In Vitro Anti-Glioblastoma Activity of Echinocereus engelmannii- and Echinocereus pectinatus-Associated Bacterial Endophyte Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Isolation of Endophytic Microorganisms from E. engelmannii and E. pectinatus
2.3. Methanol Extracts Preparation and Fermentation of Endophytic Microorganisms from E. engelmannii and E. pectinatus
2.4. Cell Lines and Culture Conditions
2.5. Effect of E. engelmannii and E. pectinatus-Associated Endophytes on Tumor Cell Growth
2.6. Antioxidant Activity
2.7. Hemolytic Activity Test
2.8. Identification of Selected Strains
2.9. Characterization of Methanol Extracts of Selected Microorganisms
2.10. Statistical Analysis
3. Results
3.1. Isolation of Microorganisms and Extraction Yields
3.2. Effect of Extracts on Tumor Cell Growth
3.3. Antioxidant and Hemolytic Activities
3.4. Identification of Selected Microorganisms
3.5. Characterization of Methanol Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical Presentation, Diagnosis, and Management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- INEGI. Estadísticas de Defunciones Registradas (EDR). 2024. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2024/EDR/EDR2024_1erT.pdf (accessed on 6 February 2025).
- Wirsching, H.-G.; Galanis, E.; Weller, M. Glioblastoma. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 381–397. [Google Scholar]
- Yin, W.; Wang, J.; Jiang, L.; Kang, Y.J. Cancer and Stem Cells. Exp. Biol. Med. 2021, 246, 1791–1801. [Google Scholar] [CrossRef]
- Mittal, S.; Ali, J.; Baboota, S. Overcoming the Challenges in the Treatment of Glioblastoma via Nanocarrier- Based Drug Delivery Approach. Curr. Pharm. Des. 2021, 27, 4539–4556. [Google Scholar] [CrossRef]
- Aasland, D.; Götzinger, L.; Hauck, L.; Berte, N.; Meyer, J.; Effenberger, M.; Schneider, S.; Reuber, E.E.; Roos, W.P.; Tomicic, M.T.; et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR–CHK1, P21, and NF-ΚB. Cancer Res. 2019, 79, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Harlev, E.; Nevo, E.; Solowey, E.; Bishayee, A. Cancer Preventive and Curative Attributes of Plants of the Cactaceae Family: A Review. Planta Med. 2013, 79, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Pensamiento-Niño, C.A.; Campos-Montiel, R.G.; Añorve-Morga, J.; Ramírez-Moreno, E.; Ascacio-Valdés, J.A.; Hernández-Fuentes, A.D. Nutritional Characterization of the Functional and Antioxidant Activity of Cactus Flowers from Hidalgo, Mexico. Appl. Sci. 2021, 11, 5965. [Google Scholar] [CrossRef]
- Gezici, S.; Şekeroğlu, N. Current Perspectives in the Application of Medicinal Plants Against Cancer: Novel Therapeutic Agents. Anticancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef]
- Salazar, J.R.; Loza-Mejía, M.A.; Soto-Cabrera, D. Chemistry, Biological Activities and In Silico Bioprospection of Sterols and Triterpenes from Mexican Columnar Cactaceae. Molecules 2020, 25, 1649. [Google Scholar] [CrossRef]
- Vicente-Vicente, L.; Prieto, M.; Morales, A. Eficacia y Seguridad de La Quercetina Como Complemento Alimenticio. Rev. Toxicol. 2013, 30, 171–181. [Google Scholar]
- Ramírez-Villalobos, J.M.; Romo-Sáenz, C.I.; Morán-Santibañez, K.S.; Tamez-Guerra, P.; Quintanilla-Licea, R.; Orozco-Flores, A.A.; Romero-Arguelles, R.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro Tumor Cell Growth Inhibition Induced by Lophocereus marginatus (DC.) S. Arias and Terrazas Endophytic Fungi Extracts. Int. J. Environ. Res. Public Health 2021, 18, 9917. [Google Scholar] [CrossRef] [PubMed]
