Abstract
Sustainably farmed Atlantic salmon could drive global food system solutions by contributing essential nutrients to the human diet while delivering high-quality protein. One of the biggest obstacles to sustainable salmon aquaculture in Chile is the prevalence of piscirickettsiosis disease caused by the Gram-negative bacteria Piscirickettsia salmonis and the excessive amount of antibiotics used to eradicate this disease. Farmed salmon products can be consumed without prior processing and therefore present a substantial risk for the transfer of resistant pathogens to humans. Antibiotics also carry the risk of antibiotic residues and damage to the environment. An alternative to antibiotics is the use of natural antimicrobials without the negative influence on the consumer’s microbiome. Here, we evaluate the potential antimicrobial activity against P. salmonis of the marine microalgae Microchloropsis gaditana. A non-genetically modified M. gaditana was grown with nitrogen deprivation to improve the synthesis of the eicosapentaenoic fatty acid (EPA). A spray-dried M. gaditana concentrate (Mg) was elaborated and given to Atlantic salmon for a period of 49 days, and serum and fillet samples were collected. Our results showed a significant increase in the nutritional quality improving the levels of EPA+ Docosapentaenoic acid (DPA) (23%) and Vitamin D3 (106%) of the fillets treated with Mg. Fish fed serum were challenged with P. salmonis, and serum antibacterial activity was measured. Sera from fish fed Mg-enriched diets showed a significant increase in antibacterial activity (85.68%) against P. salmonis. Our results indicate that Mg can be used as a viable alternative to address the critical problem of microbial resistance and to assure consumers that farm-raised Atlantic salmon is safe.
1. Introduction
Despite efforts to reduce the use of antimicrobials in salmon farming in Chile, its use increased by 18% in 2021 (463.4 tons) versus 2020 (379.6 tons). The total production of salmonids decreased by 8.35%. Of the total amount of antimicrobials used in 2021, 98.74% were administered in the marine stage (Patagonian fjords and channels), and 97.1% was Florfenicol used in Atlantic salmon (92.83%) to control piscirickettsiosis (93.17%) [1]. In 2023, this disease was responsible for 44.7% of infectious deaths in Atlantic salmon, being the most important infectious cause in this species [2]. The increased overuse of antibiotics suggests that measures used to reduce their use have failed. Antibiotic residues as well as antimicrobial-resistant bacteria from salmon production are spreading in the environment [3,4,5,6], and both wild organisms and salmon food commodities can become a source of resistant bacteria that can be transmitted to humans as foodborne contaminants [6,7,8,9]. There is thus a need for new and sustainable strategies. Antimicrobials originating from natural sources are not only safe but constitute a viable alternative to solve the problem of microbial resistance while meeting the requirement for healthy foods [10].
Piscirickettsia salmonis is the causative agent of SRS (Salmonid Rickettsial Septicemia) [11]. Salmon affected by P. salmonis respond inconsistently or poorly to treatment with Florfenicol [12,13,14,15], and several studies have reported that P. salmonis developed resistance to antibiotics [13,16] that can generate new antimicrobial resistance among bacteria, with negative effects on aquaculture and human health [17]. The evolution of resistance of P. salmonis to antibiotics has been demonstrated: A large-scale study evaluated the susceptibility profiles for Oxytetracycline and Florfenicol from 292 field isolates obtained from different farm sites over a five-year period. The results revealed resistance to Florfenicol and Oxytetracycline [18,19].
There is emerging evidence that adequate nutritional support can increase the ability of fish to resist infectious diseases by maintaining intestinal health and thus increasing immunity. Dietary components affect intestinal health through several pathways including intestinal barriers, digestive enzyme activity, oxidative status, and microbial diversity that are essential in controlling general fish immunity [20]. The essential fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have an important anti-inflammatory role in immune regulation during infections in Atlantic salmon [21,22]. The reported dietary requirement of the n-3 fatty acids EPA, alpha linolenic acid (ALA), and DHA of salmonids are 10–25 g/kg feed. Atlantic salmon fed low ratios of n-3/n-6 PUFAs (Polyunsaturated Fatty Acids) are more susceptible to infections [23]. Fish fed manufactured diets with an increasing content of plant-based products have shown metabolic disturbances with a low PUFA content in their muscles related to the composition of vegetable oils. The essential fatty acid requirements of freshwater fish can be met by adding linolenic acid (18:3n3) or linoleic acid (18:2n6), but EPA and DHA are regarded as essential fatty acids in marine fish due to the inability of all marine fish to convert 18:3n3 to EPA and DHA as well as 18:2n6 to arachidonic acid (ARA 20:4n6) [24].
The ability of Atlantic salmon to osmoregulate is directly related to the diet and is mediated through changes in tissue polar lipid fatty acid composition. Elevation of omega-3 PUFAs is an indicator of a pre-adapted fish for entry into seawater [22]. Changes in the composition of PUFAs have been shown to be involved in adaptation to seawater and have affected the production of prostaglandins that mediate the flow of electrolytes and fluids and operate synergistically with the prolactin hormone in the regulation of plasma Na+ and Ca+2 concentrations [22,25]. EPA and DHA deficiencies in the early life stages of fish impact their ability to deal with challenging conditions [26]. Non-GMO (genetically modified organism) terrestrial plant ingredients used for the formulation of fish diets affect the composition of deposited fatty acids by decreasing the concentration of EPA and DHA in turn reducing the resistance of fish to diseases and presenting physiological alterations that affect their performance. This process can also lead to lower levels of EPA and DHA in the final seafood; levels sometimes do not cover the minimum required levels of these fatty acids [27,28].
