Variability in Arterial Stiffness and Vascular Endothelial Function After COVID-19 During 1.5 Years of Follow-Up—Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion/Exclusion Criteria
2.2. Search Strategy and Inclusion/Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis and Assessment of Bias
3. Results
3.1. Study Characteristics
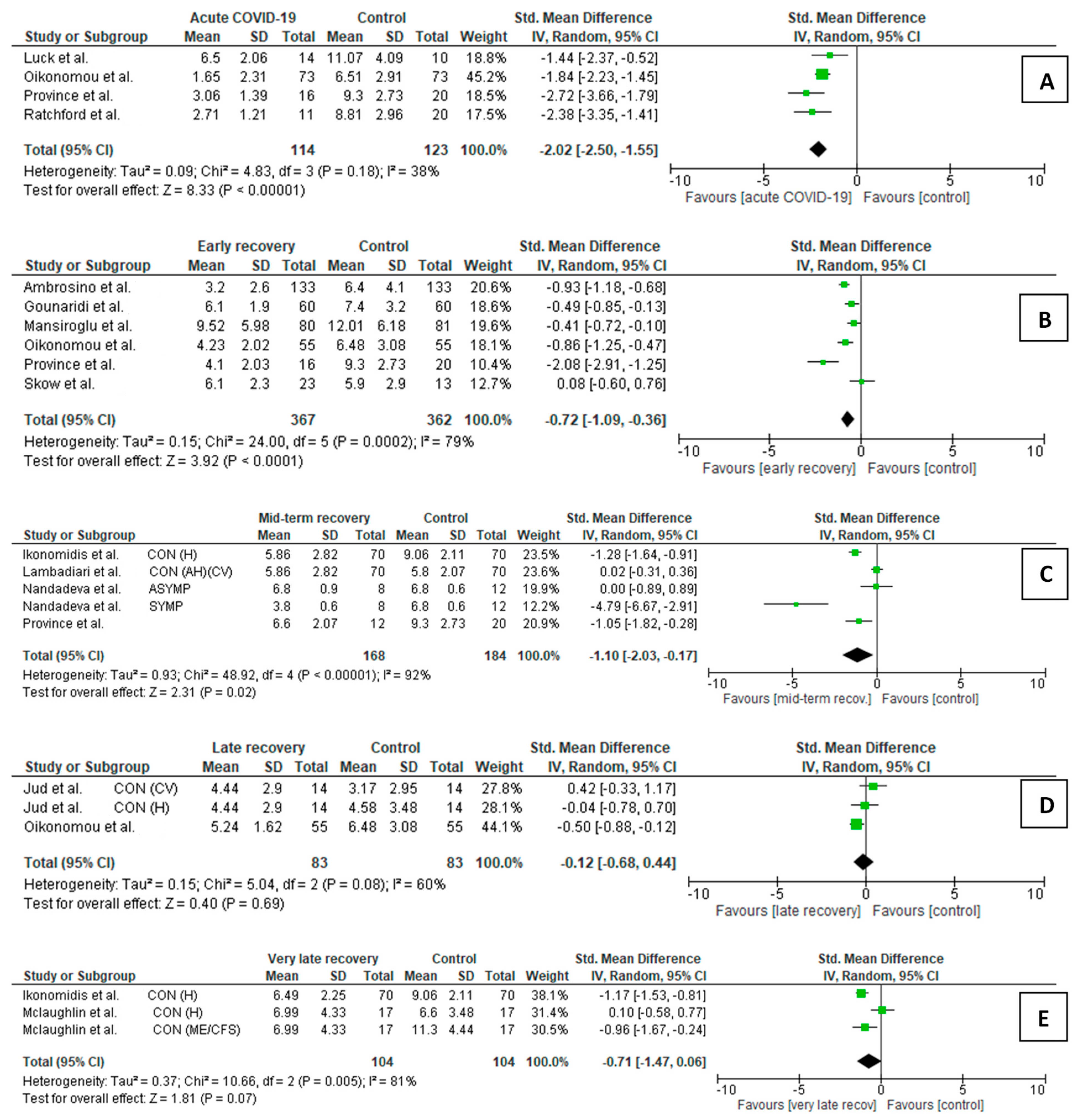
3.2. Meta-Analysis of Flow-Mediated Dilation
3.2.1. FMD Comparison Between COVID-19 Patients and Controls
- Acute/subacute COVID-19 participants vs. controls (Figure 1A)
- Early recovery post-COVID-19 participants vs. controls (Figure 1B)
- Mid-term recovery post-COVID-19 participants vs. controls (Figure 1C)
- Late recovery post-COVID-19 participants vs. controls (Figure 1D)
- Very late recovery post-COVID-19 participants vs. controls (Figure 1E)
3.2.2. FMD Changes in COVID-19 Patients During Follow-Up
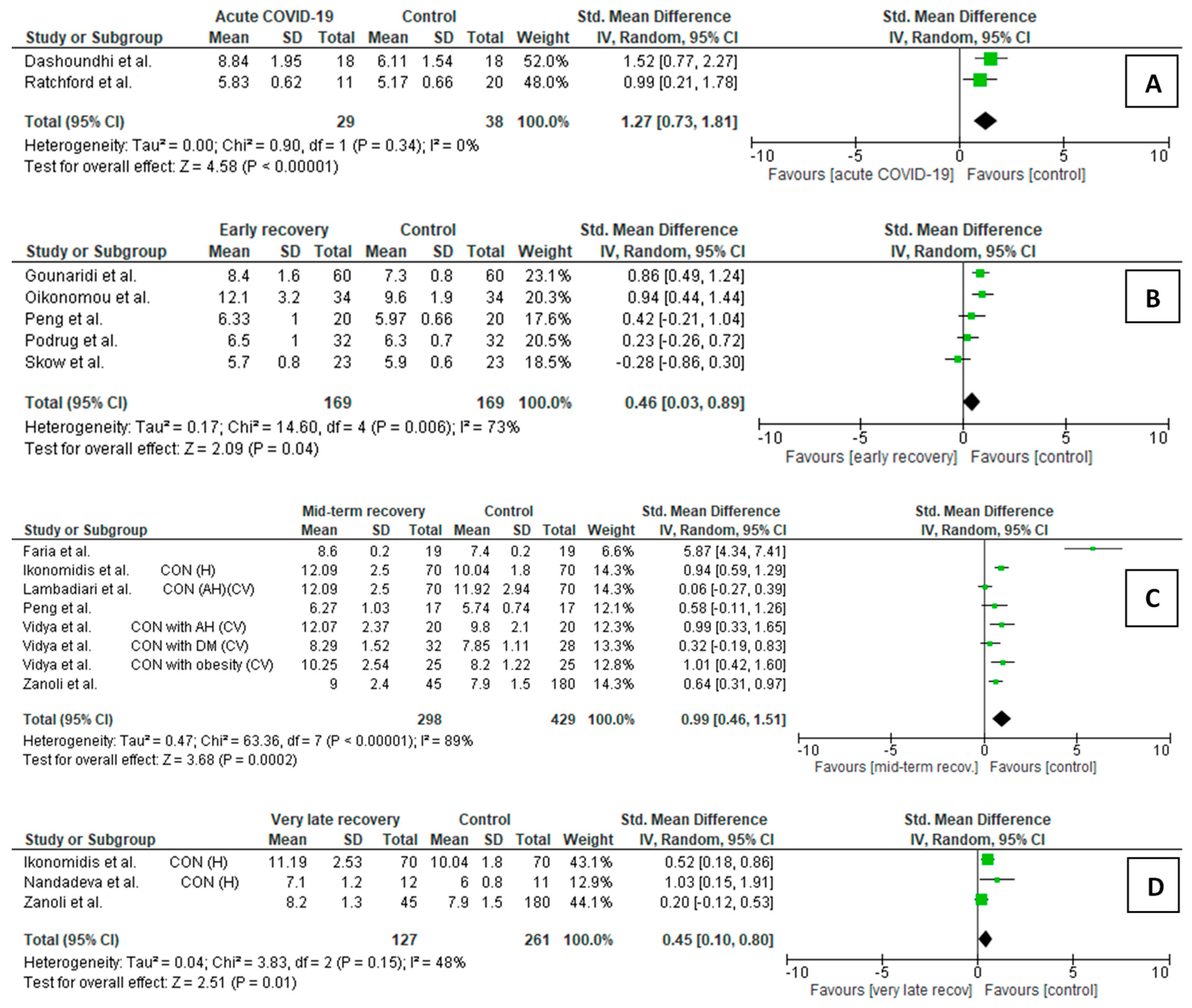
3.3. Meta-Analysis of Carotid-Femoral Pulse Wave Velocity
3.3.1. cfPWV Comparison Between COVID-19 Patients and Controls
- Acute/subacute COVID-19 vs. controls (Figure 2A)
- Early recovery post-COVID-19 participants vs. controls (Figure 2B)
- Mid-term recovery post-COVID-19 participants vs. controls (Figure 2C)
- Very late recovery post-COVID-19 participants vs. controls (Figure 2D)
3.3.2. cfPWV Changes in COVID-19 Patients During Follow-Up
4. Discussion
4.1. Differences in FMD and cfPWV Following COVID-19 Compared to Healthy Individuals
4.1.1. Acute/Subacute COVID-19
4.1.2. Early Recovery Period
4.1.3. Mid-Term Recovery Period
4.1.4. Late Recovery Period
4.1.5. Very Late Recovery Period
4.2. Differences in FMD and cfPWV Following COVID-19 Compared to Particpiants with Cardiovascular Risk Factors or Atherosclerotic Cardiovascular Diseases
4.2.1. Acute/Subacute COVID-19
4.2.2. Early Recovery Period
4.2.3. Mid-Term Recovery Period
4.2.4. Late Recovery Period
4.2.5. Very Late Recovery Period
4.3. Associations of Differences in FMD and cfPWV with Age
4.4. Associations of Changes in FMD and cfPWV with Time Since COVID-19 Onset
4.4.1. Changes Observed in Young and Middle-Aged Healthy Adults
4.4.2. Changes Observed in Middle-Aged and Elderly Participants with CV Risk Factors or ASCVD
4.5. Associations of Differences and Changes in FMD and cfPWV with the Severity of the Acute Phase of COVID-19
4.6. Associations of Differences and Changes in FMD and cfPWV with Long-Term COVID-19 Syndrome
4.7. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res. 2021, 117, 2705–2729. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C.Y.; Wang, S.I.; Wei, J.C.C. Long-Term Cardiovascular Outcomes in COVID-19 Survivors among Non-Vaccinated Population: A Retrospective Cohort Study from the TriNetX US Collaborative Networks. eClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Mathur, S.; Zhang, R.; Yan, V.K.C.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: A prospective cohort in UK Biobank. Cardiovasc. Res. 2023, 119, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Chidambaram, V.; Kumar, A.; Sadaf, M.I.; Lu, E.; Al’Aref, S.J.; Tarun, T.; Galiatsatos, P.; Gulati, M.; Blumenthal, R.S.; Leucker, T.M.; et al. COVID-19 in the Initiation and Progression of Atherosclerosis: Pathophysiology During and Beyond the Acute Phase. JACC Adv. 2024, 3, 101107. [Google Scholar] [CrossRef]
- Wu, X.; Xiang, M.; Jing, H.; Wang, C.; Novakovic, V.A.; Shi, J. Damage to endothelial barriers and its contribution to long COVID. Angiogenesis 2024, 27, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Zappa, M.; Verdecchia, P. Global burden of new-onset hypertension associated with severe acute respiratory syndrome coronavirus 2 infection. Eur. J. Intern. Med. 2024, 119, 31–33. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. ESC Scientific Document Group. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Jud, P.; Gressenberger, P.; Muster, V.; Avian, A.; Meinitzer, A.; Strohmaier, H.; Sourij, H.; Raggam, R.B.; Stradner, M.H.; Demel, U.; et al. Evaluation of Endothelial Dysfunction and Inflammatory Vasculopathy After SARS-CoV-2 Infection-A Cross-Sectional Study. Front. Cardiovasc. Med. 2021, 8, 750887. [Google Scholar] [CrossRef]
- Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Thymis, J.; Katogiannis, K.; Korakas, E.; Varlamos, C.; Andreadou, I.; Tsoumani, M.; Triantafyllidi, H.; et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur. J. Heart Fail. 2021, 23, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Castellino, N.; Longo, A.; Russo, A.; Bonfiglio, V.; Fallico, M.; Toro, M.D.; Cappellani, F.; Grillo, M.; Gaudio, A.; Lo Cicero, L.; et al. COVID-19-related retinal microvasculopathy and systemic implications in patients with severe disease: Results from the Methuselah study. Front. Med. 2024, 11, 1294432. [Google Scholar] [CrossRef]
- Saloň, A.; Neshev, R.; Teraž, K.; Šimunič, B.; Peskar, M.; Marušič, U.; Pišot, S.; Šlosar, L.; Gasparini, M.; Pišot, R.; et al. A pilot study: Exploring the influence of COVID-19 on cardiovascular physiology and retinal microcirculation. Microvasc. Res. 2023, 150, 104588. [Google Scholar] [CrossRef]
