Relationship Between Infrared Thermography and Functional Parameters in the Lower Limbs of Hemiplegic Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Participants
2.3. Familiarization
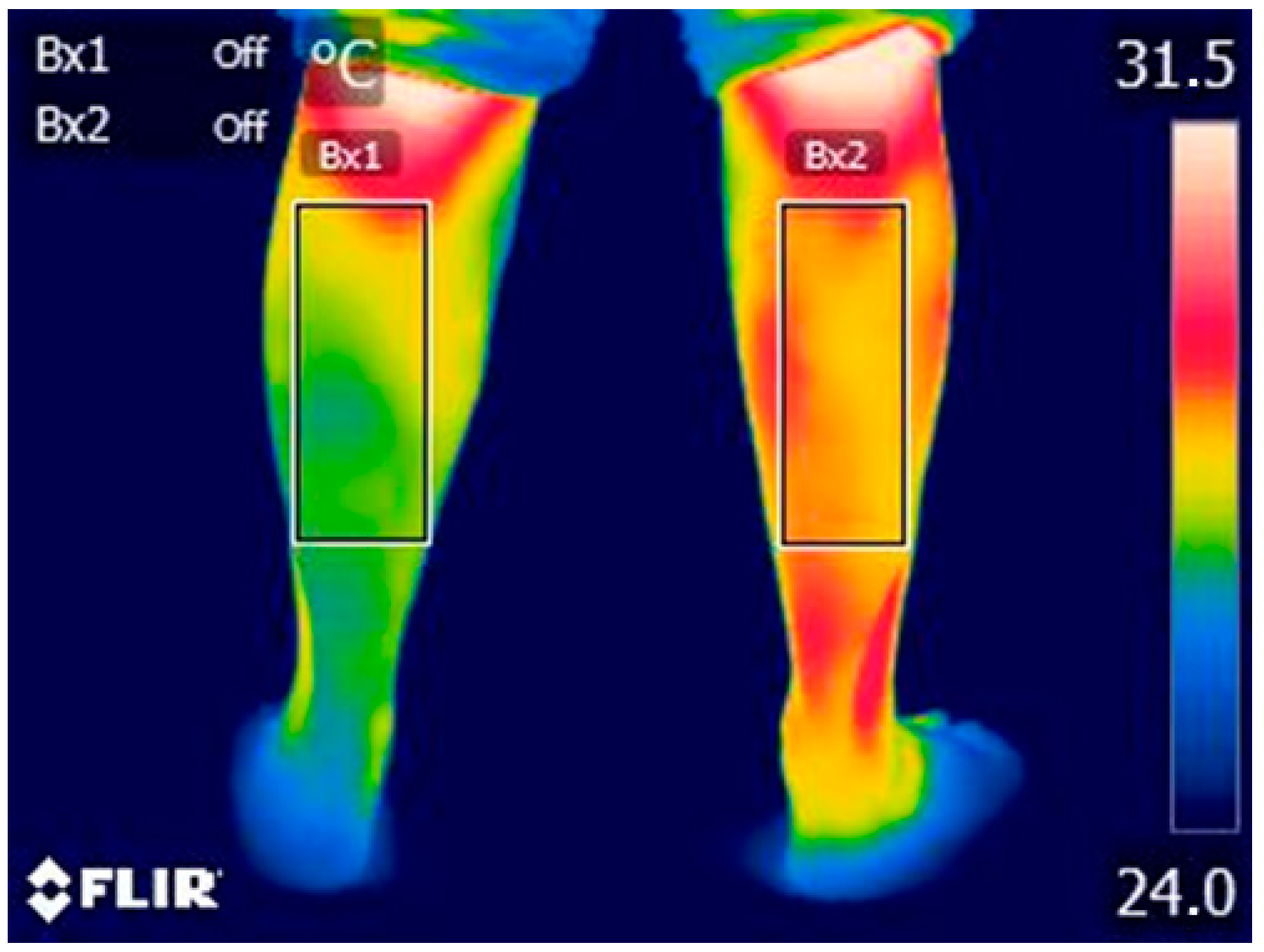
2.4. Index Test: Thermal Data Acquisition
2.5. Reference Standard
2.6. Statistical Analysis
3. Results
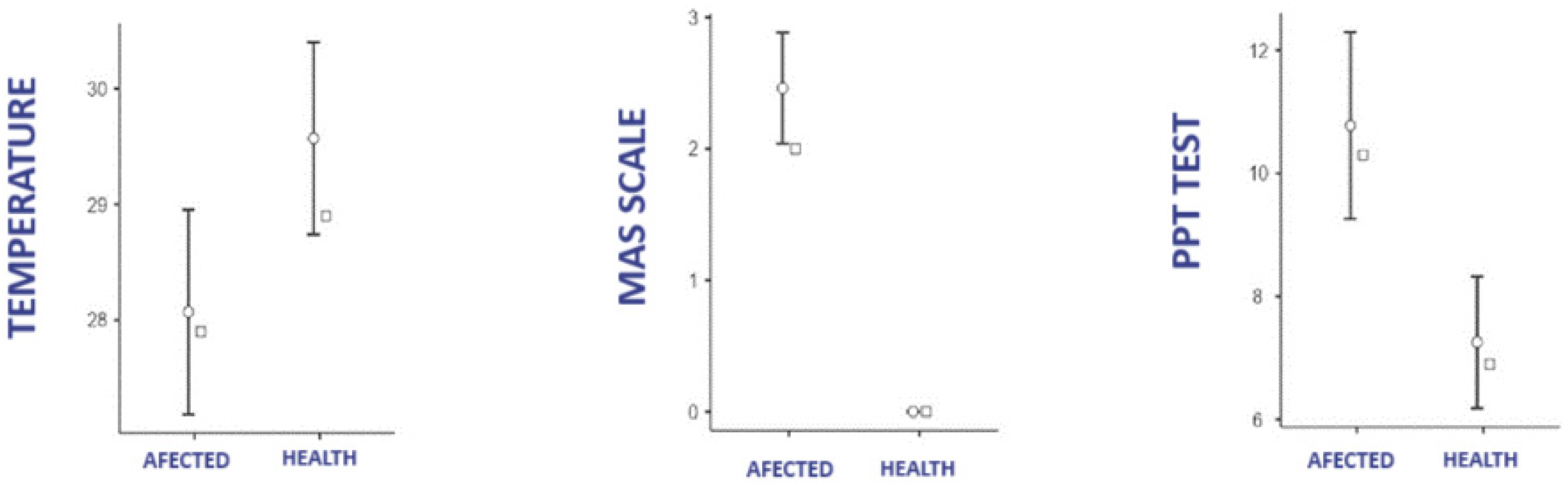
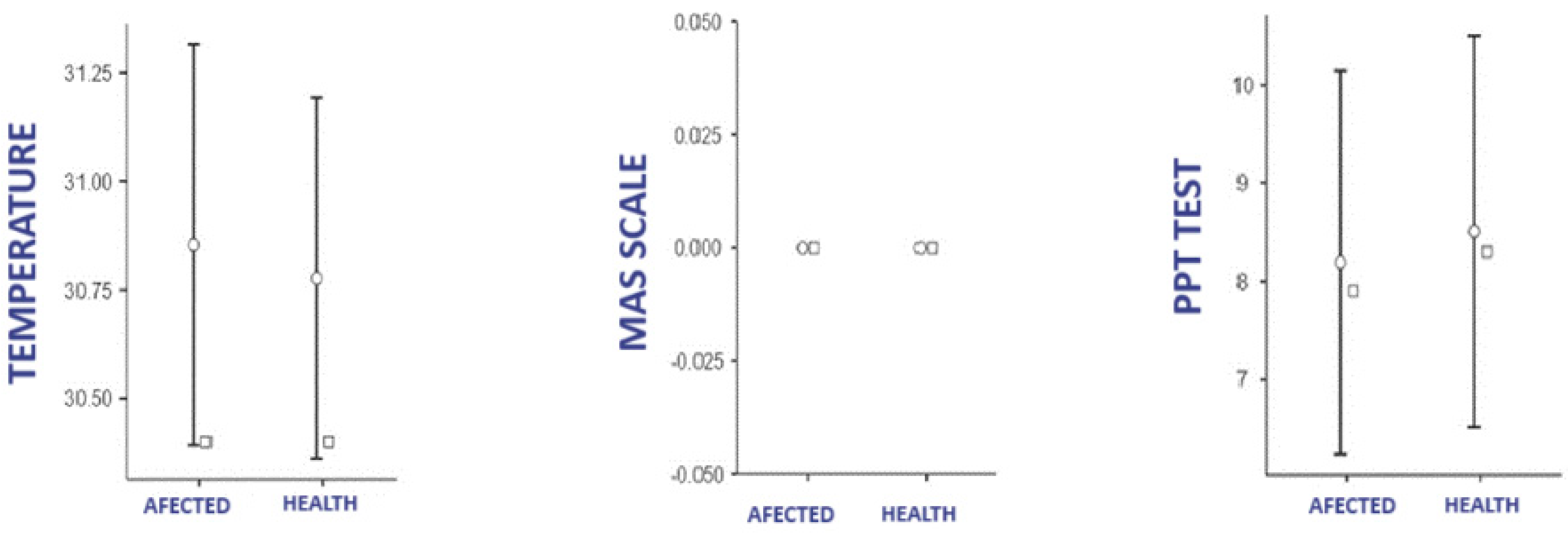
3.1. Difference in Temperature, PPT, and MAS According to Group
3.1.1. Affected Group
3.1.2. Control Group
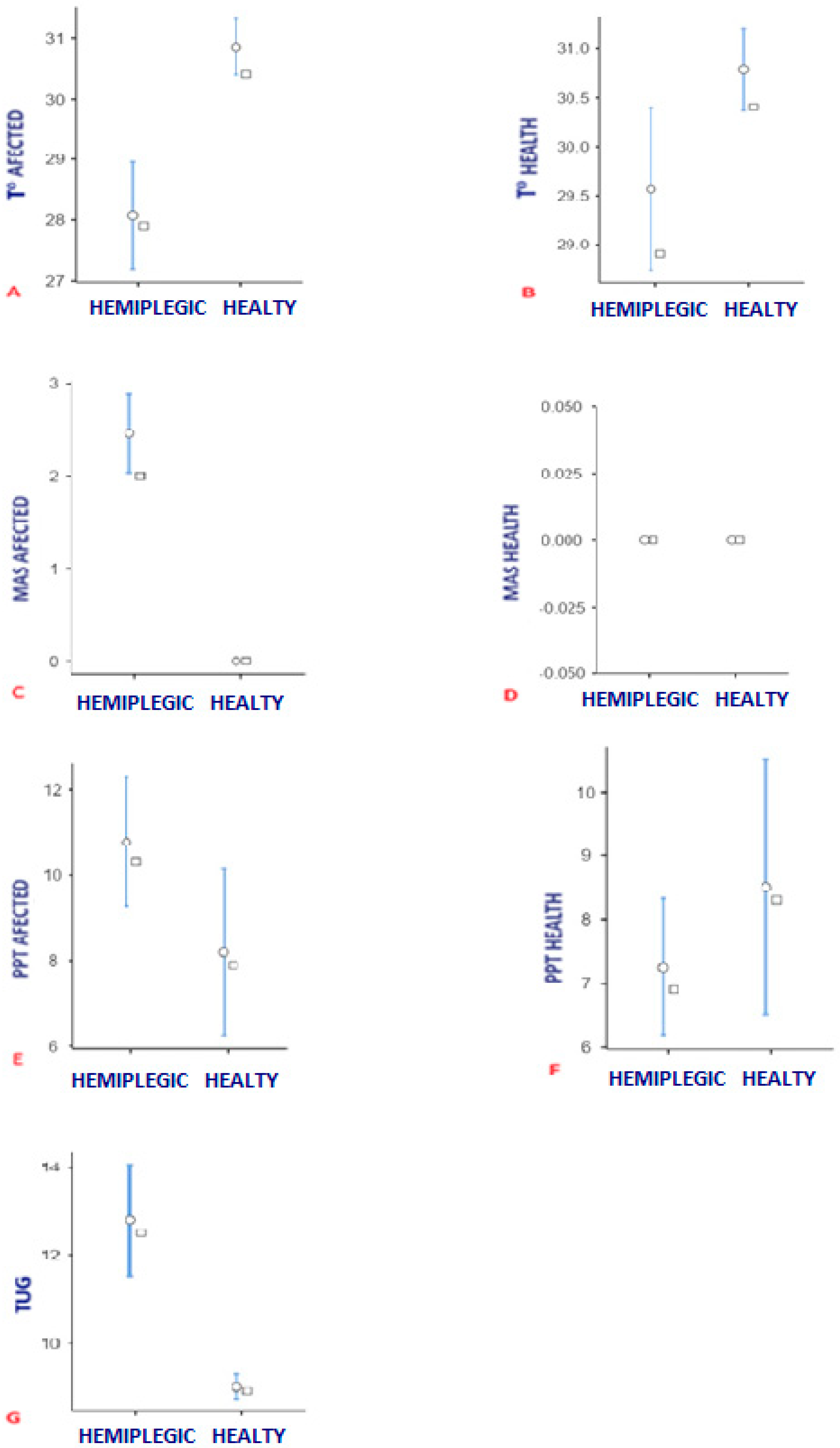