Microalgae in their natural ecosystem are producers of bioactive compounds with antimicrobial properties. Many marine algae derivatives are promising novel antimicrobial agents with multiple applications, including in the food and pharmaceutical industries [10]. Microalgae are producers of PUFAs that are subsequently consumed by other aquatic organisms and contribute to the healthy fatty acid profile in fish [28,29,30]. Microalgae are rich in oil and mineral content and can reproduce their biomass (1–3 per day). This is 50-fold higher than other terrestrial plants [31]. The microalgae Microchloropsis sp. is widely distributed in oceans worldwide [31] and is well known to produce PUFAs. It can naturally produce high levels of vitamin D3 [32]. Stress parameters (temperature, nutrients, light intensity) are known to improve the synthesis of PUFAs rich in EPA in Microchloropsis sp. [29,33], along with reasonable amounts of ARA and significant amounts of minerals. The mineral content of the biomass has been published: (mg/100 g) of magnesium (1490.1–3039.6), calcium (751.68–2338.8), potassium (190.74–1424.4), iron (91.68–214.32), zinc (23.4–52.68), manganese (11.52–75.12), copper (7.44–14.64), and nickel (3.96–4.68) [33]. Nitrogen limitations are considered the most efficient strategy to increase the content of triglycerides composed of fatty acids with a high degree of saturation in microalgae [34].
Fish do not synthesize vitamin D3 and are fully dependent on dietary sources to meet their requirements [35]. Calcium (Ca+2) concentrations in water regulate vitamin D3 in Atlantic salmon during the smoltification process and their migration from freshwater to seawater [36]. The values of vitamin D3 metabolites in plasma depend on the environmental concentrations of calcium present in both saltwater and freshwater. Lower environmental calcium concentrations induce higher conversion to a 1,25-dihydroxycholecalciferol-like compound but increased environmental calcium is associated with higher transformation to the compound 25,26-dihydroxycholecalciferol [36]. Vitamin D is known to regulate calcium homeostasis [37] and is a promising candidate to combat infections caused by bacteria (including multi-drug resistance). It exerts fundamental functions in innate immunity with broad antimicrobial effects. Vitamin D receptors are expressed on innate and adaptive immune cells. The 1,25(OH)2-vitamin D-active form of vitamin D has been shown to increase cathelicidin antimicrobial peptide gene expression in innate immune cells such as macrophages and monocytes [38,39]. The elimination of pathogens by cathelicidin may partially explain the antimicrobial properties of vitamin D [38].
The aim of this study was to evaluate the effect of two different levels of Microchloropsis gaditana (1.0 and 10.0%) with high levels of EPA as a functional anti-P. salmonis LF-89 additive to be used in salmonid diets as a sustainable nutritional strategy alternative to the use of antimicrobials—specifically Florfenicol—for the control of P. salmonis strains.
2. Methods
2.1. M. gaditana Growth Conditions, Concentrate, and Characterization
The M. gaditana strain (Lubián CCMP 527) was maintained in 250 mL flasks at 20 °C under constant illumination at 200 μE m−2 s−1 via a fluorescent lamp under UMA 5 nitrogen and phosphorous deprivation culture medium (NaNO3: 0.4 g/L, NaHCO3: 0.168 g/L, NaH2PO4: 0.034 g/L, and trace elements μmol/L, Mn 0.90, Zn 0.08, Co 0.050, Mo 0.030, and Fe 11.70, Centro de Bioinnovación, Antofagasta, Chile). The culture medium was autoclaved for 15 min at 121 °C and prepared using natural seawater with analytical-grade nutrients [34]. The M. gaditana strain was sub-cultured every ten days by adding 10% of the culture media to 90% of fresh culture medium. The lack of contamination was monitored by microscopic observations (Leica CME microscope 40×/0.65, Leica, Wetzlar, Germany). The culture was scaled to 1 L (under the same conditions) and then to an open raceway system (14.4 m3) under outdoor conditions. It was then grown in batch mode and maintained for 15 days. Cultures were hatched at 20 °C and initiated in a semi-continuous mode in which 75% of the culture medium and vitamin supplementation were deprived.
The M. gaditana was subsequently harvested (December 2018) at a dilution rate of 10% per day. Semi-continuous culture in outdoor conditions allowed to reach 2.6 g EPA/100 g biomass and volumetric productivity of 46–56 mg/L/day to be reached. Microalgal cells were harvested by continuous centrifugation (GEA Westfalia, model AS 1936076, Oelde, Germany) at a flow rate of 2 m3/h and a pressure of 3 bars. After centrifugation, the Mg was concentrated and dehydrated by spray drying (LPG-25 high-speed centrifugal spray dryer, Changzhou Yibu Drying Equipment Co., Ltd., Zengzhou, Henan, China) with a flow rate of 4 L/h, an initial inlet air temperature of 185 °C and a drying chamber temperature of 90 °C with a final temperature of 80 °C/15 s. Fatty acid profiles, minerals, and proximate analyses in Mg concentrate were determined using the official methods of analysis of the Association of Official Analytical Chemists (AOAC): crude fat [40,41]; fatty acid profile [42]; protein [43] using the calculation 5.25XN value; ash determination [44], moisture content [45]; and vitamin D3 [46] with a limit of quantification (LOQ) of 0.25 μg/100 g.