- Mahmoud, E.O.; Elsabagh, Y.A.; Abd El Ghaffar, N.; Fawzy, M.W.; Hussein, M.A. Atherosclerosis Associated with COVID-19: Acute, Tends to Severely Involve Peripheral Arteries, and May be Reversible. Angiology 2025, 76, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Nasoufidou, A.; Sagris, M.; Fragakis, N.; Tsioufis, K. Vascular Alterations Following COVID-19 Infection: A Comprehensive Literature Review. Life 2024, 14, 545. [Google Scholar] [CrossRef]
- Dolhnikoff, M.; Duarte-Neto, A.N.; de Almeida Monteiro, R.A.; da Silva, L.F.F.; de Oliveira, E.P.; Saldiva, P.H.N.; Mauad, T.; Negri, E.M. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J. Thromb. Haemost. 2020, 18, 1517–1519. [Google Scholar] [CrossRef]
- Joffre, J.; Rodriguez, L.; Matthay, Z.A.; Lloyd, E.; Fields, A.T.; Bainton, R.J.; Kurien, P.; Sil, A.; Calfee, C.S.; Woodruff, P.G.; et al. COVID-19-associated lung microvascular endotheliopathy: A “from the bench” perspective. Am. J. Respir. Crit. Care Med. 2022, 206, 961–972. [Google Scholar] [CrossRef]
- Zapata, M.Á.; Banderas García, S.; Sánchez-Moltalvá, A.; Falcó, A.; Otero-Romero, S.; Arcos, G.; Velazquez-Villoria, D.; García-Arumí, J. Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br. J. Ophthalmol. 2022, 106, 559–563. [Google Scholar] [CrossRef]
- Bois, M.C.; Boire, N.A.; Layman, A.J.; Aubry, M.-C.; Alexander, M.P.; Roden, A.C.; Hagen, C.E.; Quinton, R.A.; Larsen, C.; Erben, Y.; et al. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation 2021, 143, 230–243. [Google Scholar] [CrossRef]
- Szeghy, R.E.; Province, V.M.; Stute, N.L.; Augenreich, M.A.; Koontz, L.K.; Stickford, J.L.; Stickford, A.S.L.; Ratchford, S.M. Carotid stiffness, intima-media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp. Physiol. 2022, 107, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Gelardi, F.; Chiti, A. Vasculitis changes in COVID-19 survivors with persistent symptoms: An [18F]FDG-PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med. 2022, 10, 761–775, Erratum in Lancet Respir. Med. 2022, 10, e85. [Google Scholar] [CrossRef]
- Fan, B.E.; Wong, S.W.; Sum, C.L.L.; Lim, G.H.; Leung, B.P.; Tan, C.W.; Ramanathan, K.; Dalan, R.; Cheung, C.; Lim, X.R.; et al. Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: Assessing the long-term outcomes in COVID-19 patients. Am. J. Hematol. 2022, 97, 915–923. [Google Scholar] [CrossRef]
- Poyatos, P.; Luque, N.; Sabater, G.; Eizaguirre, S.; Bonnin, M.; Orriols, R.; Tura-Ceide, O. Endothelial dysfunction and cardiovascular risk in post-COVID-19 patients after 6- and 12-months SARS-CoV-2 infection. Infection 2024, 52, 1269–1285. [Google Scholar] [CrossRef]
- Ambrosino, P.; Calcaterra, I.; Molino, A.; Moretta, P.; Lupoli, R.; Spedicato, G.A.; Papa, A.; Motta, A.; Maniscalco, M.; Di Minno, M.N.D. Persistent Endothelial Dysfunction in Post-Acute COVID-19 Syndrome: A Case-Control Study. Biomedicines 2021, 9, 957. [Google Scholar] [CrossRef]
- Nandadeva, D.; Young, B.E.; Stephens, B.Y.; Grotle, A.K.; Skow, R.J.; Middleton, A.J.; Haseltine, F.P.; Fadel, P.J. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H479–H484. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Souvaliotis, N.; Lampsas, S.; Siasos, G.; Poulakou, G.; Theofilis, P.; Papaioannou, T.G.; Haidich, A.B.; Tsaousi, G.; Ntousopoulos, V.; et al. Endothelial dysfunction in acute and long standing COVID-19: A prospective cohort study. Vascul Pharmacol. 2022, 144, 106975. [Google Scholar] [CrossRef]
- Gao, Y.P.; Zhou, W.; Huang, P.N.; Liu, H.Y.; Bi, X.J.; Zhu, Y.; Sun, J.; Tang, Q.Y.; Li, L.; Zhang, J.; et al. Persistent Endothelial Dysfunction in Coronavirus Disease-2019 Survivors Late After Recovery. Front. Med. 2022, 9, 809033. [Google Scholar] [CrossRef]
- Gouzi, F.; Philippe, A.; Pastre, J.; Renaud, B.; Gendron, N.; Subileau, M.; Hua-Huy, T.; Planquette, B.; Sanchez, O.; Smadja, D.M.; et al. Recovery of Endothelium-dependent vascular relaxation impairment in convalescent COVID-19 patients: Insight from a pilot study. Respir. Med. Res. 2023, 84, 101044. [Google Scholar] [CrossRef]
- Durieux, J.C.; Zisis, S.N.; Mouchati, C.; Labbato, D.; Abboud, M.; McComsey, G.A. Sex Modifies the Effect of COVID-19 on Arterial Elasticity. Viruses 2024, 16, 1089. [Google Scholar] [CrossRef]
- Honchar, O.; Ashcheulova, T.; Chumachenko, T.; Chumachenko, D. Early prediction of long COVID-19 syndrome persistence at 12 months after hospitalisation: A prospective observational study from Ukraine. BMJ Open 2025, 15, e084311. [Google Scholar] [CrossRef]
- Schnaubelt, S.; Oppenauer, J.; Tihanyi, D.; Mueller, M.; Maldonado-Gonzalez, E.; Zejnilovic, S.; Haslacher, H.; Perkmann, T.; Strassl, R.; Anders, S.; et al. Arterial stiffness in acute COVID-19 and potential associations with clinical outcome. J. Intern. Med. 2021, 290, 437–443. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, S.; Kumar, A.; Bhushan, D.; Kumar, A.; Kumar, A.; Singh, V.; Singh, P.K. The COSEVAST Study Outcome: Evidence of COVID-19 Severity Proportionate to Surge in Arterial Stiffness. Indian J. Crit. Care Med. 2021, 25, 1113–1119. [Google Scholar] [CrossRef]
- Zanoli, L.; Gaudio, A.; Mikhailidis, D.P.; Katsiki, N.; Castellino, N.; Lo Cicero, L.; Geraci, G.; Sessa, C.; Fiorito, L.; Marino, F.; et al. Vascular Dysfunction of COVID-19 Is Partially Reverted in the Long-Term. Circ. Res. 2022, 130, 1276–1285. [Google Scholar] [CrossRef]