3.2. Difference of Temperature, PPT y MAS Between Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATM | Atmosphere |
| BMI | Body Mass Index |
| CNS | Nervous central system |
| DITI | Digital infrared thermal imaging |
| ΔT | Difference of temperature |
| MAS | Modified Ashworth scale |
| PPT | Pressure pain threshold |
| ROI | Region of interest |
| STARD | Standards for the Reporting of Diagnostic Accuracy Studies |
| TISEM | Thermographic Imaging in Sports and Exercise Medicine |
| TUG | Timed up and go |
| T° | Temperature |
References
- Li, Z.; Zhang, N.; Wang, Y.; Cao, S.; Huang, Z.; Hu, Y. Stair-shaped Achilles tendon lengthening in continuity—A new method to treat equinus deformity in patients with spastic cerebral palsy. Foot Ankle Surg Off. J. Eur. Soc. Foot Ankle Surg. 2019, 25, 165–168. [Google Scholar]
- Calame, A.; Singer, B.J. Inter- and Intra-Rater Reliability of the Australian Spasticity Assessment Scale in Adults with Acquired Brain Injury. Open J. Ther. Rehabil. 2015, 3, 77–86. [Google Scholar] [CrossRef]
- Volpon, J.B.; Natale, L.L. Critical evaluation of the surgical techniques to correct the equinus deformity. Rev. Col. Bras. Cir. 2019, 46, e2054. [Google Scholar]
- Firth, G.B.; Passmore, E.; Sangeux, M.; Thomason, P.; Rodda, J.; Donath, S.; Selber, P.; Graham, H.K. Multilevel surgery for equinus gait in children with spastic diplegic cerebral palsy: Medium-term follow-up with gait analysis. J. Bone Jt. Surg. Am. 2013, 95, 931–938. [Google Scholar]
- Gracies, J.M. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar]
- Xu, D.; Zhou, H.; Quan, W.; Ma, X.; Chon, T.-E.; Fernandez, J.; Gusztav, F.; Kovács, A.; Baker, J.S.; Gu, Y. New Insights Optimize Landing Strategies to Reduce Lower Limb Injury Risk. Cyborg Bionic Syst. 2024, 5, 0126. Available online: https://www.sciopen.com/article/10.34133/cbsystems.0126 (accessed on 19 March 2025).