2.2. Experimental Diets and Fish
Three diets were manufactured into 3 mm diameter extruded pellets using a Clextral BC-21 (Firminy, France). These included a control diet and two diets with Mg inclusion (1 and 10%). After extrusion, pellets were dried at 60 °C for 12 h. In those with Mg supplementation, the Mg was dissolved in fish oil and added to the extruded basal feed. The oil was incorporated according to each diet formulation using a Dinnissen VC10 vacuum oiler (Sevenum, The Netherlands). Diets were prepared in batches of 10 kg. The formulations and approximate composition of the experimental diets are shown in Table 1.

Table 1.
Formulations, proximate, fatty acid, and vitamin D3 composition of the experimental diets.
Atlantic salmon (n = 225; 104 ± 1.29 g), each from AquaGen single family (SNAQ16LSSCO), were randomly distributed in nine tanks (three tanks per diet). Prior to the start of the trial, the fish (undifferentiated sex: in typical Atlantic salmon cycle schemes, sexual maturation is reached after a period of growth in the sea, generally 1 to 4 years) were acclimatized for 15 days. Fish were fed one of three diets for 49 days (March–May 2018) at Piscicultura Iculpe-Ilihue, Lago Ranco, Chile experimental station. The fish were maintained at 8.51 ± 0.14 dissolved oxygen concentration, pH 7.11 ± 0.04, 8.6 ± 1 °C, 24 h light photoperiod and were held in 200 L tanks with fresh water supply (flow-through system). The study was approved by the Bioethics and Biosafety Committee of the Faculty of Agronomic Sciences University of Chile Nº74-2018. This study was reported following the ARRIVE guidelines [47].
The fish were fed to satiation twice a day. Feed consumption, dissolved oxygen concentration, temperature, and pH were measured daily. Fish weight and length were recorded at the beginning and at the end of the trial. Indices of fish growth and feed utilization were calculated as follows:
- -
- Weight gain (%) = 100 [(W2 − W1)/W1]where W2 = final weight and W1 = initial weight of fish
- -
- Specific growth rate (SGR, %/day) =100 × (ln W2 − ln W1)/feeding days where ln is the natural logarithm, W1 = initial weight and W2 = final weight of fish.
- -
- Feed conversion ratio (FCR) = feed consumed/ biomass increase.
- -
- Survival rate (SR%) = (final fish number/initial fish number) × 100.
- -
- Feed intake (g/day/individual) = total feed intake/(days × number of fish)
- -
- The Fulton’s condition factor (K) of the experimental fish was calculated as: K = 100 W/L3 [48] where W = weight of the fish in grams and L = total length of the fish in centimeters.
2.3. Serum and Fish Fillet Sampling
For antibacterial activity, ten fish per tank for blood samples were taken after a 12 h fast on days 0 and 49. For blood extraction, to reduce movement the fish were placed on an ice gel pack for approximately 30 s, and a 2 mL sterile syringe was used to extract 1.0 mL of blood from the caudal vein, which was collected in a 2.0 mL cryo vial. The fish were returned to the tank for recovery, which took about 1 min. Serum was obtained from blood by low-speed centrifugation (3000 rpm for 10 min at 4 °C) within 1 h after sampling. Serum samples were immediately placed in liquid nitrogen and subsequently stored at −80 °C.
For fatty acid profiles, vitamin D3, ash, and proximate analyses, three fish per tank were randomly sampled on day 49. All animals were fasted for 12 h before sampling. The fish were anesthetized with MS-22 (80 mg/L/4 min, Argent Chemical Laboratories Inc., Redmond, WA, USA) [49] before being slaughtered by cervical dislocation according to Directive 210/63/EU [50]. Skin, liver, and gut were removed and the resulting fillets were lyophilized (FDT 8632 model freeze dryer) and stored for further analysis. All procedures including treatment, euthanasia, and handling were performed according to the guidelines of the University of Chile animal welfare committee. Fatty acid profiles, minerals, and proximate analyses in fish fillets were determined using the official methods of analysis of the AOAC: crude fat [51]; fatty acid profile (Chemists AOAC 2012); protein [43] using the calculation 6.25XN value; ash determination [52], moisture content [45]; and vitamin D3 [46] with a limit of quantification (LOQ) of 0.25 μg/100 g.