- Küçük, U.; Gazi, E.; Duygu, A.; Akşit, E. Evaluation of Aortic Elasticity Parameters in Survivors of COVID-19 Using Echocardiography Imaging. Med. Princ. Pract. 2022, 31, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Szoltysek-Boldys, I.; Zielinska-Danch, W.; Loboda, D.; Wilczek, J.; Gibinski, M.; Paradowska-Nowakowska, E.; Golba, K.S.; Sarecka-Hujar, B. Photoplethysmographic Measurement of Arterial Stiffness in Polish Patients with Long-COVID-19 Syndrome-The Results of a Cross-Sectional Study. Diagnostics 2022, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Nabeel, P.M.; Raj, K.V.; Soneja, M.; Chandran, D.S.; Joseph, J.; Wig, N.; Jaryal, A.K.; Thijssen, D.; Deepak, K.K. Baroreflex sensitivity is impaired in survivors of mild COVID-19 at 3-6 months of clinical recovery; association with carotid artery stiffness. Physiol. Rep. 2023, 11, e15845. [Google Scholar] [CrossRef] [PubMed]
- Can, Y.; Kocayigit, I.; Kocayiğit, H.; Sarıbıyık Çakmak, B.; Şahinöz, M.; Akdemir, R. Ongoing Effects of SARS-CoV-2 Infection on Arterial Stiffness in Healthy Adults. Angiology 2024, 75, 116–121. [Google Scholar] [CrossRef]
- Badaras, I.; Laučytė-Cibulskienė, A. Vascular Aging and COVID-19. Angiology 2023, 74, 308–316. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Lindmark, K.; Fors Connolly, A.M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study. Lancet 2021, 398, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Rezel-Potts, E.; Douiri, A.; Sun, X.; Chowienczyk, P.J.; Shah, A.M.; Gulliford, M.C. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med. 2022, 19, e1004052. [Google Scholar] [CrossRef]
- Tintore, C.; Cuartero, J.; Camps-Vilaró, A.; Subirana, I.; Elosua, R.; Marrugat, J.; Degano, I.R. Increased risk of arrhythmias, heart failure, and thrombosis in SARS-CoV-2 positive individuals persists at one year post-infection. Comput. Struct. Biotechnol. J. 2024, 24, 476–483. [Google Scholar] [CrossRef] [PubMed]
- la Roi-Teeuw, H.M.; van Smeden, M.; Geersing, G.J.; Klungel, O.H.; Rutten, F.H.; Souverein, P.C.; van Doorn, S. Incidence and individual risk prediction of post-COVID-19 cardiovascular disease in the general population: A multivariable prediction model development and validation study. Eur. Heart J. Open 2023, 3, oead101. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Cooper, J.; Salih, A.; Raman, B.; Lee, A.M.; Neubauer, S.; Harvey, N.C.; Petersen, S.E. Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart 2023, 109, 119–126. [Google Scholar] [CrossRef]
- Rodilla, E.; López-Carmona, M.D.; Cortes, X.; Cobos-Palacios, L.; Canales, S.; Sáez, M.C.; Campos Escudero, S.; Rubio-Rivas, M.; Díez Manglano, J.; Freire Castro, S.J.; et al. Impact of Arterial Stiffness on All-Cause Mortality in Patients Hospitalized with COVID-19 in Spain. Hypertension 2021, 77, 856–867. [Google Scholar] [CrossRef]
- Loboda, D.; Sarecka-Hujar, B.; Nowacka-Chmielewska, M.; Szoltysek-Boldys, I.; Zielinska-Danch, W.; Gibinski, M.; Wilczek, J.; Gardas, R.; Grabowski, M.; Lejawa, M.; et al. Relationship of Non-Invasive Arterial Stiffness Parameters with 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Post-COVID-19 Patients—The Results of a Cross-Sectional Study. Life 2024, 14, 1105. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Ahn, Y.; Aung, N.; Ahn, H.-S. A Comprehensive Review of Clinical Studies Applying Flow-Mediated Dilation. Diagnostics 2024, 14, 2499. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart 2013, 99, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.D.; Ahmed, H.N.; Jin, Z.; Cushman, M.; Herrington, D.M.; Nelson, J.C.; Di Tullio, M.R.; Homma, S. Systemic inflammation and brachial artery endothelial function in the Multi-Ethnic Study of Atherosclerosis (MESA). Heart 2014, 100, 862–866. [Google Scholar] [CrossRef]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A metaanalysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Green, D.J.; Jones, H.; Thijssen, D.; Cable, N.T.; Atkinson, G. Flow-mediated dilation and cardiovascular event prediction: Does nitric oxide matter? Hypertension 2011, 57, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Fliser, D.; Goldsmith, D.; Covic, A.; Wiecek, A.; Ortiz, A.; Martinez-Castelao, A.; Lindholm, B.; Massy, Z.A.; Suleymanlar, G.; et al. Assessment of arterial stiffness for clinical and epidemiological studies: Methodological considerations for validation and entry into the European Renal and Cardiovascular Medicine registry. Nephrol. Dial. Transplant. 2014, 29, 232–239. [Google Scholar] [CrossRef]
- Benetos, A.; Waeber, B.; Izzo, J.; Mitchell, G.; Resnick, L.; Asmar, R.; Safar, M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: Clinical applications. Am. J. Hypertens. 2002, 15, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Bruno, R.M.; Nilsson, P.M.; Engström, G.; Wadström, B.N.; Empana, J.P.; Boutouyrie, P.; Laurent, S. Early and Supernormal Vascular Aging: Clinical Characteristics and Association with Incident Cardiovascular Events. Hypertension 2020, 76, 1616–1624. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction: An Individual Participant Meta-Analysis of Prospective Observational Data from 17,635 Subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Liu, C.; Pan, H.; Kong, F.; Yang, S.; Shubhra, Q.T.H.; Li, D.; Chen, S. Association of arterial stiffness with all-cause and cause-specific mortality in the diabetic population: A national cohort study. Front. Endocrinol. 2023, 14, 1145914. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Walia, R.; Arunachalam, V.S.; Chauhan, U.; Khapre, M.; Arora, P. Endothelial dysfunction assessed by brachial artery flow-mediated dilatation predicts severe COVID-19-related disease. J. Fam. Med. Prim. Care 2022, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Güz, G.; Demirgan, S. Lower brachial artery flow-mediated dilation is associated with a worse prognosis and more lung parenchymal involvement in COVID-19: Prospective observational study. Medicine 2022, 101, e30001. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Schiaroli, E.; Cosentini, E.; Batori, G.; Marini, E.; Sahebkar, A.; Grignani, F.; Gidari, A.; et al. Low Brachial Artery Flow-Mediated Dilation Predicts Worse Prognosis in Hospitalized Patients with COVID-19. J. Clin. Med. 2021, 10, 5456. [Google Scholar] [CrossRef]
- Santoro, L.; Falsetti, L.; Zaccone, V.; Nesci, A.; Tosato, M.; Giupponi, B.; Savastano, M.C.; Moroncini, G.; Gasbarrini, A.; Landi, F.; et al. Impaired Endothelial Function in Convalescent Phase of COVID-19: A 3 Month Follow Up Observational Prospective Study. J. Clin. Med. 2022, 11, 1774. [Google Scholar] [CrossRef]
- Ambrosino, P.; Parrella, P.; Formisano, R.; Perrotta, G.; D’Anna, S.E.; Mosella, M.; Papa, A.; Maniscalco, M. Cardiopulmonary Exercise Performance and Endothelial Function in Convalescent COVID-19 Patients. J. Clin. Med. 2022, 11, 1452. [Google Scholar] [CrossRef]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of l-Arginine Plus Vitamin C Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef]
- Cristina-Oliveira, M.; Meireles, K.; Gil, S.; Cavalcante Assis, F.; Geber-Júnior, J.C.; Shinjo, S.K.; de Souza, H.P.; Cruz Santana, A.N.; Swinton, P.A.; Drager, L.F.; et al. Carotid intima-media thickness and flow-mediated dilation do not predict acute in-hospital outcomes in patients hospitalized with COVID-19. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H906–H913. [Google Scholar] [CrossRef]
- Heubel, A.D.; Viana, A.A.; Linares, S.N.; do Amaral, V.T.; Schafauser, N.S.; de Oliveira, G.Y.O.; Ramírez, P.C.; Martinelli, B.; da Silva Alexandre, T.; Borghi-Silva, A.; et al. Determinants of endothelial dysfunction in noncritically ill hospitalized COVID-19 patients: A cross-sectional study. Obesity 2022, 30, 165–171. [Google Scholar] [CrossRef]
- Avdeeva, I.V.; Pavlenko, K.I.; Salyamova, L.I.; Lukyanova, M.V.; Oleinikov, V.E. Influence of a new coronavirus infection on the arterial stiffness in patients with hypertension. Arter. Hypertens. 2023, 29, 593–602. [Google Scholar] [CrossRef]
- Calvani, R.; Giampaoli, O.; Marini, F.; Del Chierico, F.; De Rosa, M.; Conta, G.; Sciubba, F.; Tosato, M.; Picca, A.; Ciciarello, F.; et al. Beetroot juice intake positively influenced gut microbiota and inflammation but failed to improve functional outcomes in adults with long COVID: A pilot randomized controlled trial. Clin. Nutr. 2024, 43, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Mannarino, M.R.; Cosentini, E.; Figorilli, F.; Colangelo, C.; Cellini, G.; Braca, M.; Lombardini, R.; Paltriccia, R.; Sahebkar, A.; et al. The impact of statin therapy on in-hospital prognosis and endothelial function of patients at high-to-very high cardiovascular risk admitted for COVID-19. J. Med. Virol. 2023, 95, e28678. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Santoro, L.; Crincoli, E.; Fossataro, C.; Gambini, G.; Savastano, A.; De Vico, U.; Santoliquido, A.; Nesci, A.; Landi, F.; et al. Radial Peripapillary Capillary Plexus Perfusion and Endothelial Dysfunction in Early Post-SARS-CoV-2 Infection. Vision 2022, 6, 26. [Google Scholar] [CrossRef]
- Oikonomou, E.; Lampsas, S.; Souvaliotis, N.; Sarantos, S.; Siasos, G.; Poulakou, G.; Lytra, T.; Papamikroulis, G.A.; Fountoulakis, N.; Theofilis, P.; et al. Vaccination Against SARS-CoV-2 Protects from COVID-19-induced Endothelial Dysfunction. Curr. Pharm. Des. 2022, 28, 3225–3230. [Google Scholar] [CrossRef]
- Çiftel, M.; Ateş, N.; Yılmaz, O. Investigation of Endothelial Dysfunction and Arterial Stiffness in Multisystem Inflammatory Syndrome in Children. Eur. J. Pediatr. 2022, 181, 91–97. [Google Scholar] [CrossRef]
- Astley, C.; Prado, D.M.L.D.; Sieczkowska, S.M.; Esteves, G.P.; Suguita, P.; Fink, T.; Lindoso, L.; Matsuo, O.; Martins, F.; Bain, V.; et al. Impaired cardiorespiratory fitness and endothelial function after SARS-CoV-2 infection in a sample of mainly immunocompromised youth. J. Appl. Physiol. 2023, 135, 1323–1329. [Google Scholar] [CrossRef]
- Astley, C.; Drezner, J.A.; Sieczkowska, S.M.; Ihara, A.; Franco, T.; Gil, S.; DO Prado, D.M.L.; Longobardi, I.; Suguita, P.; Fink, T.; et al. Exercise in Pediatric COVID-19: A Randomized Controlled Trial. Med. Sci. Sports Exerc. 2025, 57, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Leite, M.; Santos, F.G.C.D.; Penido, E.A.R.; Ribeiro, K.A.; Dos Santos, L.M.; Rodrigues-Machado, M.D.G.; Rezende, B.A. Impact of social isolation during COVID-19 on anthropometric data, quality of life, baseline physical activity and aortic pulse wave parameters in children and adolescents in two independent samples. Ital. J. Pediatr. 2023, 49, 154. [Google Scholar] [CrossRef]
