The Association of Histological Signs of Plaque Instability with Low eGFR, Higher Neutrophil-to-Lymphocyte Ratio, and Lower Serum MCP-1 Levels in Carotid Endarterectomy Patients—A Single-Center, Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Study of Circulating Biomarkers
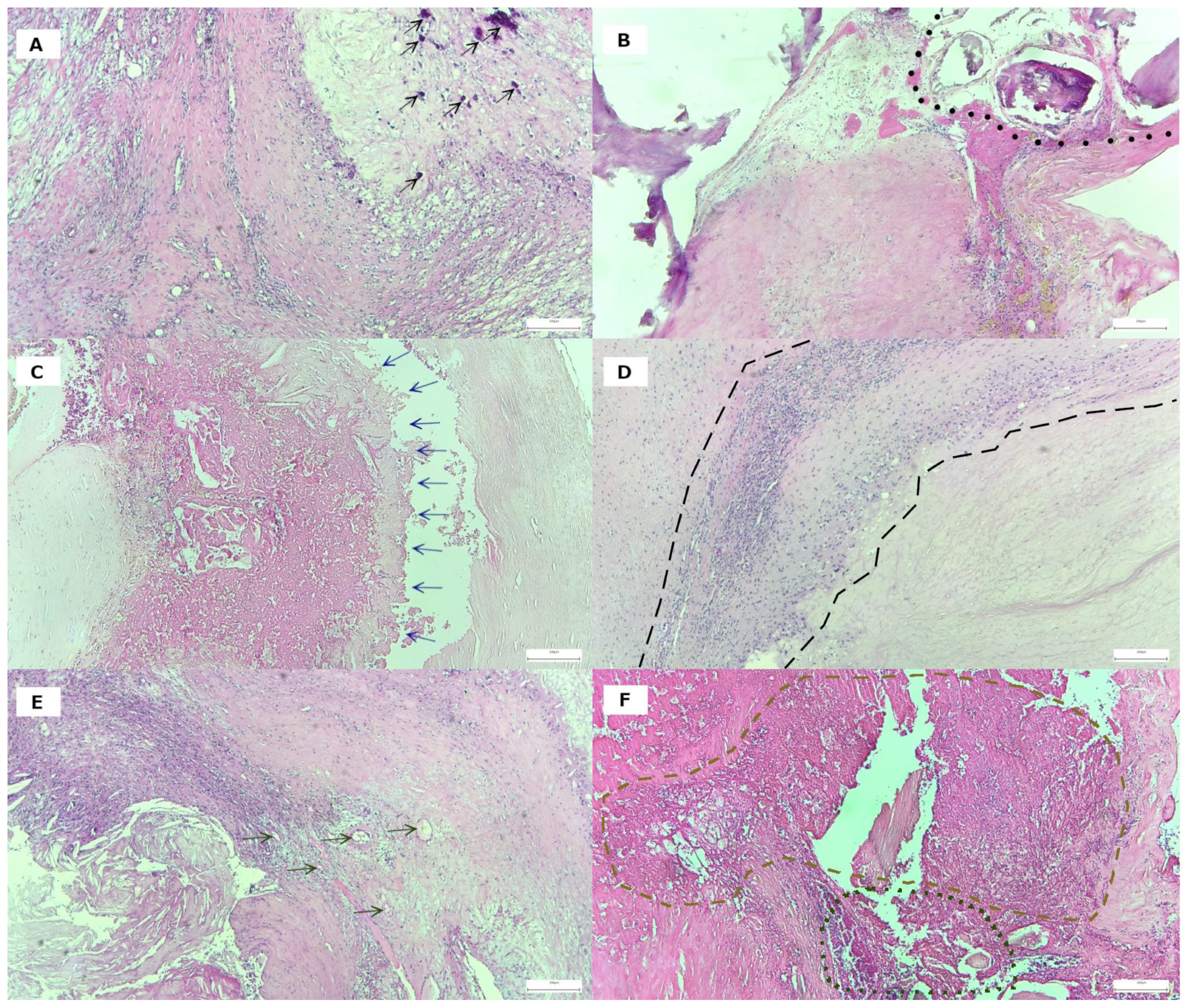
2.3. Carotid Specimens: Histological Processing
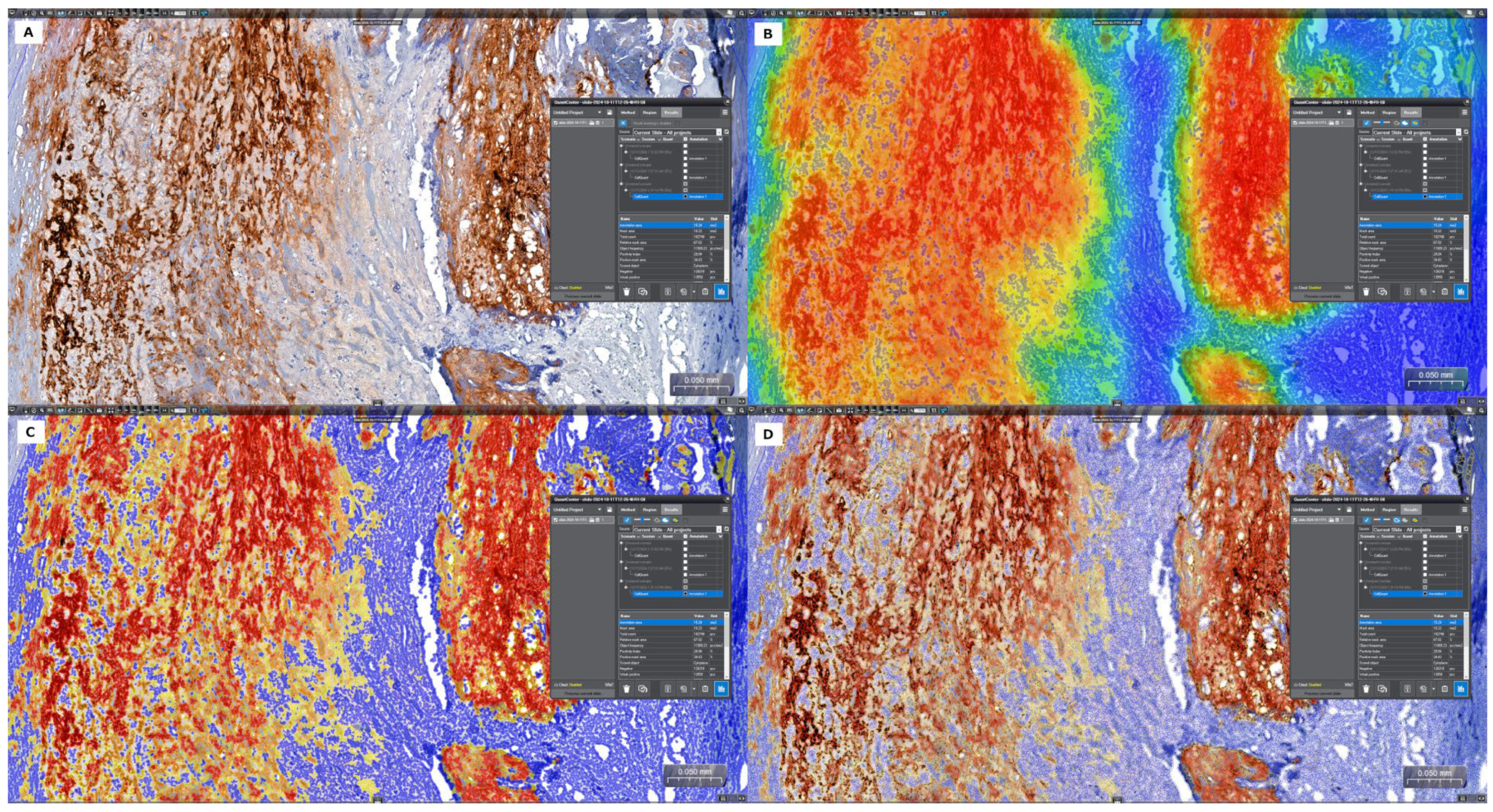
2.4. Investigation of the CRP and MMP-9 Expression Within the Atherosclerotic Plaque by Immunohistochemistry
2.5. Quantitative Digital Image Analysis: Digital Morphometry
2.6. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. Histological Features of Plaque Vulnerability in Advanced Carotid Stenosis
3.3. Analysis of In Situ Accumulation of MMP-9 and CRP by Immunohistochemistry and Digital Morphometry
3.3.1. Analysis of the Carotid Plaques with and Without Complications
3.3.2. Analysis of the Carotid Plaques with and Without Inflammatory Infiltrate
3.4. Comparative Analysis of Biological Characteristics Associated with Histological Evidence of Hemorrhagic and Any Cause Complicated Plaques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| CEA | Carotid endarterectomy |
| CBC | Complete blood count |
| eGFR | Glomerular filtration rate |
| NLR | Neutrophil/lymphocyte ratio |
| LMR | Lymphocyte/monocyte ratio |
| SIRI | Systemic inflammatory response index |
| MMP-9 | Matrix metalloproteinase-9 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| oxLDL | Oxidized forms of human low-density lipoprotein |
| hsCRP | High-sensitivity C-reactive protein |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemistry |
References
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Khalili, P.; Franklin, S.S. Blood pressure and pulse wave velocity as metrics for evaluating pathologic ageing of the cardiovascular system. Blood Press. 2014, 23, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Robert, L. Aging of the vascular-wall and atherosclerosis. Exp. Gerontol. 