2.4. Anti-Piscirickettsia salmonis Assay
The strain P. salmonis ATCC VR-1361, equivalent to LF-89, was used from the American Type Culture Collection (ATCC, Manassas, VA, USA). The bacterial strain was confirmed as P. salmonis using an indirect fluorescent antibody test (IFAT, SRS-Bios, Santiago, Chile) according to the manufacturer’s recommendation. The strains were cultivated in the Austral-TSFe agar plates and incubated at 18 °C for 8 to 10 days. Stock cultures were prepared from cells scraped off TSFe plates and resuspended in L-15 Lebovitz medium (1 mL) with 20% of fetal bovine serum (Biological Industries Ltd., Israel Beit Haemek, Israel) and 10% of dimethyl sulfoxide (Sigma-Aldrich, ≥99.7%, St. Louis, MO, USA) and stored at −80 °C. Colonies grown on Austral-TSHem plates were used to prepare the inocula of P. salmonis in sterile Austral-SRS broth [53,54]. Purity controls were used in each of the methodological steps via Gram staining. The concentration of the initial inoculum was quantified by microscopic counting and microbiological seeding while recording the number of colony-forming units (CFU/mL) in Austral-TSHem plates [54]. The cultures were diluted to an optical density (OD) of 600 nm = 0.1 and returned to the exponential growth phase (OD 600 nm = 0.18–0.2) prior to use in anti-P. salmonis assays.
The bacteria (10 μL) in exponential growth culture and 50 μL of each serum were added into a 96-well plate (NEST®, Xuzhou, Jiangsu, China) in triplicate. Negative controls had 50 μL of serum and 10 μL of bovine fetal serum (Biological Industries, Israel Beit Haemek Ltd., Israel Beit Haemek, Israel). Positive controls had 10 μL of the bacteria in 50 μL of nutrient broth.
The anti-P. salmonis activity in fish fed serum was measured using an MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay based on the reduction of MTS tetrazolium salt to a red formazan product by dehydrogenase enzymes from live cells [55]. The assay was performed by adding 20 μL of the cellTiter 96®AQueous one solution reagent (Promega, Alexandria, NSW, Australia) directly to each culture well and incubating for 2 h, followed by recording the absorbance at 490 nm with a 96-well plate reader. The quantity of formazan product was measured via the amount of 490 nm absorbance with a TECAN INFINITE® 200 PRO spectrophotometer (Tecan Trading AG, Männedorf, Switzerland). The antibacterial activity of the sera (%) was calculated using the formula:
Antibacterial activity (%) = 100 − (treatment absorbance − negative control absorbance)/(positive control absorbance × 100).
2.5. Statistical Analysis
To test for differences between dietary treatments on P. salmonis antibacterial activity, data were subjected to a univariate analysis of variance. Fish growth performance indices and fatty acid retention data were subjected to a one-way analysis of variance (ANOVA) using IBM SPSS Statistics (version 25.0, IBM Corporation, Armonk, NY, USA). Multiple comparisons were made using Dunnett’s post hoc test when significant differences were identified between groups. Significant differences were considered when p < 0.05.
3. Results
3.1. Mg Concentrate Characterization
Nutritional characterization of the Mg concentrate showed high protein, ash, and calcium content (Table 2). Analysis of the fatty acid composition in Mg concentrate showed low levels of vitamin D3 and DHA. The EPA levels reached 26.73% of total fatty acids.

Table 2.
Chemical composition, vitamin D3, and fatty acid profile of Mg concentrate.
3.2. Fish Growth Performance Indices
No toxic or pathological signs were observed; experimental diets were received well by the animals. Consumption of the diet supplemented with Mg (10%) resulted in a significant increase in weight gain and the specific growth rate at the end of the 49-day feeding study. No significant differences were observed in the condition factor and feed conversion ratio (Table 3). No exclusions were made in the data analysis.

Table 3.
Growth performance indices of Salmon salar fed on Microchloropsis gaditana concentrate (Mg)-enriched diets for 49 days.
3.3. Salmon Fillets Fatty Acid Retention
The inclusion of Mg in the diet had a significant and positive effect on the retention efficiency of the anti-inflammatory fatty acids EPA (20:5n3) and DPA (22:5n3). The results showed increased levels in the n-3 family (EPA and DPA) and decreased levels of n-6 family fatty acids, including a decrease in the oleic acid pathway in fish fed on Mg-enriched diets (Table 4). The ARA/EPA ratio was significantly decreased (Figure 1).

Table 4.
Proximate, vitamin D3, and anti-inflammatory fatty acids composition of Salmo salar fillets fed on Microchloropsis gaditana concentrate (Mg)-enriched diets for 49 days, n = 3. * Significant difference from the control, p < 0.05, values are mean ± SD.
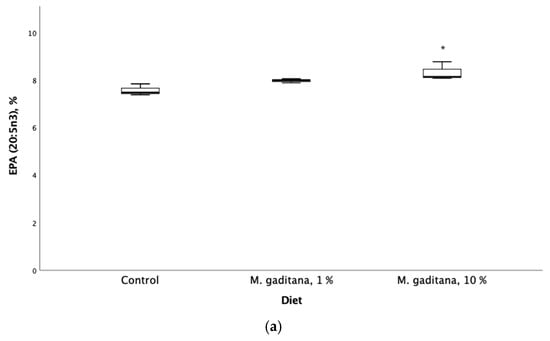
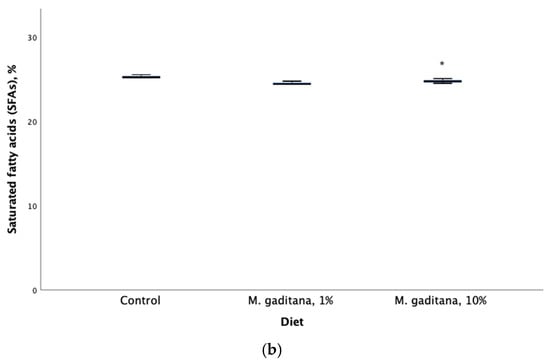
Figure 1.