- Goswami, N.; Fredriksen, P.M.; Lundin, K.E.A.; Agu, C.; Olanike Elias, S.; Motaung, K.S.; Brix, B.; Cvirn, G.; Sourij, H.; Stelzl, E.; et al. Correction: COVID-19 and its effects on endothelium in HIV-positive patients in sub-saharan Africa: Cardiometabolic risk, thrombosis and vascular function (ENDOCOVID STUDY). BMC Infect. Dis. 2024, 24, 207, Erratum in BMC Infect Dis. 2021, 21, 719. [Google Scholar] [CrossRef]
- Đogaš, T.; Novak, I.; Babić, M.; Vučković, M.; Tandara, L.; Radić, J. Associations of Serum Calprotectin, Arterial Stiffness and Long COVID Symptoms in Dalmatian Kidney Transplant Recipients. Viruses 2023, 15, 1776. [Google Scholar] [CrossRef]
- Mikhailova, L.V.; Belousova, Y.D.; Moiseeva, E.M.; Tsapkova, A.A.; Gazatova, N.D.; Plotnikova, A.R.; Rudev, D.G.; Doktorova, S.A.; Rafalskiy, V.V. Dynamics of endothelial function indexes in patients with post-Covid syndrome using a combination drug of ethylmethylhydroxyperidine succinate/vitamin B6. Res. Results Pharmacol. 2023, 9, 21–26. [Google Scholar] [CrossRef]
- Theresa, C.; Katebe, B.; Shibao, C.A.; Kirabo, A. Arterial stiffness in adults with Long COVID in sub-Saharan Africa. Physiol. Rep. 2024, 12, e70029. [Google Scholar] [CrossRef]
- Khodnapur, J.P.; Khodnapur, G.P.; Basavaraddi, I.V.; Podder, A.; Pal, R.; Patil, S.M.; Doddamani, M.P. Yoga Improves Vascular stiffness in COVID-19 Survivors of Vijayapur, Karnataka, India. Biomed. Pharmacol. J. 2024, 17. [Google Scholar] [CrossRef]
- Owens, C.D.; Pinto, C.B.; Szarvas, Z.; Muranyi, M.; da C. Pinaffi-Langley, A.C.; Peterfi, A.; Mukli, P.; Detwiler, S.; Olay, L.; Kaposzta, Z.; et al. COVID-19 Exacerbates Neurovascular Uncoupling and Contributes to Endothelial Dysfunction in Patients with Mild Cognitive Impairment. Biomolecules 2024, 14, 1621. [Google Scholar] [CrossRef]
- Ratchford, S.M.; Stickford, J.L.; Province, V.M.; Stute, N.; Augenreich, M.A.; Koontz, L.K.; Bobo, L.K.; Stickford, A.S.L. Vascular alterations among young adults with SARS-CoV-2. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H404–H410. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Back, G.D.; da Luz Goulart, C.; Domingos, B.C.; Arena, R.; Borghi-Silva, A. Endothelial function provides early prognostic information in patients with COVID-19: A cohort study. Respir. Med. 2021, 185, 106469. [Google Scholar] [CrossRef]
- Province, V.M.; Szeghy, R.E.; Stute, N.L.; Augenreich, M.A.; Behrens, C.E.; Stickford, J.L.; Stickford, A.S.L.; Ratchford, S.M. Tracking peripheral vascular function for six months in young adults following SARS-CoV-2 infection. Physiol. Rep. 2022, 10, e15552. [Google Scholar] [CrossRef]
- Dashoundhi, V.; Khodnapur, G.P.; Podder, A.; Patil, S.M.; Khodnapur, J.P. Assessment of Arterial Stiffness in Patients Recovered from Mild COVID-19 Disease using Pulse Wave Velocity: A Cross-sectional Study. J. Clin. Diagn. Res. 2023, 17, CC05–CC08. [Google Scholar] [CrossRef]
- Ciacci, P.; Paraninfi, A.; Orlando, F.; Rella, S.; Maggio, E.; Oliva, A.; Cangemi, R.; Carnevale, R.; Bartimoccia, S.; Cammisotto, V.; et al. Endothelial dysfunction, oxidative stress and low-grade endotoxemia in COVID-19 patients hospitalised in medical wards. Microvasc. Res. 2023, 149, 104557. [Google Scholar] [CrossRef] [PubMed]
- Luck, J.C.; Blaha, C.; Cauffman, A.; Gao, Z.; Arnold, A.C.; Cui, J.; Sinoway, L.I. Autonomic and vascular function testing in collegiate athletes following SARS-CoV-2 infection: An exploratory study. Front. Physiol. 2023, 14, 1225814. [Google Scholar] [CrossRef]
- Ergül, E.; Yılmaz, A.S.; Öğütveren, M.M.; Emlek, N.; Kostakoğlu, U.; Çetin, M. COVID 19 disease independently predicted endothelial dysfunction measured by flow-mediated dilatation. Int. J. Cardiovasc. Imaging 2022, 38, 25–32. [Google Scholar] [CrossRef]
- Skow, R.J.; Nandadeva, D.; Grotle, A.K.; Stephens, B.Y.; Wright, A.N.; Fadel, P.J. Impact of breakthrough COVID-19 cases during the omicron wave on vascular health and cardiac autonomic function in young adults. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H59–H64. [Google Scholar] [CrossRef]
- Mansiroglu, A.K.; Seymen, H.; Sincer, I.; Gunes, Y. Evaluation of Endothelial Dysfunction in COVID-19 with Flow-Mediated Dilatation. Arq. Bras. Cardiol. 2022, 119, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Gounaridi, M.I.; Vontetsianos, A.; Oikonomou, E.; Theofilis, P.; Chynkiamis, N.; Lampsas, S.; Anastasiou, A.; Papamikroulis, G.A.; Katsianos, E.; Kalogeras, K.; et al. The Role of Rehabilitation in Arterial Function Properties of Convalescent COVID-19 Patients. J. Clin. Med. 2023, 12, 2233. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Lampsas, S.; Theofilis, P.; Souvaliotis, N.; Papamikroulis, G.A.; Katsarou, O.; Kalogeras, K.; Pantelidis, P.; Papaioannou, T.G.; Tsatsaragkou, A.; et al. Impaired left ventricular deformation and ventricular-arterial coupling in post-COVID-19: Association with autonomic dysregulation. Heart Vessels 2023, 38, 381–393. [Google Scholar] [CrossRef]
- Tudoran, C.; Bende, F.; Bende, R.; Giurgi-Oncu, C.; Dumache, R.; Tudoran, M. Correspondence between Aortic and Arterial Stiffness, and Diastolic Dysfunction in Apparently Healthy Female Patients with Post-Acute COVID-19 Syndrome. Biomedicines 2023, 11, 492. [Google Scholar] [CrossRef]