1999, 34, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Pelisek, J.; Eckstein, H.-H.; Zernecke, A. Pathophysiological mechanisms of carotid plaque vulnerability: Impact on ischemic stroke. Arch. Immunol. Ther. Exp. 2012, 60, 431–442. [Google Scholar] [CrossRef]
- Anbar, R.; Sultan, S.R.; Saikhan, L.A.; Alkharaiji, M.; Chaturvedi, N.; Hardy, R.; Richards, M.; Hughes, A. Is carotid artery atherosclerosis associated with poor cognitive function assessed using the Mini-Mental State Examination? A systematic review and meta-analysis. BMJ Open 2022, 12, e055131. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Eng. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Puz, P.; Lasek-Bal, A.; Ziaja, D.; Kazibutowska, Z.; Ziaja, K. Inflammatory markers in patients with internal carotid artery stenosis. Arch. Med. Sci. 2013, 2, 254–260. [Google Scholar] [CrossRef]
- Baroncini, L.A.V.; Nakao, L.S.; Ramos, S.G.; Filho, A.P.; Murta, L.O.; Ingberman, M.; Tefé-Silva, C.; Précoma, D.B. Assessment of MMP-9, TIMP-1, and COX-2 in normal tissue and in advanced symptomatic and asymptomatic carotid plaques. Thromb. J. 2011, 9, 6. [Google Scholar] [CrossRef]
- Potter, T.B.H.; Tannous, J.; Vahidy, F.S. A contemporary review of epidemiology, risk factors, etiology, and outcomes of premature stroke. Curr. Atheroscler. Rep. 2022, 24, 939–948. [Google Scholar] [CrossRef]
- Kelly, D.M.; Ademi, Z.; Doehner, W.; Lip, G.Y.H.; Mark, P.; Toyoda, K.; Wong, C.X.; Sarnak, M.; Cheung, M.; Herzog, C.A.; et al. Chronic kidney disease and cerebrovascular disease. Stroke 2021, 52, e328–e346. [Google Scholar] [CrossRef]
- Chen, X.; Yang, D.; Zhao, H.; Zhang, H.; Hong, P. Stroke-Induced Renal Dysfunction: Underlying mechanisms and challenges of the Brain–Kidney Axis. CNS Neurosci. Ther. 2024, 30, e70114. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Li, L.; Burgess, A.I.; Poole, D.L.; Duerden, J.M.; Rothwell, P.M. Associations of blood biomarkers with glomerular filtration rate in patients with TIA and stroke: Population-based study. Stroke Vasc. Neurol. 2021, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Krassilnikova, M.; Tighiouart, H.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. CKD classification based on estimated GFR over three years and subsequent cardiac and mortality outcomes: A cohort study. BMC Nephrol. 2009, 10, 26. [Google Scholar] [CrossRef]
- Sandsmark, D.K.; Messé, S.R.; Zhang, X.; Roy, J.; Nessel, L.; Hamm, L.L.; He, J.; Horwitz, E.J.; Jaar, B.G.; Kallem, R.R.; et al. Proteinuria, but not EGFR, predicts stroke risk in chronic kidney disease. Stroke 2015, 46, 2075–2080. [Google Scholar] [CrossRef]
- van Dam-Nolen, D.H.K.; Truijman, M.T.B.; van der Kolk, A.G.; Liem, M.I.; Schreuder, F.H.B.M.; Boersma, E.; Daemen, M.J.A.P.; Mess, W.H.; van Oostenbrugge, R.J.; van der Steen, A.F.W.; et al. Carotid plaque characteristics predict recurrent ischemic stroke and TIA. JACC Cardiovasc. Imaging 2022, 15, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Kopczak, A.; Schindler, A.; Bayer-Karpinska, A.; Koch, M.L.; Sepp, D.; Zeller, J.; Strecker, C.; Hempel, J.-M.; Yuan, C.; Malik, R.; et al. Complicated carotid artery plaques as a cause of cryptogenic stroke. J. Am. Coll. Cardiol. 2020, 76, 2212–2222. [Google Scholar] [CrossRef]
- Redgrave, J.N.E.; Lovett, J.K.; Gallagher, P.J.; Rothwell, P.M. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms. Circulation 2006, 113, 2320–2328. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Coupland, A.P.; Thapar, A.; Qureshi, M.I.; Jenkins, H.; Davies, A.H. The definition of stroke. J. R. Soc. Med. 2017, 110, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- El Brihi, J.; Pathak, S. Normal and Abnormal Complete Blood Count with Differential [Updated 2024 Jun 8]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK604207/ (accessed on 25 April 2025).