- Clark-Pérez, D.L.; Romo-Sáenz, C.I.; Ramírez-Villalobos, J.M.; Tamez-Guerra, P.; Caballero-Hernández, D.; Delgado-Miranda, A.L.; García, A.; Elizondo-Luevano, J.H.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro and In Vivo Antitumor Activity of Lophocereus marginatus (DC.) S. Arias & Terrazas Endophytic Aspergillus versicolor and Metarhizium anisopliae Extracts Against the Murine Lymphoma L5178Y-R. Microorganisms 2024, 12, 2310. [Google Scholar] [CrossRef]
- Romero-Arguelles, R.; Romo-Sáenz, C.I.; Morán-Santibáñez, K.; Tamez-Guerra, P.; Quintanilla-Licea, R.; Orozco-Flores, A.A.; Ramírez-Villalobos, J.M.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro Antitumor Activity of Endophytic and Rhizosphere Gram-Positive Bacteria from Ibervillea sonorae (S. Watson) Greene against L5178Y-R Lymphoma Cells. Int. J. Environ. Res. Public Health 2022, 19, 894. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Garza, N.E.; Gomez-Flores, R.; Quintanilla-Licea, R.; Elizondo-Luévano, J.H.; Romo-Sáenz, C.I.; Marín, M.; Sánchez-Montejo, J.; Muro, A.; Peláez, R.; López-Abán, J. In Vitro Anthelmintic Effect of Mexican Plant Extracts and Partitions Against Trichinella spiralis and Strongyloides venezuelensis. Plants 2024, 13, 3484. [Google Scholar] [CrossRef]
- Majc, B.; Novak, M.; Kopitar-Jerala, N.; Jewett, A.; Breznik, B. Immunotherapy of Glioblastoma: Current Strategies and Challenges in Tumor Model Development. Cells 2021, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular Targeted Therapy of Glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Jezierzański, M.; Nafalska, N.; Stopyra, M.; Furgoł, T.; Miciak, M.; Kabut, J.; Gisterek-Grocholska, I. Temozolomide (TMZ) in the Treatment of Glioblastoma Multiforme—A Literature Review and Clinical Outcomes. Curr. Oncol. 2024, 31, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Ashfaque, A.; Hanif, F.; Simjee, S.; Bari, M.; Faizi, S.; Zehra, S.; Mirza, T.; Begum, S.; Khan, L. Opuntiol Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells by Upregulating Active Caspase 3 Expression. Asian Pac. J. Cancer Prev. 2021, 22, 3607–3613. [Google Scholar] [CrossRef]
- Salazar, J.R.; Martínez-Vazquez, M.; Cespedes, C.L.; Ramírez-Apan, T.; Nieto-Camacho, A.; Rodríguez-Silverio, J.; Flores-Murrieta, F. Anti-Inflammatory and Cytotoxic Activities of Chichipegenin, Peniocerol, and Macdougallin Isolated from Myrtillocactus geometrizans (Mart. Ex Pfeiff.) Con. Z. Naturforschung C 2011, 66, 24–30. [Google Scholar] [CrossRef]
- Malishev, R.; Shaham-Niv, S.; Nandi, S.; Kolusheva, S.; Gazit, E.; Jelinek, R. Bacoside-A, an Indian Traditional-Medicine Substance, Inhibits β-Amyloid Cytotoxicity, Fibrillation, and Membrane Interactions. ACS Chem. Neurosci. 2017, 8, 884–891. [Google Scholar] [CrossRef]
- Nagpal, I.; Yuan, Z.M. The Basally Expressed P53-Mediated Homeostatic Function. Front. Cell Dev. Biol. 2021, 9, 775312. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of In-Vitro Bioassay Methods: Application in Herbal Drug Research. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar] [CrossRef]
- Mansour, K.A.; El-Neketi, M.; Lahloub, M.-F.; Elbermawi, A. Nanoemulsions of Jasminum Humile L. and Jasminum Grandiflorum L. Essential Oils: An Approach to Enhance Their Cytotoxic and Antiviral Effects. Molecules 2022, 27, 3639. [Google Scholar] [CrossRef] [PubMed]
- La Cruz-Jiménez, L.D.; Hernández-Torres, M.A.; Monroy-García, I.N.; Rivas-Morales, C.; Verde-Star, M.J.; Gonzalez-Villasana, V.; Viveros-Valdez, E. Biological Activities of Seven Medicinal Plants Used in Chiapas, Mexico. Plants 2022, 11, 1790. [Google Scholar] [CrossRef]