- Balci, B.P. Spasticity Measurement. Arch. Neuropsychiatry 2018, 55 (Suppl. S1), S49–S53. [Google Scholar]
- Huang, H.P.; Kuo, C.C.; Lu, T.W.; Wu, K.W.; Kuo, K.N.; Wang, T.M. Bilateral symmetry in leg and joint stiffness in children with spastic hemiplegic cerebral palsy during gait. J. Orthop. Res. 2020, 38, 2006–2014. [Google Scholar]
- Lieber, R.L.; Runesson, E.; Einarsson, F.; Fridén, J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 2003, 28, 464–471. [Google Scholar] [CrossRef]
- Gómez-Soriano, J.; Cano-De-La-Cuerda, R.; Muñoz-Hellin, E.; Ortiz-Gutiérrez, R.; Taylor, J.S. [Evaluation and quantification of spasticity: A review of the clinical, biomechanical and neurophysiological methods]. Rev. Neurol. 2012, 55, 217–226. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987, 67, 206–207. [Google Scholar] [PubMed]
- Ansari, N.N.; Naghdi, S.; Moammeri, H.; Jalaie, S. Ashworth Scales are unreliable for the assessment of muscle spasticity. Physiother. Theory Pr. 2006, 22, 119–125. [Google Scholar]
- Nakamura, K.; Morrison, S.F. A thermosensory pathway mediating heat-defense responses. Proc. Natl. Acad. Sci. USA 2010, 107, 8848–8853. [Google Scholar] [PubMed]
- Cabizosu, A.; Grotto, D.; López López, A.; Castañeda Vozmediano, R. Thermography Sensor to Assess Motor and Sensitive Neuromuscular Sequels of Brain Damage. Sensors 2024, 24, 1723. [Google Scholar] [CrossRef]
- Jones, B.F. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans. Med. Imaging 1998, 17, 1019–1027. [Google Scholar]
- Vardasca, R.; Simoes, R. Current Issues in Medical Thermography. In Topics in Medial Image Processing and Computational Vision; Lecture Notes in Computational Vision and Biomechanics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 223–238. [Google Scholar]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 202, 78–98. Available online: https://pubmed.ncbi.nlm.nih.gov/26945597/ (accessed on 3 October 2023).
- Purup, M.M.; Knudsen, K.; Karlsson, P.; Terkelsen, A.J.; Borghammer, P. Skin Temperature in Parkinson’s Disease Measured by Infrared Thermography. Park. Dis. 2020, 2020, 2349469. Available online: https://pubmed.ncbi.nlm.nih.gov/32774831/ (accessed on 3 October 2023). [CrossRef]
- Volovik, M.; Sheiko, G.; Kuznetsov, A. Thermal imaging signs of spastic forms of cerebral palsy in children 4–7 years: Preliminary results. Thermol. Int. 2021, 31, 111–112. [Google Scholar]
- Satomura, S.; Yokota, I.; Tatara, K.; Naito, E.; Ito, M.; Kuroda, Y. Paradoxical weight loss with extra energy expenditure at brown adipose tissue in adolescent patients with Duchenne muscular dystrophy. Metabolism 2001, 50, 1181–1185. [Google Scholar]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; De Vet, H.C.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.; Costa, C.M.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [PubMed]
- Arif, M.; Gaur, D.K.; Gemini, N.; Iqbal, Z.A.; Alghadir, A.H. Correlation of Percentage Body Fat, Waist Circumference and Waist-to-Hip Ratio with Abdominal Muscle Strength. Healthcare 2022, 10, 2467. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Gezmen-Karadağ, M. Association of FTO common variant (rs9939609) with body fat in Turkish individuals. Lipids Heal. Dis. 2019, 18, 212. [Google Scholar]