Eicosapentaenoic acid (EPA) expressed as percent of total fatty acids (a) and saturated fatty acids (b) retention in Salmo salar fed on M. gaditana concentrate (Mg)-enriched diets for 49 days. * Significant difference from the control, p < 0.05, n = 3.
3.4. Effect of M. gaditana in Anti-P. salmonis Assay
After performing the ex vivo assay, we next completed a P. salmonis challenge protocol. Positive and negative controls showed the expected results. Bacterial counts detected average concentrations of 4.5 × 108 bacteria/mL and 4 × 105 CFU/mL. Serum from fish fed the Mg-enriched diets showed higher anti-P. salmonis activity (Table 5). Sera from S. salar fed the diet with a 10% Mg inclusion level had a significant increase in antibacterial activity (%) from the control diet at 49 days of feeding (Figure 2).

Table 5.
P. salmonis antibacterial activity in sera from S. salar fed experimental diets, values are mean ± SD, n = 10. * Significantly different from control (p < 0.05), values are mean ± SD.
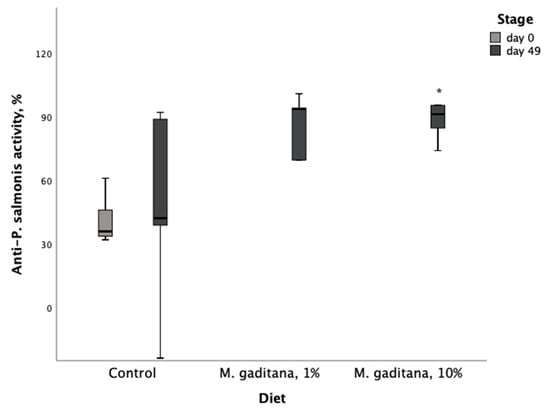
Figure 2.
Anti-P. salmonis (LF-89) activity (%) in serum of S. salar fed on M. gaditana (Mg)-enriched diets for 49 days. * Significant difference from the control, p < 0.05, n = 10.
4. Discussion
Our results showed that M. gaditana grown with nitrogen deprivation culture conditions is an important source of polyunsaturated anti-inflammatory fatty acid EPA (26.73%), sodium (5.40%), ash (21.26%), and calcium (4.11%). These results are consistent with EPA values reported for dehydrated samples by centrifugal spray-drying Nannochlorpsis oceanica (28.9%) [56] and ash content (24.47%) reported for Nannochloropsis oculata [31]. The N. gaditana can accumulate large amounts of EPA during nitrogen starvation. With controlled photoperiod conditions, EPA values of up to 37% have been reported for N. gaditana [33]. The resulting fish performance data from our study indicate that the inclusion of Mg-concentrate up to 10% in the diet of S. salar has no negative effect on performance parameters. These results agree with a previous study where the incorporation of 10% N. oculata in a commercial Atlantic salmon diet did not affect feed utilization and growth [57]. Another study achieved a 31% greater weight gain in Atlantic salmon when fed a diet containing 5% Schizochytrium sp. [58]. However, our fish performance data do not agree with a previous report where the use of N. oceanica with a 30% inclusion and a 60-day feeding generated significantly lower weight gains and a specific growth rate in Atlantic salmon [59]. This may be due to the inclusion percentage, differences in the nutritional content of the microalgae used in this study, or variations in microalgae processing during the preparation of the diets.
Our study showed a significant increase in the essential nutrient vitamin D3, which increased by 100% in fish fed Mg-concentrate relative to fish fed a control diet. This may be due to the presence of calcium (Ca+2) and its effects on plasma D-vitamin metabolites values, which have been reported to be dependent on ambient calcium concentrations in water in Atlantic salmon [36]. Previous studies have shown that N. oceanica can naturally produce high levels of vitamin D3 (up to 1 ± 0.3 μg/g DM) by exposure to ultraviolet-B light [32]. The potential role of vitamin D3 in inducing innate immunity and improving resistance against different pathogens is well-documented [60,61,62]. Vitamin D3 modulates the immune response of the infected host by promoting an autolysosome function [60], producing antimicrobial peptides, and inducing cell-specific receptors related to the elimination of pathogens. Vitamin D3 has a direct influence on macrophages and assists neutrophil motility and phagocytic function [61]. There is also clear evidence that D3 positively regulates the innate and adaptive immunity in fish [62].
Nannochloropsis sp. is a source of high-value essential n-3 long-chain PUFAs such as EPA and alpha linolenic acid [59,63]. Our results for ARA and EPA fatty acids are consistent with values previously reported when comparing Microchloropsis-treated fish with the control diet [59]. There was an increase in EPA content and a decrease in ARA content resulting in a significant decrease in the ARA/EPA ratio. Values of up to 35% lower in DHA (7.64 ± 0.53) and EPA (5.83 ± 0.85) levels have been reported for Chilean salmon fillets compared to those obtained in our study [64]. It has been reported that the fatty acid content of total lipid extract from the green microalgae Coelastrella sp. showed therapeutic potential against Gram-negative bacteria [65]. In addition, the bactericidal activity of C20 series fatty acid has shown increased potency with the increase in unsaturation, reaching maxima for EPA [66].