- Rodríguez-Garza, N.E.; Quintanilla-Licea, R.; Romo-Sáenz, C.I.; Elizondo-Luevano, J.H.; Tamez-Guerra, P.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro Biological Activity and Lymphoma Cell Growth Inhibition by Selected Mexican Medicinal Plants. Life 2023, 13, 958. [Google Scholar] [CrossRef]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; van der Lelie, D.; Dow, J.M. The Versatility and Adaptation of Bacteria from the Genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of Metabolic Activity, Beneficial and Pathogenic Aspects of Burkholderia spp. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Qian, Y.; Tan, B.; Li, K.; Hong, X.; Wong, C.C.; Fu, L.; Zhang, J.; Li, N.; Wu, J.-L. In-Depth Mapping Carboxylic Acid Metabolome Reveals the Potential Biomarkers in Colorectal Cancer through Characteristic Fragment Ions and Metabolic Flux. Anal. Chim. Acta 2020, 1128, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, N.A.; Ravi, A.; Joseph, B.J.; Jose, A.; Jithesh, O.; Krishnankutty, R.E. Phenazine 1-Carboxylic Acid Producing Seed Harbored Endophytic Bacteria from Cultivated Rice Variety of Kerala and Its Broad Range Antagonism to Diverse Plant Pathogens. Probiotics Antimicrob. Proteins 2023, 15, 516–523. [Google Scholar] [CrossRef]
- Medica, A.J.; Aitken, R.J.; Nicolson, G.L.; Sheridan, A.R.; Swegen, A.; De Iuliis, G.N.; Gibb, Z. Glycerophospholipids Protect Stallion Spermatozoa from Oxidative Damage in Vitro. Reprod. Fertil. 2021, 2, 199–209. [Google Scholar] [CrossRef]
- Jadoon, M.S.K.; Pelletier, J.; Sévigny, J.; Iqbal, J. Synthesis of New Class of Indole Acetic Acid Sulfonate Derivatives as Ectonucleotidases Inhibitors. RSC Adv. 2023, 13, 29496–29511. [Google Scholar] [CrossRef]
- Uvarani, C.; Sankaran, M.; Jaivel, N.; Chandraprakash, K.; Ata, A.; Mohan, P.S. Bioactive Dimeric Carbazole Alkaloids from Murraya koenigii. J. Nat. Prod. 2013, 76, 993–1000. [Google Scholar] [CrossRef]
- Grande, F.; Ioele, G.; Caruso, A.; Occhiuzzi, M.A.; El-Kashef, H.; Saturnino, C.; Sinicropi, M.S. Carbazoles: Role and Functions in Fighting Diabetes. Appl. Sci. 2022, 13, 349. [Google Scholar] [CrossRef]
- Paudel, B.; Maharjan, R.; Rajbhandari, P.; Aryal, N.; Aziz, S.; Bhattarai, K.; Baral, B.; Malla, R.; Bhattarai, H.D. Maculosin, a Non-Toxic Antioxidant Compound Isolated from Streptomyces sp. KTM18. Pharm. Biol. 2021, 59, 931–934. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Luo, Z.; Gao, L.; Li, R.; Zhang, Y.; Kalaji, H.M.; Qiang, S.; Chen, S. Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis. J. Fungi 2022, 8, 168. [Google Scholar] [CrossRef]
- Karanam, G.; Arumugam, M.K.; Natesh, N.S. Anticancer Effect of Marine Sponge-Associated Bacillus pumilus AMK1 Derived Dipeptide Cyclo (-Pro-Tyr) in Human Liver Cancer Cell Line Through Apoptosis and G2/M Phase Arrest. Int. J. Pept. Res. Ther. 2020, 26, 445–457. [Google Scholar] [CrossRef]
- Yan, P.-S.; Song, Y.; Sakuno, E.; Nakajima, H.; Nakagawa, H.; Yabe, K. Cyclo (l-leucyl-l-prolyl) Produced by Achromobacter xylosoxidans Inhibits Aflatoxin Production by Aspergillus parasiticus. Appl. Environ. Microbiol. 2004, 70, 7466–7473. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan Metabolism as a Common Therapeutic Target in Cancer, Neurodegeneration and Beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 131752725, N-(1-Deoxy-1-Fructosyl)Isoleucine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/N-_1-Deoxy-1-fructosyl_isoleucine (accessed on 14 December 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 6992261, Cyclo(L-Pro-L-Val). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/cyclo_L-Pro-L-Val (accessed on 14 December 2024).