- Podrug, M.; Koren, P.; Dražić Maras, E.; Podrug, J.; Čulić, V.; Perissiou, M.; Bruno, R.M.; Mudnić, I.; Boban, M.; Jerončić, A. Long-Term Adverse Effects of Mild COVID-19 Disease on Arterial Stiffness, and Systemic and Central Hemodynamics: A Pre-Post Study. J. Clin. Med. 2023, 12, 2123. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Guo, W.; Li, P.; Leng, L.; Gao, D.; Yu, Z.; Huang, J.; Guo, J.; Wang, S.; Hu, M.; et al. Long-term effects of COVID-19 on endothelial function, arterial stiffness, and blood pressure in college students: A pre-post-controlled study. BMC Infect. Dis. 2024, 24, 742. [Google Scholar] [CrossRef] [PubMed]
- Riou, M.; Oulehri, W.; Momas, C.; Rouyer, O.; Lebourg, F.; Meyer, A.; Enache, I.; Pistea, C.; Charloux, A.; Marcot, C.; et al. Reduced Flow-Mediated Dilatation Is Not Related to COVID-19 Severity Three Months after Hospitalization for SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, K.M.; Bakker, E.A.; Schuijt, T.J.; Joseph, J.; Kavousi, M.; Geersing, G.J.; Rutten, F.H.; Hartman, Y.A.W.; Thijssen, D.H.J.; Eijsvogels, T.M.H. Long-term cardiovascular health status and physical functioning of nonhospitalized patients with COVID-19 compared with non-COVID-19 controls. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H47–H56. [Google Scholar] [CrossRef]
- Faria, D.; Moll-Bernardes, R.J.; Testa, L.; Moniz, C.M.V.; Rodrigues, E.C.; Rodrigues, A.G.; Araujo, A.; Alves, M.J.N.N.; Ono, B.E.; Izaias, J.E.; et al. Sympathetic Neural Overdrive, Aortic Stiffening, Endothelial Dysfunction, and Impaired Exercise Capacity in Severe COVID-19 Survivors: A Mid-Term Study of Cardiovascular Sequelae. Hypertension 2023, 80, 470–481. [Google Scholar] [CrossRef]
- Vidya, G.; Sowganthikashri, A.; Madhuri, T.; Anil, K.B.; Nitin, A.J. Arterial Stiffness and COVID-19: Potential Association with Diabetes, Hypertension and Obesity: A Cross Sectional Study. Maedica 2023, 18, 447–454. [Google Scholar] [CrossRef]
- Nandadeva, D.; Skow, R.J.; Stephens, B.Y.; Grotle, A.K.; Georgoudiou, S.; Barshikar, S.; Seo, Y.; Fadel, P.J. Cardiovascular and cerebral vascular health in females with postacute sequelae of COVID-19. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H713–H720. [Google Scholar] [CrossRef]
- Mclaughlin, M.; Sanal-Hayes, N.E.M.; Hayes, L.D.; Berry, E.C.; Sculthorpe, N.F. People with Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Exhibit Similarly Impaired Vascular Function. Am. J. Med. 2023, 138, 560–566. [Google Scholar] [CrossRef]
- World Health Organization. Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/newsroom/fact-sheets/item/post-covid-19-condition (accessed on 1 February 2025).
- Szeghy, R.E.; Stute, N.L.; Province, V.M.; Augenreich, M.A.; Stickford, J.L.; Stickford, A.S.L.; Ratchford, S.M. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J. Appl. Physiol. 2022, 132, 1297–1309. [Google Scholar] [CrossRef]
- Belcaro, G.; Cornelli, U.; Cesarone, M.R.; Scipione, C.; Scipione, V.; Hu, S.; Feragalli, B.; Corsi, M.; Cox, D.; Cotellese, R.; et al. Preventive effects of Pycnogenol® on cardiovascular risk factors (including endothelial function) and microcirculation in subjects recovering from coronavirus disease 2019 (COVID-19). Minerva Med. 2022, 113, 300–308. [Google Scholar] [CrossRef]
- Teixeira DO Amaral, V.; Viana, A.A.; Heubel, A.D.; Linares, S.N.; Martinelli, B.; Witzler, P.H.C.; Orikassa DE Oliveira, G.Y.; Zanini, G.S.; Borghi Silva, A.; Mendes, R.G.; et al. Cardiovascular, Respiratory, and Functional Effects of Home-Based Exercise Training after COVID-19 Hospitalization. Med. Sci. Sports Exerc. 2022, 54, 1795–1803. [Google Scholar] [CrossRef]
- Maruhashi, T.; Kajikawa, M.; Kishimoto, S.; Hashimoto, H.; Takaeko, Y.; Yamaji, T.; Harada, T.; Han, Y.; Aibara, Y.; Mohamad Yusoff, F.; et al. Diagnostic Criteria of Flow-Mediated Vasodilation for Normal Endothelial Function and Nitroglycerin-Induced Vasodilation for Normal Vascular Smooth Muscle Function of the Brachial Artery. J. Am. Heart Assoc. 2020, 9, e013915. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Bapir, M.; Skene, S.S.; Sies, H.; Kelm, M. Flow-Mediated Dilation Reference Values for Evaluation of Endothelial Function and Cardiovascular Health. Cardiovasc. Res. 2023, 119, 283–293. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Rev. Esp. Cardiol. (Engl. Ed.) 2022, 75, 429. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Romiti, S.; Fattouch, K.; De Bellis, A.; Greco, E. Atherosclerosis as Pathogenetic Substrate for Sars-Cov2 Cytokine Storm. J. Clin. Med. 2020, 9, 2095. [Google Scholar] [CrossRef]
- Kerch, G. Severe COVID-19-A Review of Suggested Mechanisms Based on the Role of Extracellular Matrix Stiffness. Int. J. Mol. Sci. 2023, 24, 1187. [Google Scholar] [CrossRef]
- Tavares, C.A.M.; Bailey, M.A.; Girardi, A.C.C. Biological Context Linking Hypertension and Higher Risk for COVID-19 Severity. Front. Physiol. 2020, 11, 599729. [Google Scholar] [CrossRef]
- Korakas, E.; Ikonomidis, I.; Kousathana, F.; Balampanis, K.; Kountouri, A.; Raptis, A.; Palaiodimou, L.; Kokkinos, A.; Lambadiari, V. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E105–E109. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.M.; Charlton, P.H.; Grillo, A.; et al. Vascular ageing: Moving from bench towards bedside. Eur. J. Prev. Cardiol. 2023, 30, 1101–1117, Erratum in Eur. J. Prev. Cardiol. 2023, 30, 1165. [Google Scholar] [CrossRef]