Compared with the fish fed a control diet, fish fed a 10% inclusion of Mg-concentrate diet had total saturated fatty acids (SFAs) that were significantly decreased (2%). Our results agree with a previous study on the muscle tissues of Nile tilapia (O. niloticus) fed diets supplemented with 0.75% of microalgae mix containing Nannochloropsis oculate, Schizochytrium, and Spirulina species in equal proportions (1:1:1). The study reported a decrease of 2.49% in SFAs, and showed that the DHA and EPA components of the microalgae mixture used have antibacterial properties against Aeromonas hydrophila [67].
Diets including 10% Mg-concentrate showed significant increases in antibacterial activity against P. salmonis relative to the control diet. Marine green microalgae antibacterial activity has been described previously against Staphylococcus sp., Vibrio angullarum, A. hydrophila, Lactobacillus sp., and Aeromonas salmonicida [68]. In addition, various studies have already shown that Chlorella sp., Tetraselmis sp., Navicula sp., Phaeodactylum tricornutum, Porphyridium cruentum, Microchloropsis gaditana, Dunaliella salina, Lobosphaera sp., and Schizochytrium sp. improve immunostimulatory capacities, resistance to infectious diseases, and tolerance to environmental stress in fish [69]. Phenolic compounds obtained from microalgae have shown inhibitory and antimicrobial effects on bacterial growth, depending on their structural properties and their amount in the environment. Studies conducted with N. oceania show that water-soluble polysaccharides produced by this organism can be used as antimicrobial agents [70] and that fatty acids present in microalgae N. oculata ably inhibit the growth of bacteria [71].
Our results indicate that M. gaditana grown under controlled conditions are suitable for sustainable nutrition against P. salmonis in Atlantic salmon due to the presence of essential nutrients that play important roles in the immune system of fish. DHA and EPA are important components of plasma membranes that are integral in controlling membrane-signaling pathways [72] and are precursors for anti-inflammatory lipid mediators [73,74] that modulate the duration and intensity of inflammatory responses in humans and fish [72]. Dietary fat is also linked to inflammation by promoting the translocation of gut microbiome metabolites from the gut into the bloodstream [73].
Lipopolysaccharide (LPS) is often described as an endotoxin and is an important virulence factor. It represents 75% of the total surface area of Gram-negative bacteria [75]. LPS is found in their outer membrane where their hydrophobic structures are composed of fatty acids. LPS plays a crucial role in bacteria–host interactions by modulating host immune system responses [73,75]. In salmon macrophage-like cells infected with P. salmonis LF-89, LPS has been identified to vary between stages of infection. In the vacuolization stage, proteins belonging to the “endotoxin” virulence factor family are abundant, suggesting the bacteria modifies or overproduce surface components, including LPS, allowing survival inside the host [76]. High-density lipoprotein-associated LPS favors its elimination through the liver and bile, thus preventing LPS-induced toxicity [75,77]. In the case of low high-density lipoprotein levels, most of the LPS is associated with very low-density lipoproteins with a lower neutralizing capacity [75].
The acute postprandial inflammatory response associated with fat consumption is mediated by endotoxins. These are mainly derived from the intestinal microflora [73]. In addition, gut dysbiosis induces gut permeability defects, leading to spontaneous endotoxemia [78]. In the case of P. salmonis, previous studies have apparently concluded that is an opportunistic environmental pathogen with low levels of pathogenicity and virulence and an endogenous pathobiont colonizing the salmonid microbiome [17]. SFAs induce inflammation in part by mimicking the actions of LPS. They are an essential structural component of bacterial endotoxins associated with the toxicity caused by these molecules. The substitution of SFAs with MUFAs (Monounsaturated fatty acids) or PUFAs eliminates the pro-inflammatory activity of LPS [73]. Macrophages are considered the main effectors in the defense against bacterial infection capable of responding to interactions with Gram-negative cells. Macrophages recognize these organisms in the absence of specific immune system components, and there are many effects of Gram-negative bacteria on macrophages that are mediated by bacterial lipopolysaccharides and their biologically active lipid fraction [79]. SFAs are also characterized by their role in inflammatory responses in macrophages and can induce inflammation, either extracellularly via Toll-like receptors or intracellularly via products of SFA metabolism [80]. Our results show a significant decrease in SFAs and a significant increase in PUFAs. Our results suggest a synergistic effect between EPA, vitamin D3, and the substitution of saturated fatty acids in anti-P. salmonis activity (Figure 3).
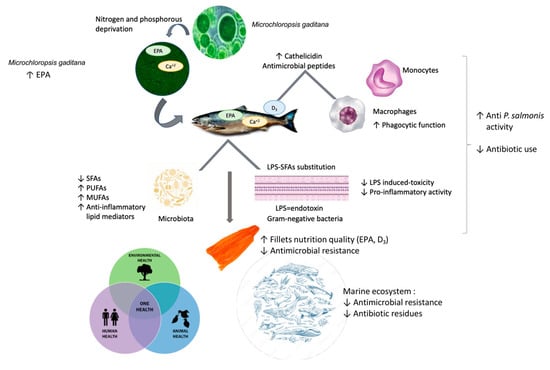
Figure 3.