- Balmos, I.A.; Hutanu, A.; Horváth, E.; Calburean, P.-A.; Molnár, G.B.; Muresan, A.V. The Role of Serum Biomarkers in the Prediction of Immediate Postoperative Complications after Carotid Endarterectomy. J. Cardiovasc. Emergencies 2025, 11, 26–35. [Google Scholar] [CrossRef]
- Balmos, I.A.; Horváth, E.; Brinzaniuc, K.; Muresan, A.V.; Olah, P.; Molnár, G.B.; Nagy, E.E. Inflammation, Microcalcification, and Increased Expression of Osteopontin Are Histological Hallmarks of Plaque Vulnerability in Patients with Advanced Carotid Artery Stenosis. Biomedicines 2023, 11, 881. [Google Scholar] [CrossRef]
- Balmos, I.A.; Slevin, M.; Brinzaniuc, K.; Muresan, A.V.; Suciu, H.; Molnár, G.B.; Mocian, A.; Szabó, B.; Nagy, E.E.; Horváth, E. Intraplaque Neovascularization, CD68+ and iNOS2+ Macrophage Infiltrate Intensity Are Associated with Atherothrombosis and Intraplaque Hemorrhage in Severe Carotid Atherosclerosis. Biomedicines 2023, 11, 3275. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, J.; Dou, M.; Cheng, X.; Chen, X.; Ma, L.; Xie, J. Inhibition of CCR2 attenuates NLRP3-dependent pyroptosis after myocardial ischaemia–reperfusion in rats via the NF-kB pathway. Int. Immunopharmacol. 2025, 145, 113803. [Google Scholar] [CrossRef]
- HealthMatters.io LLC. Oxidized LDL. Available online: https://healthmatters.io/understand-blood-test-results/oxidized-ldl (accessed on 25 April 2025).
- Mathew, A.; Eliasziw, M.; Devereaux, P.J.; Merino, J.G.; Barnett, H.J.M.; Garg, A.X. Carotid Endarterectomy Benefits Patients with CKD and Symptomatic High-Grade Stenosis. J. Am. Soc. Nephrol. 2010, 21, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Pearce, W.H.; Rodriguez, H.E.; Helenowski, I.B.; Eskandari, M.K. Durability of Stroke Prevention with Carotid Endarterectomy and Carotid Stenting. Surgery 2018, 164, 1271–1278. [Google Scholar] [CrossRef]
- Van Lammeren, G.W.; Moll, F.L.; De Borst, G.J.; De Kleijn, D.P.V.; De Vries, J.-P.P.M.; Pasterkamp, G. Atherosclerotic plaque biomarkers: Beyond the horizon of the vulnerable plaque. Curr. Cardiol. Rev. 2011, 7, 22–27. [Google Scholar] [CrossRef][Green Version]
- Brott, T.G.; Howard, G.; Roubin, G.S.; Meschia, J.F.; Mackey, A.; Brooks, W.; Moore, W.S.; Hill, M.D.; Mantese, V.A.; Clark, W.M.; et al. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N. Eng. J. Med. 2016, 374, 1021–1031. [Google Scholar] [CrossRef]
- Koziarska-Rościszewska, M.; Gluba-Brzózka, A.; Franczyk, B.; Rysz, J. High-Sensitivity C-Reactive Protein Relationship with Metabolic Disorders and Cardiovascular Diseases Risk Factors. Life 2021, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.P.; Tio, M.C.; Li, X.; Adams-Huet, B.; De Lemos, J.A.; Hedayati, S.S. Association of Monocyte Chemoattractant Protein-1 with Death and Atherosclerotic Events in Chronic Kidney Disease. Am. J. Nephrol. 2018, 47, 395–405. [Google Scholar] [CrossRef]
- Ohtake, T.; Kobayashi, S. Chronic kidney Disease and atherosclerosis: An important implication of carotid Intima-Media Thickness. J. Atheroscler. Thromb. 2021, 28, 471–473. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Y.; Li, W.; Deng, F.; Liu, X.; Wang, X.; Gui, Y.; Qin, L.; Hu, C.; Chen, L. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima–media thickness. Atherosclerosis 2014, 232, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Imamura, T.; Kitamura, K.; Ogawa, T.; Fujimoto, S.; Kakitsubata, Y.; Ishikawa, T.; Asada, Y.; Kodama, T. Associations of Plasma Pentraxin 3 and Monocyte Chemoattractant Protein-1 Concentrations with Cardiovascular Disease in Patients with Chronic Kidney Disease. Ren. Fail. 2011, 33, 398–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Georgakis, M.K.; van der Laan, S.W.; Asare, Y.; Mekke, J.M.; Haitjema, S.; Schoneveld, A.H.; de Jager, S.C.A.; Nurmohamed, N.S.; Kroon, J.; Stroes, E.S.G.; et al. Monocyte-chemoattractant protein-1 levels in human atherosclerotic lesions associate with plaque vulnerability. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2038–2048. [Google Scholar] [CrossRef]
