implants. J. Arthroplast. 2011, 26, 109–113. [Google Scholar] [CrossRef]
- Goswami, K.; Austin, M.S. Intraoperative Povidone-Iodine Irrigation for Infection Prevention. Arthroplast. Today 2019, 5, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.M.; Hart, A.; Taunton, M.J.; Osmon, D.R.; Mabry, T.M.; Abdel, M.P.; Perry, K.I. Use of Povidone-Iodine Irrigation Prior to Wound Closure in Primary Total Hip and Knee Arthroplasty: An Analysis of 11,738 Cases. J. Bone Jt. Surg. 2019, 101, 1144–1150. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Wu, M.; Cochrane, N.H.; Belay, E.; Myntti, M.F.; James, G.A.; Ryan, S.P.; Seyler, T.M. Efficacy of Common Antiseptic Solutions against Clinically Relevant Microorganisms in Biofilm. Bone Jt. J. 2021, 103-B, 908–915. [Google Scholar] [CrossRef]
- Argenson, J.N.; Arndt, M.; Babis, G.; Battenberg, A.; Budhiparama, N.; Catani, F.; Chen, F.; de Beaubien, B.; Ebied, A.; Esposito, S.; et al. Hip and Knee Section, Treatment, Debridement and Retention of Implant: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S399–S419. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, 1st ed.; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-0-470-69951-5. [Google Scholar]
- Rooney, A. Extending a Risk-of-Bias Approach to Address In Vitro Studies; National Toxicology Program Office of Health Assessment and Translation: Raleigh, NC, USA, 2015.
- Tshwang, M.D. A Robust Test for Multivariate Publication Bias in Meta-Analysis. Master’s Thesis, Botswana International University of Science and Technology, Palapye, Botswana, 2019. [Google Scholar]
- Márquez-Gómez, M.; Díaz-Navarro, M.; Visedo, A.; Hafian, R.; Matas, J.; Muñoz, P.; Vaquero, J.; Guembe, M.; Sanz-Ruíz, P. An In Vitro Study to Assess the Best Strategy for the Chemical Debridement of Periprosthetic Joint Infection. Antibiotics 2023, 12, 1507. [Google Scholar] [CrossRef]
- Moussa, F.W.; Gainor, B.J.; Anglen, J.O.; Christensen, G.; Simpson, W.A. Disinfecting Agents for Removing Adherent Bacteria From Orthopaedic Hardware. Clin. Orthop. 1996, 329, 255–262. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; De Jonge, S.; De Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO Recommendations on Intraoperative and Postoperative Measures for Surgical Site Infection Prevention: An Evidence-Based Global Perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Surgical Site Infections: Prevention and Treatment; NICE Guideline: London, UK, 2019. [Google Scholar]
- Kataoka, M.; Tsumura, H.; Kaku, N.; Torisu, T. Toxic Effects of Povidone–Iodine on Synovial Cell and Articular Cartilage. Clin. Rheumatol. 2006, 25, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Von Keudell, A.; Canseco, J.A.; Gomoll, A.H. Deleterious Effects of Diluted Povidone–Iodine on Articular Cartilage. J. Arthroplast. 2013, 28, 918–921. [Google Scholar] [CrossRef]
- Romano, V.; Di Gennaro, D.; Sacco, A.M.; Festa, E.; Roscetto, E.; Basso, M.A.; Ascione, T.; Balato, G. Cell Toxicity Study of Antiseptic Solutions Containing Povidone–Iodine and Hydrogen Peroxide. Diagnostics 2022, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.V.; Rath, T.; Radtke, C. Hydrogen Peroxide (H2O2): A Review of Its Use in Surgery. Wien. Med. Wochenschr. 2019, 169, 222–225. [Google Scholar] [CrossRef]
- Gonzalez Alonso, M.; Guerra González, A.; Villar Suárez, V.; Lázaro, J. In vitro efficacy of different irrigation solutions against biofilm in orthopaedic surgery: A systematic review and network meta-analysis. Orthop. Proc. 2024, 106-B, 65. [Google Scholar] [CrossRef]
| Study | Márquez-Gómez (2023) [20] | Premkumar (2021) [13] | O’Donnell (2021) [14] | Moussa (1996) [21] |
|---|---|---|---|---|
| Surfaces | Stainless Steel | Ti6Al4V *; PMMA †; Polystyrene | Titanium disc | Stainless Steel |
| Irrigation solutions | PI 0.3%; PI 10%; H2O2 3%; AA 3%; Bactisure; saline | PI 10%; PI 0.3%; GCH 0.05%; PI10% + H2O2 3%; Prontosan 0.04%; Bactisure; saline | Bactisure; CG 0.05%; PI 0.35%; Vashe; Prontosan; saline | Benzalkonium Chloride 0.1%; saline |
| Exposure times | 1′; 3′; 5′ | 1′; 3′ | 1′ | 1′; 5′ |
| Bacteria species | MSSA ‡; MRSA §; S. epidermidis; P. aeuriginosa | MSSA ‡ | MSSA ‡; S. epidermidis; P. aeruginosa; C. acnes | P. aeruginosa; S. epidermidis; MSSA ‡ |
| Biofilm maturation time | 24 h | 72 h | 72 h | 24 h |
| Studies | D1 | D2 | D3 | D4 | D5 | D6 |
|---|---|---|---|---|---|---|
| Márquez-Gómez 2023 [20] | ||||||
| O’Donnell 2021 [14] | ||||||
| Premkumar 2021 [13] | ||||||
| Moussa 1996 [21] |
 Definitely low;
Definitely low;  Probably low;
Probably low;  Probably high;
Probably high;  Definitely high.