Microchloropsis gaditana as a natural antimicrobial with a One Health approach to food safety in farmed salmon. Synergistic effect between EPA, vitamin D3, and the substitution of saturated fatty acids in the anti-P salmonis activity.
5. Conclusions
M. gaditana grown under controlled conditions can improve the synthesis of the anti-inflammatory eicosapentaenoic acid (EPA), which in turn can be used as a sustainable natural antimicrobial against P. salmonis. This approach confronts the issue of resistance to antibiotics, which impacts environmental and food safety risks. This natural therapy can reduce the cost of salmon meat production, mainly due to the reduction in the costs of smolts and antimicrobials, and can impact aquaculture sustainability as related to salmon farming. Our study provides evidence that M. gaditana is suitable for sustainable anti-P. salmonis feed additives for use in S. salar and a good candidate to test their efficacy in seawater challenge trials due to the presence of essential nutrients that play important roles in the fish immune system. M. gaditana provides new modalities to guarantee food safety and increase the nutritional quality of farmed Atlantic salmon.
Author Contributions
Conceptualization: N.D., I.L.-M. and C.R.; Funding acquisition, C.R., S.M. and N.D., Investigation, I.L.-M., A.M. and C.R.; Methodology, N.D., C.R., A.M. and I.L.-M.; Writing—Original draft, I.L.-M. and C.R.; Writing—Review and editing, I.L.-M., C.R. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministerio de Ciencias, Agencia Nacional de Investigación y Desarrollo, (Fondef ID14I20110).
Institutional Review Board Statement
The study was approved by the Bioethics and Biosafety Committee of the Faculty of Agronomic Sciences University of Chile Nº74-2018, approval date: 11 October 2018. This study was reported following the ARRIVE guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
The authors thank BioMar Chile-Jaime Carrasco for their support with the ingredients for the production of the experimental feeds, Aquagen Chile-Matias Medina for providing the experimental fish from a single family, and Cultivos Acuáticos Manantiales-Claudio Padilla for well-run fish feeding trials.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- SERNAPESCA. Informe Sobre Uso de Antimicrobianos en la Salmonicultura Nacional. Subdirección de Acuicultura, Departamento de Salud Animal. 2022. Available online: http://www.sernapesca.cl/sites/default/files/informe_sobre_uso_de_antimicrobianos_en_la_salmonicultura_nacional_ano_2021.pdf (accessed on 3 January 2025).
- SERNAPESCA. Informe con Antecedentes Sanitarios de Agua Dulce y Mar año, 1er Semestre 2023. 2023. Available online: https://www.sernapesca.cl/app/uploads/2023/12/Informe-Sanitario-1S-2023-Publicacion-002.pdf (accessed on 3 January 2025).
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Ivanova, L.; Shah, S.Q.A.; Sørum, H.; Tomova, A. Freshwater salmon aquaculture in Chile and transferable antimicrobial resistance. Environ. Microbiol. 2020, 22, 559–563. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Cabello, F.; Good, C.M.; Lunestad, B.T. Veterinary drug use in United States net pen Salmon aquaculture: Implications for drug use policy. Aquaculture 2020, 518, 734820. [Google Scholar] [CrossRef]
- Ramírez, C.; Gutiérrez, M.S.; Venegas, L.; Sapag, C.; Araya, C.; Caruffo, M.; López, P.; Reyes-Jara, A.; Toro, M.; González-Rocha, G.; et al. Microbiota composition and susceptibility to florfenicol and oxytetracycline of bacterial isolates from mussels (Mytilus spp.) reared on different years and distance from salmon farms. Environ. Res. 2022, 204, 112068. [Google Scholar] [CrossRef]
- Lozano, I.; Díaz, N.F.; Muñoz , S.; Riquelme, C. Antibiotics in Chilean Aquaculture: A Review. Antibiot. Use Anim. 2018, 3, 25–44. [Google Scholar] [CrossRef]
- Millanao, A.R.; Barrientos-Schaffeld, C.; Siegel-Tike, C.D.; Tomova, A.; Ivanova, L.; Godfrey, H.P.; Dölz, H.J.; Buschmann, A.H.; Cabello, F.C.; Millanao, A.R.; et al. Antimicrobial resistance in Chile and The One Health paradigm: Dealing with threats to human and veterinary health resulting from antimicrobial use in salmon aquaculture and the clinic. Rev. Chil. Infectol. 2018, 35, 299–308. [Google Scholar] [CrossRef]
- Lozano-Muñoz, I.; Wacyk, J.; Kretschmer, C.; Vásquez-Martínez, Y.; Cortez-San Martin, M. Antimicrobial resistance in Chilean marine-farmed salmon: Improving food safety through One Health. One Health 2021, 12, 100219. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Cvitanich, J.D.; Garate n, O.; Smith, C.E. The isolation of a rickettsia-like organism causing disease and mortality in Chilean salmonids and its confirmation by Koch’s postulate. J. Fish Dis. 1991, 14, 121–145. [Google Scholar] [CrossRef]