- Jager, N.A.; De Vries, B.M.W.; Hillebrands, J.-L.; Harlaar, N.J.; Tio, R.A.; Slart, R.H.J.A.; Van Dam, G.M.; Boersma, H.H.; Zeebregts, C.J.; Westra, J. Distribution of matrix metalloproteinases in human atherosclerotic carotid plaques and their production by smooth muscle cells and macrophage subsets. Mol. Imaging Biol. 2015, 18, 283–291. [Google Scholar] [CrossRef]
- Fanni, D.; Gerosa, C.; Nurchi, V.M.; Suri, J.S.; Nardi, V.; Congiu, T.; Coni, P.; Ravarino, A.; Cerrone, G.; Piras, M.; et al. Trace elements and the carotid plaque: The GOOD (Mg, Zn, Se), the UGLY (Fe, Cu), and the BAD (P, Ca)? DOAJ (DOAJ Dir. Open Access J.) 2021, 25, 3772–3790. [Google Scholar] [CrossRef]
- Sef, D.; Kovacevic, M.; Jernej, B.; Novacic, K.; Slavica, M.; Petrak, J.; Medved, I.; Milosevic, M. Immunohistochemical analysis of MMP-9 and COX-2 expression in carotid atherosclerotic plaques among patients undergoing carotid endarterectomy: A prospective study. J. Stroke Cerebrovasc. Dis. 2022, 31, 106731. [Google Scholar] [CrossRef]
- Puig, N.; Jiménez-Xarrié, E.; Camps-Renom, P.; Benitez, S. Search for reliable circulating biomarkers to predict carotid plaque vulnerability. Int. J. Mol. Sci. 2020, 21, 8236. [Google Scholar] [CrossRef]
- Pelisek, J.; Rudelius, M.; Zepper, P.; Poppert, H.; Reeps, C.; Schuster, T.; Eckstein, H.-H. Multiple biological predictors for vulnerable carotid lesions. Cerebrovasc. Dis. 2009, 28, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Łacheta, D.; Kubiak-Tomaszewska, G. Matrix metalloproteinases as biomarkers of atherosclerotic plaque instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef]
- Bhargava, S.; De La Puente-Secades, S.; Schurgers, L.; Jankowski, J. Lipids and lipoproteins in cardiovascular diseases: A classification. Trends Endocrinol. Metab. 2022, 33, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Satała, J.; Gorzelak-Pabiś, P.; Pawlos, A.; Broncel, M.; Kaźmierski, P.; Woźniak, E. OxLDL as a prognostic biomarker of plaque instability in patients qualified for carotid endarterectomy. J. Cell. Mol. Med. 2024, 28, e18459. [Google Scholar] [CrossRef]
- Liao, M.; Liu, L.; Bai, L.; Wang, R.; Liu, Y.; Zhang, L.; Han, J.; Li, Y.; Qi, B. Correlation between novel inflammatory markers and carotid atherosclerosis: A retrospective case-control study. PLoS ONE 2024, 19, e0303869. [Google Scholar] [CrossRef]
- Tmoyan, N.A.; Afanasieva, O.I.; Ezhov, M.V. The role of lipoprotein(A) in the development of peripheral and carotid atherosclerosis. Kardiologiia 2018, 17, 70–78. [Google Scholar] [CrossRef]
- Ruan, W.; Wang, M.; Sun, C.; Yao, J.; Ma, Y.; Ma, H.; Ding, J.; Lian, X. Correlation between neutrophil-to-lymphocyte ratio and stability of carotid plaques. Clin. Neurol. Neurosurg. 2022, 212, 107055. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wu, G. Relationship of Neutrophil-to-Lymphocyte Ratio with Carotid Plaque Vulnerability and Occurrence of Vulnerable Carotid Plaque in Patients with Acute Ischemic Stroke. BioMed Res. Int. 2021, 2021, 6894623. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Zhao, Q.; Bai, Y.; Ruan, L. Remnant Cholesterol and Common Carotid Artery Intima-Media Thickness in Community Population with Normal Low-Density Lipoprotein Cholesterol. Cerebrovasc. Dis. 2023, 52, 487–494. [Google Scholar] [CrossRef]