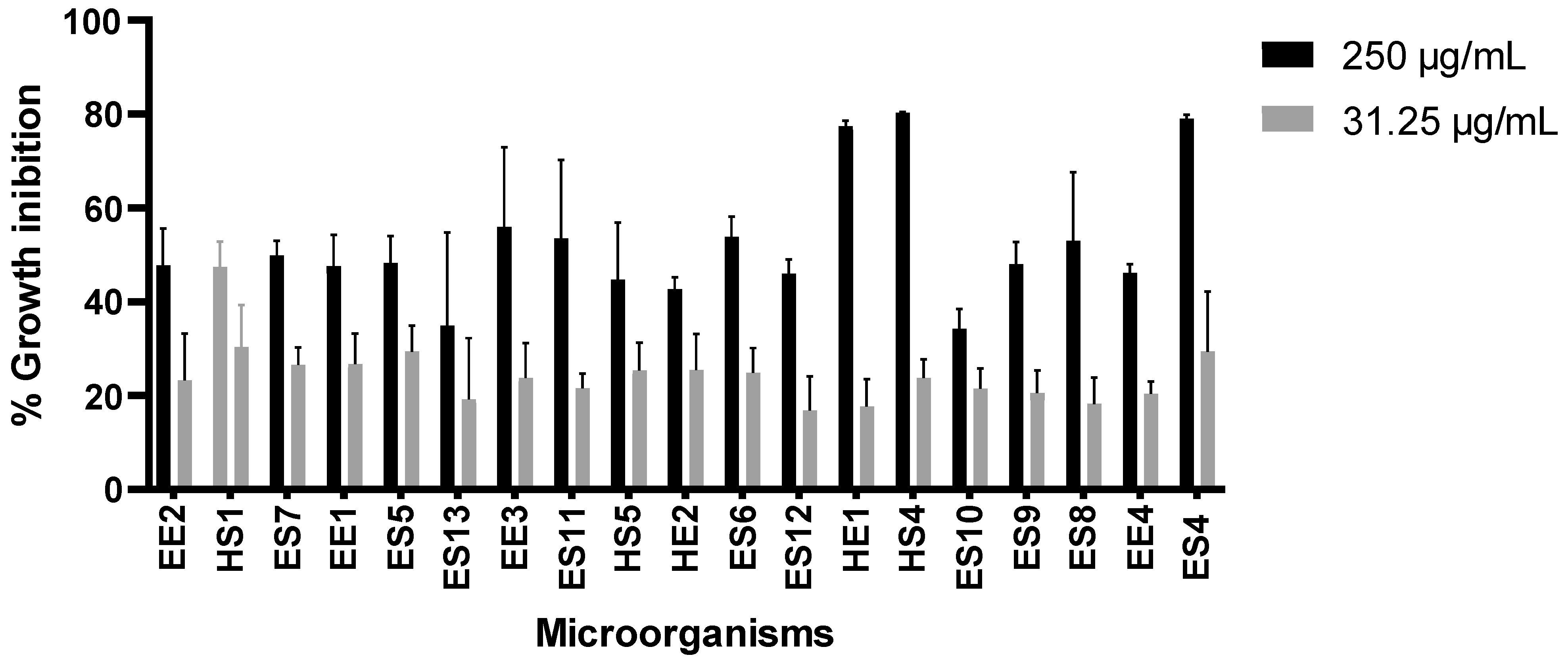
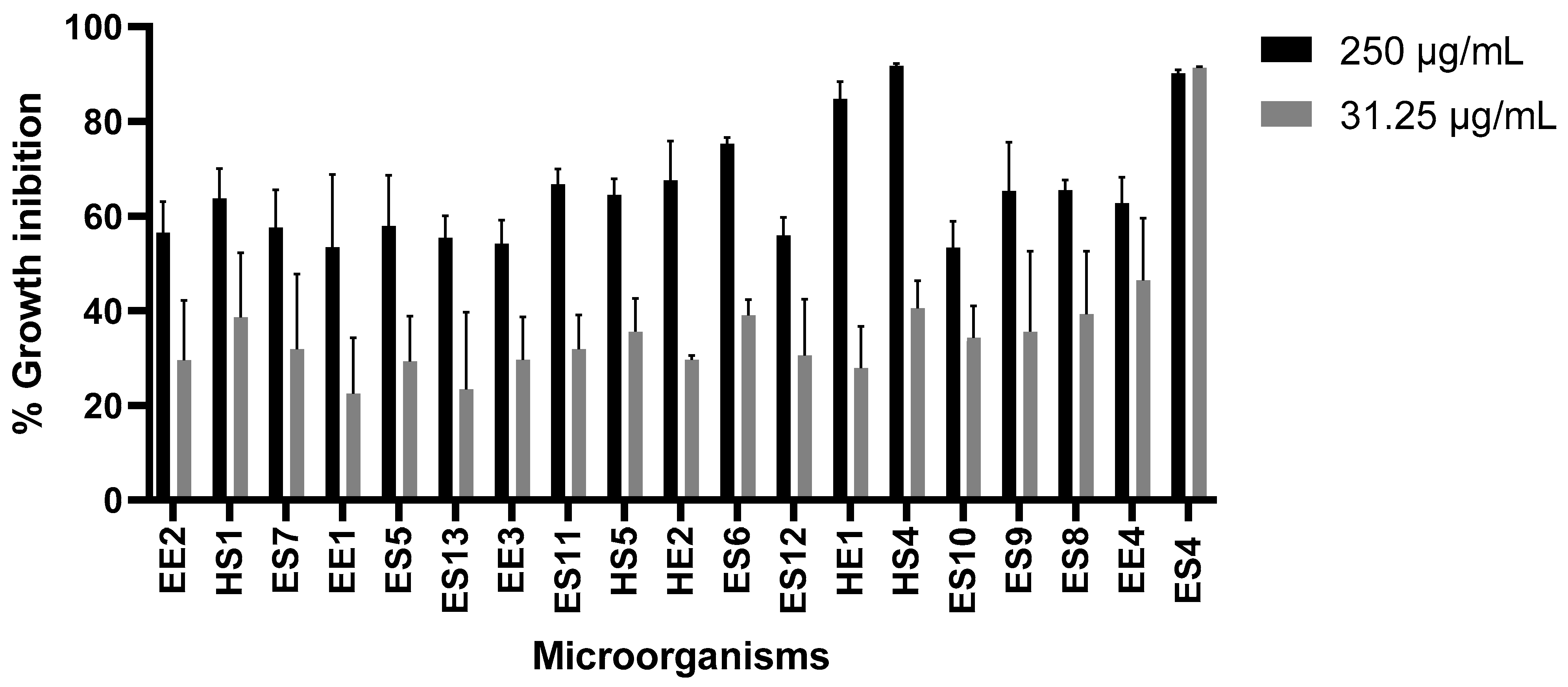
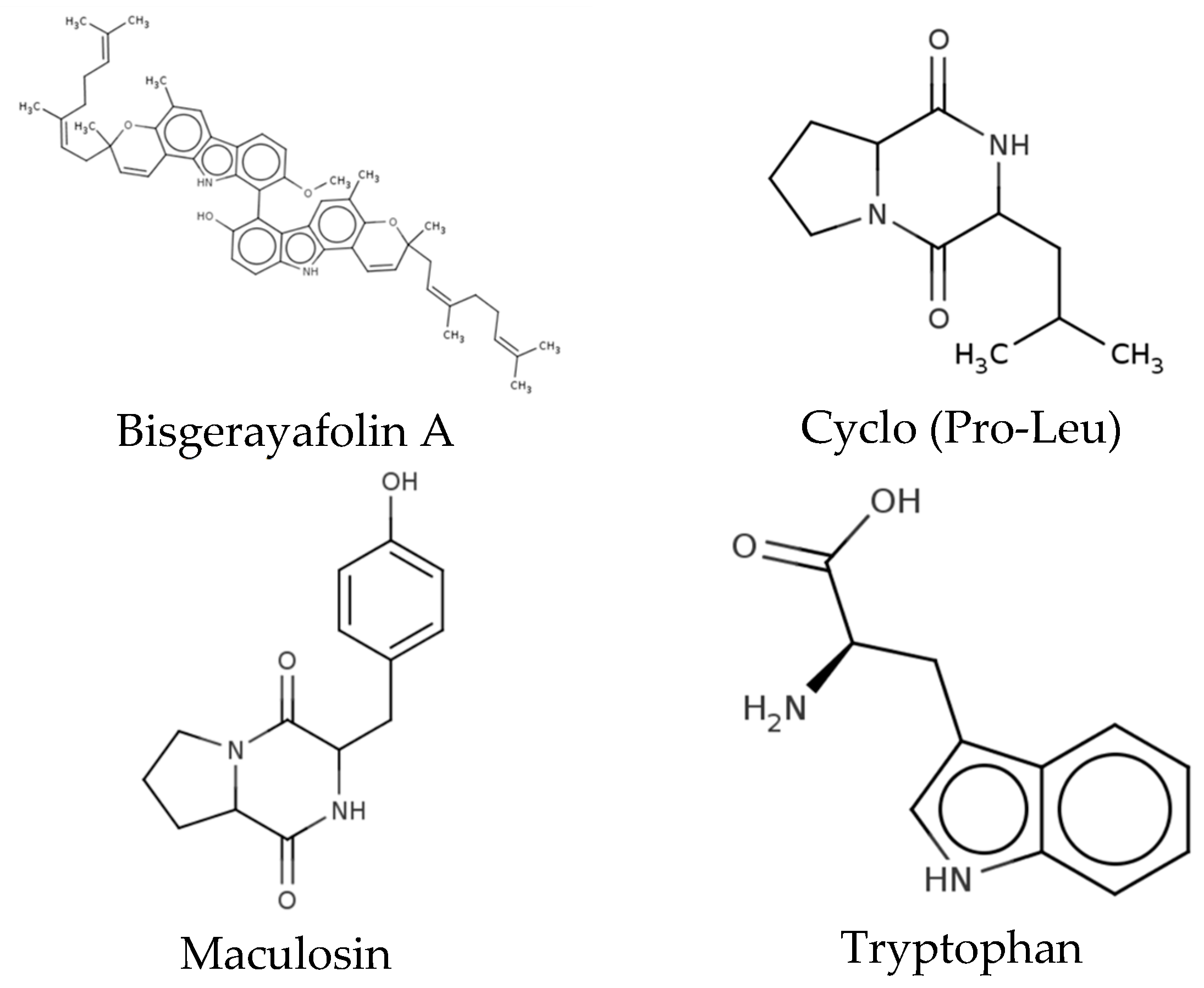
| Microorganism | Yield (%) |
|---|---|
| ES4 | 1.17 |
| ES5 | 0.79 |
| ES6 | 0.67 |
| ES7 | 6.30 |
| ES8 | 0.77 |
| ES9 | 2.73 |
| ES10 | 1.46 |
| ES11 | 0.90 |
| ES12 | 0.50 |
| ES13 | 0.56 |
| EE1 | 0.36 |
| EE2 | 1.34 |
| EE3 | 0.79 |
| EE4 | 2.70 |
| HE1 | 0.67 |
| HE2 | 0.57 |
| HS1 | 0.71 |
| HS4 | 3.63 |
| HS5 | 0.56 |
| Microorganism | IC50 (µg/mL) | SI | ||
|---|---|---|---|---|
| Schwan Healthy Cell Line | U87 Tumoral Cell Line | |||
| HS4 | 152.80 ± 2.20 | 87.66 ± 1.54 | 1.75 | |
| ES4 | 53.87 ± 1.73 | 45.11 ± 1.87 | 1.19 | |
| Microorganism | IC50 (µg/mL) | SI | |
|---|---|---|---|
| Schwan Healthy Cell Line | SH-S5Y5 Tumoral Cell Line | ||
| HS4 | 152.80 ± 2.20 | 117.90 ± 1.99 | 1.30 |
| ES4 | 53.87 ± 1.73 | 17.31 ± 1.73 | 3.11 |
| Microorganism | IC50 (μg/mL) | |
|---|---|---|
| DPPH | Hemolysis | |
| HS4 | >250 | >250 |
| ES4 | >250 | >250 |