- Lahiri, B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar]
- Ammer, K.; Ring, F. The Thermal Human Body: A Practical Guide to Thermal Imaging; Jenny Stanford Publishing: New York, NY, USA, 2019; 272p. [Google Scholar]
- Fernández-Cuevas, I.; Marins, J.C.B.; Lastras, J.A.; Carmona, P.M.G.; Cano, S.P.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar]
- Charlton, M.; Stanley, S.A.; Whitman, Z.; Wenn, V.; Coats, T.J.; Sims, M.; Thompson, J.P. The effect of constitutive pigmentation on the measured emissivity of human skin. PLoS ONE 2020, 15, e0241843. [Google Scholar]
- Fernández-Cuevas, I.; Torres, G.; Sillero-Quintana, M.; Navandar, A. Thermographic assessment of skin response to strength training in young participants. J. Therm. Anal. Calorim. 2023, 148, 3407–3415. [Google Scholar]
- Basso, O.; Johnson, D.P.; Amis, A.A. The anatomy of the patellar tendon. Knee Surg. Sports Traumatol. Arthrosc. 2001, 9, 2–5. [Google Scholar] [CrossRef]
- Williams, H.J.; Duncan, J.S.; Grove-White, D.H.; Mahen, P.J.; Gillespie, A.V. Repeatability and Feasibility of Pressure Algometry for Quantifying Mechanical Nociceptive Threshold in the Thoracic Region of Calves. Front. Vet. Sci. 2020, 7, 442. [Google Scholar]
- Chan, P.P.; Tou, J.I.S.; Tse, M.M.; Ng, S.S. Reliability and Validity of the Timed Up and Go Test with a Motor Task in People with Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, T.; Goljar Kregar, N.; Puh, U. Reliability of the Modified Ashworth Scale After Stroke for 13 Muscle Groups. Arch. Phys. Med. Rehabil. 2023, 104, 1606–1611. [Google Scholar] [PubMed]
- Ng, S.S.; Hui-Chan, C.W. The timed up & go test: Its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar]
- Harb, A.; Kishner, S. Modified Ashworth Scale. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554572/ (accessed on 3 October 2023).
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test: Normative Reference Values for Ages 20 to 59 Years and Relationships With Physical and Mental Health Risk Factors. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Hegedűs, B. The Potential Role of Thermography in Determining the Efficacy of Stroke Rehabilitation. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2018, 27, 309–314. [Google Scholar]
- Nowak, I.; Mraz, M.; Mraz, M. Thermography assessment of spastic lower limb in patients after cerebral stroke undergoing rehabilitation. J. Therm. Anal. Calorim. 2020, 140, 755–762. [Google Scholar]
- Noble, J.J.; Charles-Edwards, G.D.; Keevil, S.F.; Lewis, A.P.; Gough, M.; Shortland, A.P. Intramuscular fat in ambulant young adults with bilateral spastic cerebral palsy. BMC Musculoskelet. Disord. 2014, 15, 236. [Google Scholar]
- Niu, H.H.; Lui, P.W.; Hu, J.S.; Ting, C.K.; Yin, Y.C.; Lo, Y.L.; Liu, L.; Lee, T.Y. Thermal symmetry of skin temperature: Normative data of normal subjects in Taiwan. Zhonghua Yi Xue Za Zhi Chin. Med. J. Free. China Ed. 2001, 64, 459–468. [Google Scholar]
- Cabizosu, A.; Marín-Pagán, C.; Martínez-Serrano, A.; Alcaraz, P.E.; Martínez-Noguera, F.J. Myotendinous Thermoregulation in National Level Sprinters after a Unilateral Fatigue Acute Bout-A Descriptive Study. Sensors 2023, 23, 9330. [Google Scholar] [CrossRef] [PubMed]