- Cosarca, M.; Hălmaciu, I.; Muresan, A.; Suciu, B.; Molnar, C.; Russu, E.; Horvath, E.; Niculescu, R.; Puscasiu, L.; Bacalbașa, N.; et al. Neutrophil-to-lymphocyte, platelet-to-lymphocyte and lymphocyte-to-monocyte ratios are associated with amputation rates in patients with peripheral arterial disease and diabetes mellitus who underwent revascularization: A Romanian regional center study. Exp. Ther. Med. 2022, 24, 703. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. LekáRske Listy/Bratisl. Med. J. 2021, 122, 474–488. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, M.; Crean, D.; Barry, M.; Belton, O. M1- and M2-Type Macrophage Responses Are Predictive of Adverse Outcomes in Human Atherosclerosis. Front. Immunol. 2016, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Z.; Sirolli, V.; Bonomini, M.; Gallina, S.; Renda, G. Hallmarks for Thrombotic and Hemorrhagic Risks in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2024, 25, 8705. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T. Atherosclerotic diseases in chronic kidney disease. J. Atheroscler. Thromb. 2025, 32, 111–119. [Google Scholar] [CrossRef]
- Pelisek, J.; Hahntow, I.N.; Eckstein, H.-H.; Ockert, S.; Reeps, C.; Heider, P.; Luppa, P.B.; Frank, H. Impact of chronic kidney disease on carotid plaque vulnerability. J. Vasc. Surg. 2011, 54, 1643–1649. [Google Scholar] [CrossRef]
- Huang, M.-J.; Wei, R.-B.; Wang, Y.; Su, T.-Y.; Di, P.; Li, Q.-P.; Yang, X.; Li, P.; Chen, X.-M. Blood coagulation system in patients with chronic kidney disease: A prospective observational study. BMJ Open 2017, 7, e014294. [Google Scholar] [CrossRef]
- Pénzes, K.; Hurják, B.; Katona, É.; Becs, G.; Balla, J.; Muszbek, L. Terminal Phase Components of the Clotting Cascade in Patients with End-Stage Renal Disease Undergoing Hemodiafiltration or Hemodialysis Treatment. Int. J. Mol. Sci. 2020, 21, 8426. [Google Scholar] [CrossRef]
- Lim, H.Y.; Lui, B.; Tacey, M.; Barit, D.; Patel, S.K.; Donnan, G.; Nandurkar, H.; Burrell, L.M.; Ho, P. Global coagulation assays in patients with chronic kidney disease and their role in predicting thrombotic risk. Thromb. Res. 2023, 226, 127–135. [Google Scholar] [CrossRef]
- Zahran, M.; Nasr, F.M.; Metwaly, A.A.; El-Sheikh, N.; Khalil, N.S.A.; Harba, T. The Role of Hemostatic Factors in Atherosclerosis in Patients with Chronic Renal Disease. DOAJ (DOAJ Dir. Open Access J.) 2015, 7, 1270–1276. [Google Scholar] [CrossRef]
- Selwaness, M.; Bos, D.; Van Den Bouwhuijsen, Q.; Portegies, M.L.P.; Ikram, M.A.; Hofman, A.; Franco, O.H.; Van Der Lugt, A.; Wentzel, J.J.; Vernooij, M.W. Carotid atherosclerotic plaque characteristics on magnetic resonance imaging relate with history of stroke and coronary heart disease. Stroke 2016, 47, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ninomiya, T.; Sumiyoshi, S.; Onimaru, M.; Fujii, H.; Itabe, H.; Nakashima, Y.; Sueishi, K.; Tsuruya, K.; Oda, Y.; et al. Chronic kidney disease is associated with neovascularization and intraplaque hemorrhage in coronary atherosclerosis in elders: Results from the Hisayama Study. Kidney Int. 2013, 84, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Boucher, R.E.; Sun, J.; Balu, N.; Chonchol, M.; Navaneethan, S.; Chertow, G.M.; Townsend, R.; Haley, W.; Cheung, A.K.; et al. Chronic kidney disease, atherosclerotic plaque characteristics on carotid magnetic resonance imaging, and cardiovascular outcomes. BMC Nephrol. 2021, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, M.; Van Koeverden, I.D.; Van Lammeren, G.W.; Van Der Laan, S.W.; Haitjema, S.; De Vries, J.-P.P.M.; Ruijter, H.M.D.; De Jager, S.C.A.; Hoefer, I.; Blankestijn, P.; et al. Impaired kidney function is associated with intraplaque hemorrhage in patients undergoing carotid endarterectomy. Atherosclerosis 2017, 266, 128–135. [Google Scholar] [CrossRef]