| Microorganism | Strain | Score |
|---|---|---|
| HS4 | Stenotrophomonas maltophilia | 1.93 |
| ES4 | Stenotrophomonas maltophilia | 1.54 |
| No. | Compound | Experimental Mass | Theoretical Mass | Adduct | Mass Error (ppm) | Formula | RT | Chemical Class |
|---|---|---|---|---|---|---|---|---|
| 1 | N-Fructosyl isoleucine | 294.155 | 294.155 | [M + H]+ | 1.18 | C12H23NO7 | 1.3 | CAD |
| 2 | Pyroglutamylproline | 227.103 | 227.103 | [M + H]+ | 1.95 | C10H14N2O4 | 1.7 | CAD |
| 3 | Tryptophan | 205.096 | 205.097 | [M + H]+ | −5.25 | C11H12N2O2 | 3.2 | IND |
| 4 | Maculosin | 261.123 | 261.123 | [M + H]+ | −1.12 | C14H16N2O3 | 3.4 | CAD |
| 5 | Cyclo(Pro-Val) | 197.128 | 197.128 | [M + H]+ | −1.92 | C10H16N2O2 | 3.8 | CAD |
| 6 | Cyclo(Pro-Leu) | 211.144 | 211.144 | [M + H]+ | −0.13 | C11H18N2O2 | 8.8 | CAD |
| 7 | Phe-Pro | 245.129 | 245.129 | [M − H2O + H]+ | −0.01 | C14H18N2O3 | 10.9 | CAD |
| 8 | Lyso PE(16:0/0:0) | 454.293 | 454.293 | [M + H]+ | 0.57 | C21H44NO7P | 26.4 | GPL |