- Dindorf, C.; Bartaguiz, E.; Janowicz, E.; Fröhlich, M.; Ludwig, O. Effects of Unilateral Muscle Fatigue on Thermographic Skin Surface Temperature of Back and Abdominal Muscles-A Pilot Study. Sports 2022, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Freire, B.; Valle, M.B.D.; Lanferdini, F.J.; Foschi, C.V.S.; Abou, L.; Pietta-Dias, C. Cut-off score of the modified Ashworth scale corresponding to walking ability and functional mobility in individuals with chronic stroke. Disabil. Rehabil. 2022, 45, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Park, C. Effect of turning direction on Timed Up and Go test results in stroke patients. Eur. J. Phys. Rehabil. Med. 2019, 55, 35–39. [Google Scholar] [CrossRef]
- Bonnyaud, C.; Pradon, D.; Zory, R.; Bensmail, D.; Vuillerme, N.; Roche, N. Gait parameters predicted by Timed Up and Go performance in stroke patients. NeuroRehabilitation 2015, 36, 73–80. [Google Scholar] [CrossRef]
- Cabizosu, A.; Carboni, N.; Andreo, A.M.-A.; Casu, G.; Sánchez, C.R.; Vegara-Meseguer, J. Relationship between infrared skin radiation and muscular strength tests in patients affected by Emery-Dreifuss muscular dystrophy. Med. Hypotheses 2020, 138, 109592. [Google Scholar] [CrossRef]
- Cabizosu, A.; Carboni, N.; Andreo, A.M.-A.; Vegara-Meseguer, J.; Marziliano, N.; Carrasco, G.G.; Casu, G. Theoretical basis for a new approach of studying Emery-Dreifuss muscular dystrophy by means of thermography. Med. Hypotheses 2018, 118, 103–106. [Google Scholar] [CrossRef]
- Cabizosu, A.; Grotto, D.; Lopez Esteban, M.J.; Castañeda Vozmediano, R. The assessment of neuromuscular sequels post brain damage by thermography. A pilot study. Cuest. Fisioter. 2024, 52, 8–21. Available online: http://cuestionesdefisioterapia.com/index.php/es (accessed on 12 December 2024).
- Zhang, Y.-H.; Xu, H.-R.; Wang, Y.-C.; Hu, G.-W.; Ding, X.-Q.; Shen, X.-H.; Yang, H.; Rong, J.-F.; Wang, X.-Q. Pressure pain threshold and somatosensory abnormalities in different ages and functional conditions of post-stroke elderly. BMC Geriatr. 2022, 22, 830. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9617357/ (accessed on 22 February 2024). [CrossRef]
- Salom-Moreno, J.; Sánchez-Mila, Z.; Ortega-Santiago, R.; Palacios-Ceña, M.; Truyol-Domínguez, S.; Fernández-De-Las-Peñas, C. Changes in spasticity, widespread pressure pain sensitivity, and baropodometry after the application of dry needling in patients who have had a stroke: A randomized controlled trial. J. Manip. Physiol. Ther. 2014, 37, 569–579. [Google Scholar]
- Iheanacho, F.; Vellipuram, A.R. Physiology, Mechanoreceptors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541068/ (accessed on 27 May 2024).
- Maksimovic, S.; Nakatani, M.; Baba, Y.; Nelson, A.M.; Marshall, K.L.; Wellnitz, S.A.; Firozi, P.; Woo, S.-H.; Ranade, S.; Patapoutian, A.; et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014, 509, 617–621. [Google Scholar] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.S.; McNamara, J.O.; Williams, S.M. The Somatic Sensory Cortex. In Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11153/ (accessed on 19 March 2025).