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; De Borst, G.J.; Bulbulia, R.; Halliday, A.; Van Herzeele, I.; Koncar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur. Stroke J. 2021, 6, I–XLVII. [Google Scholar] [CrossRef]


| Variable | Median (Min–Max) or Frequency n (%) |
|---|---|
| Demographic and lifestyle variables | |
| Age (years) | 67/(42–80) |
| Gender (f/m) | 12 (29.27)/29 (70.73) |
| Smoking (yes/no) | 16 (39.02)/25 (60.98) |
| Disease characteristics and comorbidities | |
| Grade of stenosis (%) | 83.47 (70–95) |
| Stroke history (y/n) | 26 (63.41)/15 (36.5) |
| Hypertension (y/n) | 39 (95.12)/2 (4.88) |
| Diabetes (y/n) | 15 (36.59)/26 (63.41) |
| Polyvascular disease (2 or 3/1 arterial beds affected) | 36 (87.8)/5 (12.2) |
| Ischemic heart disease (y/n) | 34 (82.92)/7 (17.08) |
| Chronic kidney disease (CKD) (y/n) | 9 (21.95)/32 (78.04) |
| eGFR | 85 (24–114) |
| GFR-GROUP A | 95.5 (90–114) |
| GFR-GROUP B | 78 (61–87) |
| GFR-GROUP C | 43 (24–58) |
| Body mass index | 28.37 (20–47.26) |
| Biological variables | |
| Dyslipidemia (yes/no) | 38 (92.68)/3 (7.32) |
| Abs. neutrophil count (×103/µL) | 5.16 (2.4–9.95) |
| Abs. lymphocyte count (×103/µL) | 1.93 (0.82–5.27) |
| Abs. monocyte count (×103/µL) | 0.62 (0.36–1.09) |
| Neutrophil-to-lymphocyte ratio (NLR) | 2.4 (1.1–5.02) |
| Lymphocyte-to-monocyte ratio (LMR) | 3.21 (1.77–7.98) |
| Systemic Inflammation Response Index (SIRI) | 1.59 (0.59–5.48) |
| High-sensitivity C-reactive protein (hsCRP), (mg/l) | 1.01 (0.16–29.2) |
| Monocyte chemoattractant protein-1 (MCP-1) (pg/mL) | 193.67 (75.97–847.06) |
| Matrix metalloproteinase-9 (MMP-9) (ng/mL) | 67.98 (1.94–452.5) |
| Oxidized low-density lipoprotein (ng/mL) | 51.89 (17.59–234.51) |
| Signs of plaque vulnerability | |
| Inflammatory infiltrate | 32 (78.1) |
| Macro-/microcalcification | 30 (73.2) |
| Lipid core | 18 (43.9) |
| Neovascularization | 35 (85.4) |
| Hemorrhage | 27 (65.8) |
| Thrombosis | 4 (17.1) |
| Ulceration | 4 (9.7) |
| Variable | COMPL+ Group (n = 28) | COMPL− Group (n = 13) | p Values |
|---|---|---|---|
| Demographic and lifestyle variables | |||
| Age (years) | 66.5 (42–80) | 67 (57–78) | 0.970 |
| BMI | 28.28 (21.22–47.26) | 29.39 (20–35.06) | 0.533 |
| Smoking (yes/no) | 11 (26.82)/17 (41.46) | 4 (9.75)/9 (21.95) | 0.434 |
| Disease characteristics and comorbidities | |||
| Stroke history (y/n) | 18 (43.9)/10 (24.39) | 8 (19.51)/5 (12.19) | 0.615 |
| Hypertension (y/n) | 26 (63.41)/2 (4.87) | 13 (31.7)/0 | 0.307 |
| Diabetes (y/n) | 8 (19.51)/20 (48.78) | 7 (17.07)/6 (14.63) | 0.224 |
| Polyvascular disease (2 or 3/1 arterial beds affected) | 25 (60.97)/3 (7.31) | 10 (24.39)/3(7.31) | 0.276 |
| Plaque charcteristics | |||
| Inflammation (y/n) | 23 (82.14)/5 (17.85) | 9 (69.23)/4 (30.76) | 0.428 |
| Microcalcification (y/n) | 3 (10.71)/25 (89.28) | 2 (15.38)/11 (84.61) | 0.643 |
| CRP + surface | 67.04 (45.9–76.92) | 66.04 (45.71–75.69) | 0.857 |
| CRP-H-score | 16.11 (1–128.24) | 5.12 (1.41–93.08) | 0.095 |
| MMP-9 + surface | 62.89 (48.67–75.76) | 60.93 (51.12–73.45) | 0.310 |
| MMP-9-H-score | 32.63 (4.27–141.17) | 35.02(3.8–135.8) | 0.608 |
| Biological variables | |||
| Abs. neutrophil count (×103/µL) | 5.33 (2.4–9.95) | 4.93(2.82–7.59) | 0.729 |
| Abs. lymphocyte count (×103/µL) | 1.83 (1.01–5.27) | 2.09 (0.82–3.43) | 0.814 |
| Abs. monocyte count (×103/µL) | 0.625 (0.37–0.48) | 0.59 (0.36–1.08) | 0.708 |
| Neutrophil-to-lymphocyte ratio (NLR) | 2.48(1.1–5.02) | 2.18 (1.65–4.56) | 0.688 |