- Seals, D.R.; Kaplon, R.E.; Gioscia-Ryan, R.A.; LaRocca, T.J. You’re only as old as your arteries: Translational strategies for preserving vascular endothelial function with aging. Physiology 2014, 29, 250–264. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid.-Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Sadat-Ebrahimi, S.R.; Ala, A.; Nabavi, S.M.; Banach, M.; Ghaffari, S. A close-up view of dynamic biomarkers in the setting of COVID-19: Striking focus on cardiovascular system. J. Cell. Mol. Med. 2022, 26, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Bruno, R.M.; Spronck, B.; Hametner, B.; Hughes, A.; Lacolley, P.; Mayer, C.C.; Muiesan, M.L.; Rajkumar, C.; Terentes-Printzios, D.; Weber, T.; et al. COVID-19 Effects on ARTErial StIffness and Vascular AgeiNg: CARTESIAN Study Rationale and Protocol. Artery Res. 2020, 27, 59. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2023, 401, e21–e33. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Nowakowska, E.; Łoboda, D.; Gołba, K.S.; Sarecka-Hujar, B. Long COVID-19 Syndrome Severity According to Sex, Time from the Onset of the Disease, and Exercise Capacity—The Results of a Cross-Sectional Study. Life 2023, 13, 508. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.R.; Olde Hartman, T.C.; Rosmalen, J.G.M.; Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Laurence, J. Long COVID endotheliopathy: Hypothesized mechanisms and potential therapeutic approaches. J. Clin. Investig. 2022, 132, e161167. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Compared Study Groups | Age (SD) | Sex M/F |
|---|---|---|---|---|
| Ambrosino et al. [26], 2021 | Italy | 133 post-COV in early recovery (non-CR) | 61.6 (10.6) | 108M/25F |
| 133 CON (CV) | 60.4 (11.5) | 107M/26F | ||
| Ciacci et al. [93], 2023 | Italy | 20 acute COV | 70 (17) | 14M/6F |
| 20 CON (CV) | 74 (5) | 15M/5F | ||
| 20 CON (PN) | 71 (16) | 12M/8F | ||
| Dashoundhi et al. [92], 2023 | India | 18 acute COV | 31.83 (9.75) | 7M/11F |
| 18 CON (H) | 30.61 (10.11) | 7M/11F | ||
| Ergül et al. [95], 2022 | Turkey | 63 post-COV in early recovery | 44.4 (14.4) | - |
| 29 CON | ||||
| Faria et al. [105], 2023 | Brazil | 19 post-COV in mid-term recovery (LC) | 47.0 (8.0) | 12M/7F |
| 19 CON (H) | 43.0 (10.0) | 11M/8F | ||
| Gao et al. [29], 2022 | China | 86 post-COV in very late recovery (LC) | 58 (39–70) | 32M/54F |
| 28 CON (H) | 56 (37–65) | 10M/18F | ||
| 30 CON (CV) | 62 (39–67) | 11M/19F | ||
| Gounaridi et al., 2023 [98] | Greece | 60 post-COV in early recovery | 52.2 (12.3) | 29M/31F |
| 60 CON (CV) | 55.3 (9.8) | 32M/28F | ||
| Ikonomidis et al. [12], 2022 | Greece | 70 post-COV in very late recovery | 54.53 (9.07) | 44M/26F |
| 70 CON (H) | 54.77 (8.95) | 44M/26F | ||
| Jud et al. [10], 2021 | Austria | 14 post-COV in late recovery | 68.7 (12.0) | 7M/7F |
| 14 CON (H) | 30.7 (4.2) | 7M/7F | ||
| 14 CON (CV) | 66.9 (10.9) | 7M/7F | ||
| Lambadiari et al. [11], 2021 | Greece | 70 post-COV in mid-term recovery (LC) | 54.53 (9.07) | 44M/26F |
| 70 CON (H) | 54.77 (8.95) | 44M/26F | ||
| 70 CON (CV) | 54.47 (8.83) | 44M/26F | ||
| Luck et al. [94], 2023 | Pennsylvania, US | 14 subacute COV | 20 (1) | 10M/4F |
| 10 CON (H) | 22 (2) | 7M/3F | ||
| Mansiroglu et al. [97], 2022 | Turkey | 80 post-COV in early recovery | 32.10 (5.87) | 32M/48F |
| 81 CON (H) | 30.51 (7.33) | 36M/45F | ||
| Mclaughlin et al. [108], 2023 | Scotland, UK | 17 post-COV in very late recovery (LC) | 47.52 (9.60) | 4M/13F |
| 17 CON (H) | 49.05 (13.77) | 7M/10F | ||
| 17 CON (ME/CFS) | 49.7 (9.78) | 7M/10F | ||
| Nandadeva et al. [27] 2021 | Texas, US | 8 post-COV in mid-term recovery (non-LC) | 22 (4) | 5M/3F |
| 8 post-COV in mid-term recovery (LC) | 24 (3) | 1M/7F | ||
| 12 CON (H) | 23 (3) | 6M/6F | ||
| Nandadeva et al. [107] 2023 | Texas, US | 12 post-COV in very late recovery (LC) | 48 (9) | 0M/12F |
| 11 CON (H) | 50 (13) | 0M/11F | ||
| Oikonomou et al. [28], 2022 | Greece | 73 acute COV | 60.0 (12.7) | 46M/27F |
| 73 CON (CV) | 62.9 (14.0) | 49M/24F | ||
| 55 post-COV in early recovery | 57.8 (12.7) | 32M/23F | ||
| 55 post-COV in late recovery (LC) | 57.8 (12.7) | 32M/23F | ||
| 55 CON (CV) | 62.6 (16.1) | 29M/21F | ||
| Oikonomou et al. [99], 2023 | Greece | 34 post-COV in early recovery | 57.2 (12.9) | 26M/8F |
| 30 post-COV in late recovery (LC) | - | - | ||
| 34 CON (CV) | 57.4 (12.8) | 23M/11F | ||
| Oliveira et al. [90], 2021 | Brazil | 98 acute COV | 61 (16) | 55M/43F |
| 82 CON (PN) | 63 (17) | 40M/42F | ||
| Province et al. [91], 2022 | North Carolina, US | 16 subacute COV | 21 (1.0) | 8M/8F |
| 16 post-COV in early recovery | ||||
| 12 post-COV in mid-term recovery | 21 (1.0) | 7M/5F | ||
| 20 CON (H) | 23 (1.0) | 5M/15F | ||
| Ratchford et al. [89], 2021 | North Carolina, US | 11 subacute COV | 20.2 (1.1) | 4M/7F |
| 20 CON (H) | 23.0 (1.3) | 5M/15F | ||
| Riou et al. [103], 2021 | France | 27 post-COV in mid-term recovery | 57 (49–66) | 17M/10F |
| 9 CON (CV) | 59 (54–62) | 5M/4F | ||
| Schnaubelt et al. [33], 2021 | Austria | 22 acute COV | 76.5 (67.0–84.0) | 11M/11F |
| 22 CON (PN) (CV) | 76.5 (67.0–83.0) | 10M/12F | ||
| Skow et al. [96], 2022 | Texas, US | 23 post-COV in early recovery | 23 (3) | 9M/14F |
| 13 CON (H) | 26 (4) | 6M/7F | ||
| Tudoran et al. [100], 2023 | Romania | 54 post-COV in early recovery (non-MS) | 47.76 (5.43) | 0M/54F |
| 67 post-COV in early recovery (MS) | 50.59 (4.53) | 0M/54F | ||
| 40 CON (H) | 49.47 (5.14) | 0M/54F | ||
| van der Sluijs et al. [104], 2023 | The Netherlands | 31 post-COV in mid-term recovery (LC) | 58 (51–63) | 17M/14F |