| 9 | Bisgerayafoline A | 815.486 | 815.478 | [M + H]+ | 9.62 | C55H62N2O4 | 28.8 | IND |
| 10 | PE(15:0/16:0) | 678.507 | 678.507 | [M + H]+ | 0.36 | C36H72NO8P | 28.8 | GPL |
| 11 | PE(16:0/14:0) | 664.492 | 664.491 | [M + H]+ | 1.35 | C35H70NO8P | 29 | GPL |
| 12 | PE(16:0/16:1) | 690.505 | 690.507 | [M + H]+ | −2.54 | C37H72NO8P | 29.8 | GPL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Miranda, A.L.; Gomez-Flores, R.; Rodríguez-Garza, N.E.; Pérez-González, O.; Tamez-Guerra, P.; Caballero-Hernández, D.; Clark-Pérez, D.L.; Quintanilla-Licea, R.; García, A.; Romo-Sáenz, C.I. In Vitro Anti-Glioblastoma Activity of Echinocereus engelmannii- and Echinocereus pectinatus-Associated Bacterial Endophyte Extracts. Life 2025, 15, 519. https://doi.org/10.3390/life15040519
Delgado-Miranda AL, Gomez-Flores R, Rodríguez-Garza NE, Pérez-González O, Tamez-Guerra P, Caballero-Hernández D, Clark-Pérez DL, Quintanilla-Licea R, García A, Romo-Sáenz CI. In Vitro Anti-Glioblastoma Activity of Echinocereus engelmannii- and Echinocereus pectinatus-Associated Bacterial Endophyte Extracts. Life. 2025; 15(4):519. https://doi.org/10.3390/life15040519
Chicago/Turabian StyleDelgado-Miranda, Ana L., Ricardo Gomez-Flores, Nancy E. Rodríguez-Garza, Orquídea Pérez-González, Patricia Tamez-Guerra, Diana Caballero-Hernández, Diana L. Clark-Pérez, Ramiro Quintanilla-Licea, Andrés García, and César I. Romo-Sáenz. 2025. "In Vitro Anti-Glioblastoma Activity of Echinocereus engelmannii- and Echinocereus pectinatus-Associated Bacterial Endophyte Extracts" Life 15, no. 4: 519. https://doi.org/10.3390/life15040519
APA StyleDelgado-Miranda, A. L., Gomez-Flores, R., Rodríguez-Garza, N. E., Pérez-González, O., Tamez-Guerra, P., Caballero-Hernández, D., Clark-Pérez, D. L., Quintanilla-Licea, R., García, A., & Romo-Sáenz, C. I. (2025). In Vitro Anti-Glioblastoma Activity of Echinocereus engelmannii- and Echinocereus pectinatus-Associated Bacterial Endophyte Extracts. Life, 15(4), 519. https://doi.org/10.3390/life15040519