Definitely high.| Antiseptic Solutions, Concentration, and Exposure Times |
|---|
| Acetic Acid 3% 1′ |
| Acetic Acid 3% 3′ |
| Acetic Acid 3% 5′ |
| Benzalkonium Chloride 1:1000 2′ |
| Bactisure 1′ |
| Bactisure 3′ |
| Bactisure 5′ |
| Chlorhexidine Gluconate 0.05% 1′ |
| Chlorhexidine Gluconate 0.05% 3′ |
| Hydrogen Peroxide 1′ |
| Hydrogen Peroxide 3′ |
| Hydrogen Peroxide 5′ |
| Prontosan 0.04% 3′ |
| Povidone Iodine 0.3% 1′ |
| Povidone Iodine 0.3% 3′ |
| Povidone Iodine 0.3% 5′ |
| Povidone Iodine 10% 1′ |
| Povidone Iodine 10% 3′ |
| Povidone Iodine 10% 5′ |
| Povidone Iodine 10% + Hydrogen Peroxide 4% 3′ |
| Prontosan 1′ |
| Vashe 1′ |
| Saline Solution |
| Irrigation Solutions Ranking | p-Score |
|---|---|
| Povidone Iodine 10% 5′ | 0.977 |
| Povidone Iodine 10% 3′ | 0.932 |
| Povidone Iodine 10% 1′ | 0.887 |
| Povidone Iodine 10% + Hydrogen Peroxide 4% 3′ | 0.836 |
| Povidone Iodine 0.3% 5′ | 0.761 |
| Povidone Iodine 0.3% 3′ | 0.712 |
| Bactisure 5′ | 0.673 |
| Hydrogen Peroxide 5′ | 0.655 |
| Bactisure 3′ | 0.637 |
| Hydrogen Peroxide 3′ | 0.581 |
| Bactisure 1′ | 0.519 |
| Chlorhexidine Gluconate 0.05% 1′ | 0.507 |
| Benzalkonium Chloride 0.1% 2′ | 0.499 |
| Povidone Iodine 0.3% 1′ | 0.437 |
| Prontosan 1′ | 0.381 |
| Prontosan 0.04% 3′ | 0.321 |
| Vashe 1′ | 0.298 |
| Chlorhexidine Gluconate 0.05% 3′ | 0.276 |
| Acetic Acid 3% 5′ | 0.213 |
| Saline | 0.134 |
| Hydrogen Peroxide 1′ | 0.120 |
| Acetic Acid 3% 3′ | 0.103 |
| Acetic Acid 3% 1′ | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Alonso, M.; Guerra-González, A.; Villar-Suárez, V.; Fernández-Castilla, B.; Sánchez-Lázaro, J.A. Efficacy of Different Irrigation Solutions on Bacterial Biofilm in Periprosthetic Joint Infections: A Systematic Review and Network Meta-Analysis. Life 2025, 15, 568. https://doi.org/10.3390/life15040568
González-Alonso M, Guerra-González A, Villar-Suárez V, Fernández-Castilla B, Sánchez-Lázaro JA. Efficacy of Different Irrigation Solutions on Bacterial Biofilm in Periprosthetic Joint Infections: A Systematic Review and Network Meta-Analysis. Life. 2025; 15(4):568. https://doi.org/10.3390/life15040568
Chicago/Turabian StyleGonzález-Alonso, Marcos, Adrián Guerra-González, Vega Villar-Suárez, Belén Fernández-Castilla, and Jaime A. Sánchez-Lázaro. 2025. "Efficacy of Different Irrigation Solutions on Bacterial Biofilm in Periprosthetic Joint Infections: A Systematic Review and Network Meta-Analysis" Life 15, no. 4: 568. https://doi.org/10.3390/life15040568
APA StyleGonzález-Alonso, M., Guerra-González, A., Villar-Suárez, V., Fernández-Castilla, B., & Sánchez-Lázaro, J. A. (2025). Efficacy of Different Irrigation Solutions on Bacterial Biofilm in Periprosthetic Joint Infections: A Systematic Review and Network Meta-Analysis. Life, 15(4), 568. https://doi.org/10.3390/life15040568