| 31 CON | 57 (50–62) | 17M/14F | ||
| 97 post-COV in mid-term recovery | - | - | ||
| 49 CON | - | - | ||
| Vidya et al. [106], 2023 | India | IA: 32 post-COV in mid-term recovery with DM | 30–50 | - |
| IB: 28 CON with DM (CV) | ||||
| IIA: 20 post-COV in mid-term recovery with AH | ||||
| IIB: 20 CON with AH (CV) | ||||
| IIIA: 25 post-COV in mid-term recovery with obesity | ||||
| IIIB: 25 CON with obesity (CV) | ||||
| Zanoli et al. [35] (Study 1), 2022 | Italy | 45 post-COV in mid-term recovery (LC) | 55 (11) | 25M/20F |
| 45 post-COV in very late recovery | 54 (13) | 27M/18F | ||
| 180 CON (H) | 55 (13) | 97M/83F |
| Study | Country | Study Group in F/U | Age (SD) | Sex M/F |
|---|---|---|---|---|
| Belcaro et al. [111], 2022 | Italy | 30 post-COV in the early recovery period (non-Pycnogenol® group) | 35–70 | - |
| 30 post-COV in the mid-term recovery period (non-Pycnogenol® group) | ||||
| Gounaridi et al. [98], 2023 | Greece | 30 post-COV in the early recovery period (non-CR group) | 49.10 (12.70) | 18M/12F |
| 30 post-COV in the mid-term recovery period (non-CR group) | ||||
| Lambadiari et al. [11], 2021 Ikonomidis et al. [12], 2022 | Greece | 70 post-COV in the mid-term recovery period | 54.53 (9.07) | 44M/26F |
| 70 post-COV in the very late recovery period | ||||
| Oikonomou et al. [28], 2022 | Greece | 55 in the acute COV phase | 57.8 (12.7) | 32M/23F |
| 55 post-COV in the early recovery period | ||||
| 55 post-COV in the late recovery period | ||||
| Oikonomou et al. [99], 2023 | Greece | 34 post-COV in the early recovery period | 57.2 (12.9) | 26M/8F |
| 34 post-COV in the late recovery period | ||||
| Peng et al. [102], 2024 | China | 37 in the pre-COV period | 21.35 (1.99) | 27M/10F |
| 20 post-COV in the early recovery period | ||||
| 17 post-COV in the mid-term recovery period | ||||
| Podrug et al. [101], 2023 | Croatia | 32 in the pre-COV period | 36.6 (12.6) | 18M/14F |
| 32 post-COV in the early recovery period | ||||
| Province et al. [91], 2022 | North Carolina, US | 16 in the subacute COV phase | 21 (1.0) | 8M/8F |
| 16 post-COV in the early recovery period | ||||
| 12 post-COV in the mid-term recovery period | ||||
| Saloň et al. [14], 2023 | Norway | 35 in the subacute COV phase | 60 (10) | 30M/5F |
| 35 post-COV in the early recovery period | ||||
| Szeghy et al. [110], 2022 | North Carolina, US | 14 in the subacute COV phase | 21 (1.0) | 7M/7F |
| 14 post-COV in the early recovery period | 21 (1.0) | 7M/7F | ||
| 12 post-COV in the mid-term recovery period | 21 (1.0) | 7M/5F | ||
| Teixeira DO Amaral et al. [112], 2022 | Brazil | 20 post-COV in the early recovery period (non-CR group) | 53.30 (11.60) | 8M/12F |
| 20 post-COV in the mid-term recovery period (non-CR group) | ||||
| Zanoli et al. [35] (Study 2), 2022 | Italy | 41 post-COV in the mid-term recovery period | 54 (12) | 21M/20F |
| 41 post-COV in the very late recovery period |
| Comparison | Egger’s Test | Begg’s Test | |||
|---|---|---|---|---|---|
| Intercept | 95% CI | p | Kendall’s Tau | p | |
| Acute/subacute COVID-19 patients vs. controls | −1.306 | −9.058 to 6.445 | 0.544 | −0.333 | 0.497 |
| Early recovery post-COVID-19 patients vs. controls | −0.563 | −8.732 to 7.605 | 0.858 | −0.067 | 0.851 |
| Mid-term recovery post-COVID-19 vs. controls | −3.562 | −14.758 to 7.634 | 0.386 | −0.400 | 0.327 |
| Late recovery post-COVID-19 vs. controls | 3.912 | −16.060 to 23.884 | 0.243 | 0.999 | 0.117 |
| Very late recovery post-COVID-19 vs. controls | 4.422 | −51.384 to 60.229 | 0.498 | 0.333 | 0.601 |
| Comparison | Egger’s Test | Begg’s Test | |||
|---|---|---|---|---|---|
| Intercept | 95% CI | p | Kendall’s Tau | p | |
| Early recovery post-COVID-19 patients vs. controls | −6.278 | −19.952 to 7.396 | 0.240 | −0.400 | 0.327 |
| Mid-term recovery post-COVID-19 vs. controls | 5.682 | 0.044 to 11.320 | 0.049 | 0.357 | 0.216 |
| Very late recovery post-COVID-19 vs. controls | 2.641 | −25.036 to 30.319 | 0.439 | 0.999 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loboda, D.; Golba, K.S.; Gurowiec, P.; Bredelytė, A.; Razbadauskas, A.; Sarecka-Hujar, B. Variability in Arterial Stiffness and Vascular Endothelial Function After COVID-19 During 1.5 Years of Follow-Up—Systematic Review and Meta-Analysis. Life 2025, 15, 520. https://doi.org/10.3390/life15040520
Loboda D, Golba KS, Gurowiec P, Bredelytė A, Razbadauskas A, Sarecka-Hujar B. Variability in Arterial Stiffness and Vascular Endothelial Function After COVID-19 During 1.5 Years of Follow-Up—Systematic Review and Meta-Analysis. Life. 2025; 15(4):520. https://doi.org/10.3390/life15040520
Chicago/Turabian StyleLoboda, Danuta, Krzysztof S. Golba, Piotr Gurowiec, Aelita Bredelytė, Artūras Razbadauskas, and Beata Sarecka-Hujar. 2025. "Variability in Arterial Stiffness and Vascular Endothelial Function After COVID-19 During 1.5 Years of Follow-Up—Systematic Review and Meta-Analysis" Life 15, no. 4: 520. https://doi.org/10.3390/life15040520
APA StyleLoboda, D., Golba, K. S., Gurowiec, P., Bredelytė, A., Razbadauskas, A., & Sarecka-Hujar, B. (2025). Variability in Arterial Stiffness and Vascular Endothelial Function After COVID-19 During 1.5 Years of Follow-Up—Systematic Review and Meta-Analysis. Life, 15(4), 520. https://doi.org/10.3390/life15040520