- Bolognini, N.; Russo, C.; Edwards, D.J. The sensory side of post-stroke motor rehabilitation. Restor. Neurol. Neurosci. 2016, 34, 571–586. [Google Scholar] [CrossRef]
- Meier, M.L.; Vrana, A.; Schweinhardt, P. Low Back Pain: The Potential Contribution of Supraspinal Motor Control and Proprioception. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2019, 25, 583–596. [Google Scholar]
- King, C.D.; Mano, K.E.J.; Barnett, K.A.B.; Pfeiffer, M.B.; Ting, T.V.; Kashikar-Zuck, S. Pressure Pain Threshold and Anxiety in Adolescent Females with and Without Juvenile Fibromyalgia: A Pilot Study. Clin. J. Pain 2017, 33, 620–626. [Google Scholar] [PubMed]
- Corey, D.L.; Comeau, D. Cervical radiculopathy. Med. Clin. North Am. 2014, 98, 791–799. [Google Scholar]
| Characteristics | Brain Damage Group (N = 13) | Control Group (N = 13) |
|---|---|---|
| Sex Woman Man | 46% 53% | 46% 53% |
| Age | 30.5 ± 6.8 | 24.6 ± 3.8 |
| BMI | 26 ± 6.5 | 26.4 ± 5.7 |
| Affectionate side Right Left | 61% 39% | / / |
| Group | Mean ± SD | IQR [25–75%] | |
|---|---|---|---|
| T° Affected Calf | Hemiplegic | 28.0 ± 1.6 | 26.9–29.5 |
| Healthy | 30.8 ± 0.8 | 30.3–31.6 | |
| T° Health Calf | Hemiplegic | 29.5 ± 1.5 | 28.5–30.8 |
| Healthy | 30.7 ± 0.7 | 30.2–31.3 | |
| PPT Affected | Hemiplegic | 10.7 ± 2.7 | 8.7–11.8 |
| Healthy | 8.1 ± 3.5 | 5.3–9.30 | |
| PPT Health | Hemiplegic | 7.2 ± 1.9 | 5.9–8.20 |
| Healthy | 8.5 ± 3.6 | 6.2–9.70 | |
| MAS Affected | Hemiplegic | 2.4 ± 0.7 | 2.0–3.0 |
| Healthy | 0.0 ± 0.0 | 0.0–0.0 | |
| MAS Health | Hemiplegic | 0.0 ± 0.0 | 0.0–0.0 |
| Healthy | 0.0 ± 0.0 | 0.0–0.0 | |
| TUG | Hemiplegic | 12.7 ± 2.3 | 11.6–13.8 |
| Healthy | 8.9 ± 0.5 | 8.6–9.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabizosu, A.; López-López, A.; Grotto, D.; Vegara-Meseguer, J.M. Relationship Between Infrared Thermography and Functional Parameters in the Lower Limbs of Hemiplegic Patients. Life 2025, 15, 542. https://doi.org/10.3390/life15040542
Cabizosu A, López-López A, Grotto D, Vegara-Meseguer JM. Relationship Between Infrared Thermography and Functional Parameters in the Lower Limbs of Hemiplegic Patients. Life. 2025; 15(4):542. https://doi.org/10.3390/life15040542
Chicago/Turabian StyleCabizosu, Alessio, Alberto López-López, Daniele Grotto, and Josefina Maria Vegara-Meseguer. 2025. "Relationship Between Infrared Thermography and Functional Parameters in the Lower Limbs of Hemiplegic Patients" Life 15, no. 4: 542. https://doi.org/10.3390/life15040542
APA StyleCabizosu, A., López-López, A., Grotto, D., & Vegara-Meseguer, J. M. (2025). Relationship Between Infrared Thermography and Functional Parameters in the Lower Limbs of Hemiplegic Patients. Life, 15(4), 542. https://doi.org/10.3390/life15040542