| Lymphocyte-to-monocyte ratio (LMR) | 3.51(1.77–7.98) | 3.04 (2.28–6.23) | 0.857 |
| eGFR | 79.5 (24–110) | 94 (69–114) | 0.004 |
| Systemic Inflammation Response Index (SIRI) | 1.59 (0.59–5.48) | 1.45 (0.63–2.69) | 0.750 |
| High-sensitivity C-reactive protein (hsCRP) (mg/L) | 2.17 (0.16–29.2) | 0.7 (0.16–22.0) | 0.167 |
| Monocyte chemoattractant protein-1 (MCP-1) (pg/mL) | 181.94 (75.97–847.06) | 228.57 (151.18–626.89) | 0.135 |
| Matrix metalloproteinase-9 (MMP-9) (ng/mL) | 62.38 (1.94–452.5) | 85.87 (33.24–249.48) | 0.167 |
| Oxidized low-density lipoprotein (ng/mL) | 53.98 (17.59–234.21) | 46.12 (27.03–68.95) | 0.445 |
| Laboratory Parameters | Patients Without Inflammation (n = 9) | Patients with Inflammation (n = 32) | p-Value |
|---|---|---|---|
| Abs. neutrophil count (×103/µL) | 5.93 (5.5–6.46) | 4.82 (4.05–5.82) | 0.144 |
| Abs. lymphocyte count (×103/µL) | 1.56 (1.46–2.95) | 1.94 (1.65–2.73) | 0.362 |
| Neutrophil-to-lymphocyte ratio (NLR) | 3.05 (2.18–4.06) | 2.39 (1.75–3.13) | 0.384 |
| Abs. monocyte count (×103/µL) | 0.59 (0.49–0.76) | 0.63 (0.48–0.82) | 0.718 |
| Lymphocyte-to-monocyte ratio (LMR) | 3.04 (2.64–3.95) | 3.59 (2.74–4.12) | 0.362 |
| Systemic Inflammation Response Index (SIRI) | 1.87 (1.14–2.36) | 1.54 (1.09–1.85) | 0.362 |
| Creatinine (mg/dL) | 0.9 (0.88–1.12) | 0.98 (0.82–1.13) | 0.659 |
| High-sensitivity C-reactive protein (hs-CRP) (mg/L) | 1.01 (0.54–6.32) | 1.06 (0.55–5.89) | 0.936 |
| Monocyte chemoattractant protein-1 (MCP-1) (pg/mL) | 254.77 (204.95 –309.87) | 177.99 (151.30–231.97) | 0.01 |
| Matrix metalloproteinase-9 (MMP-9) (ng/mL) | 59.37 (47.89–86.89) | 71.02 (41.34–101.90) | 0.952 |
| Oxidized low-density lipoprotein (oxLDL) (ng/mL) | 51.73 (41.09–55.53) | 52.16 (33.61–66.5) | 0.887 |
| eGFR | COMPL− Group (n = 13) | COMPL+ Group (n = 28) | IPH− Group (n = 14) | IPH+ Group (n = 27) |
|---|---|---|---|---|
| C | 0 (0) | 9 (21.9) | 0 (0) | 9 (21.9) |
| B | 3 (7.3) | 11 (26.8) | 6 (14.6) | 8 (19.5) |
| A | 10 (24.4) | 8 (19.5) | 8 (19.5) | 10 (24.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmos, I.A.; Huțanu, A.; Muresan, A.V.; Nagy, E.E.; Brinzaniuc, K.; Molnár, G.B.; Szodorai, R.; Horváth, E. The Association of Histological Signs of Plaque Instability with Low eGFR, Higher Neutrophil-to-Lymphocyte Ratio, and Lower Serum MCP-1 Levels in Carotid Endarterectomy Patients—A Single-Center, Prospective Cohort Study. Life 2025, 15, 1008. https://doi.org/10.3390/life15071008
Balmos IA, Huțanu A, Muresan AV, Nagy EE, Brinzaniuc K, Molnár GB, Szodorai R, Horváth E. The Association of Histological Signs of Plaque Instability with Low eGFR, Higher Neutrophil-to-Lymphocyte Ratio, and Lower Serum MCP-1 Levels in Carotid Endarterectomy Patients—A Single-Center, Prospective Cohort Study. Life. 2025; 15(7):1008. https://doi.org/10.3390/life15071008
Chicago/Turabian StyleBalmos, Ioan Alexandru, Adina Huțanu, Adrian Vasile Muresan, Előd Ernő Nagy, Klara Brinzaniuc, Gyopár Beáta Molnár, Rita Szodorai, and Emőke Horváth. 2025. "The Association of Histological Signs of Plaque Instability with Low eGFR, Higher Neutrophil-to-Lymphocyte Ratio, and Lower Serum MCP-1 Levels in Carotid Endarterectomy Patients—A Single-Center, Prospective Cohort Study" Life 15, no. 7: 1008. https://doi.org/10.3390/life15071008
APA StyleBalmos, I. A., Huțanu, A., Muresan, A. V., Nagy, E. E., Brinzaniuc, K., Molnár, G. B., Szodorai, R., & Horváth, E. (2025). The Association of Histological Signs of Plaque Instability with Low eGFR, Higher Neutrophil-to-Lymphocyte Ratio, and Lower Serum MCP-1 Levels in Carotid Endarterectomy Patients—A Single-Center, Prospective Cohort Study. Life, 15(7), 1008. https://doi.org/10.3390/life15071008