- Sandoval, R.; Oliver, C.; Valdivia, S.; Valenzuela, K.; Haro, R.E.; Sánchez, P.; Olavarría, V.H.; Valenzuela, P.; Avendaño-Herrera, R.; Romero, A. Resistance-nodulation-division efflux pump acrAB is modulated by florfenicol and contributes to drug resistance in the fish pathogen Piscirickettsia salmonis. FEMS Microbiol. Lett. 2016, 363, fnw102. [Google Scholar] [CrossRef]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: Risks, current concern, and future thinking. Environ. Sci. Pollut. Res. 2022, 29, 11054–11075. [Google Scholar] [CrossRef] [PubMed]
- Mardones, F.O.; Paredes, F.; Medina, M.; Tello, A.; Valdivia, V.; Ibarra, R.; Correa, J.; Gelcich, S. Identification of research gaps for highly infectious diseases in aquaculture: The case of the endemic Piscirickettsia salmonis in the Chilean salmon farming industry. Aquaculture 2018, 482, 211–220. [Google Scholar] [CrossRef]
- Contreras-Lynch, S.; Smith, P.; Olmos, P.; Loy, M.E.; Finnegan, W.; Miranda, C.D. A Novel and Validated Protocol for Performing MIC Tests to Determine the Susceptibility of Piscirickettsia salmonis Isolates to Florfenicol and Oxytetracycline. Front. Microbiol. 2017, 8, 1255. [Google Scholar] [CrossRef]
- Cartes, C.; Isla, A.; Lagos, F.; Castro, D.; Muñoz, M.; Yañez, A.; Haussmann, D.; Figueroa, J. Search and analysis of genes involved in antibiotic resistance in Chilean strains of Piscirickettsia salmonis. J. Fish Dis. 2016, 40, 1025–1039. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P. Salmon aquaculture, Piscirickettsia salmonis virulence, and one health: Dealing with harmful synergies between heavy antimicrobial use and piscine and human health. Aquaculture 2019, 507, 451–456. [Google Scholar] [CrossRef]
- Saavedra, J.; Hernandez, N.; Osses, A.; Castillo, A.; Cancino, A.; Grothusen, H.; Navas, E.; Henriquez, P.; Bohle, H.; Bustamante, F.; et al. Prevalence, geographic distribution and phenotypic differences of Piscirickettsia salmonis EM-90-like isolates. J. Fish Dis. 2017, 40, 1055–1063. [Google Scholar] [CrossRef]
- Henríquez, P.; Kaiser, M.; Bohle, H.; Bustos, P.; Mancilla, M. Comprehensive antibiotic susceptibility profiling of Chilean Piscirickettsia salmonis field isolates. J. Fish Dis. 2016, 39, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Arnemo, M.; Kavaliauskis, A.; Andresen, A.M.S.; Bou, M.; Berge, G.M.; Ruyter, B.; Gjøen, T. Effects of dietary n-3 fatty acids on Toll-like receptor activation in primary leucocytes from Atlantic salmon (Salmo salar). Fish Physiol. Biochem. 2017, 43, 1065–1080. [Google Scholar] [CrossRef]
- Miao, L.H.; Remø, S.C.; Espe, M.; Philip, A.J.P.; Hamre, K.; Fjelldal, P.G.; Skjærven, K.; Holen, E.; Vikeså, V.; Sissener, N.H. Dietary plant oil supplemented with arachidonic acid and eicosapentaenoic acid affects the fatty acid composition and eicosanoid metabolism of Atlantic salmon (Salmo salar L.) during smoltification. Fish Shellfish Immunol. 2022, 123, 194–206. [Google Scholar] [CrossRef]
- Andresen, A.M.S.; Lutfi, E.; Ruyter, B.; Berge, G.; Gjøen, T. Interaction between dietary fatty acids and genotype on immune response in Atlantic salmon (Salmo salar) after vaccination: A transcriptome study. PLoS ONE 2019, 14, e0219625. [Google Scholar] [CrossRef] [PubMed]
- Roques, S.; Deborde, C.; Richard, N.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Metabolomics and fish nutrition: A review in the context of sustainable feed development. Rev. Aquac. 2020, 12, 261–282. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bell, J.G.; Dick, J.R.; Henderson, R.J.; McGhee, F.; Michell, D.; Morris, P.C. Polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation and the effects of dietary linseed and rapeseed oils. Fish Physiol. Biochem. 2000, 23, 59–73. [Google Scholar] [CrossRef]
- Bou, M.; Berge, G.M.; Baeverfjord, G.; Sigholt, T.; Østbye, T.-K.; Romarheim, O.H.; Hatlen, B.; Leeuwis, R.; Venegas, C.; Ruyter, B. Requirements of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L.): Effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. Br. J. Nutr. 2017, 117, 30–47. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef]
- Sissener, N.H. Are we what we eat? Changes to the feed fatty acid composition of farmed salmon and its effects through the food chain. J. Exp. Biol. 2018, 221, jeb161521. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Derwenskus, F.; Gille, A.; Louis, S.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Bioavailability and safety of nutrients from the microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 mice. Nutrients 2018, 10, 965. [Google Scholar] [CrossRef]
- Lozano-Muñoz, I.; Muñoz, S.; Díaz, N.F.; Medina, A.; Bazaes, J.; Riquelme, C. Nutritional Enhancement of Farmed Salmon Meat for Human Healthvia Non-GMO Nannochloropsis gaditana: Eicosapentaenoic Acid (EPA, 20:5n-3), Docosapentaenoic Acid (DPA, 22:5n-3) and Vitamin D3. Molecules 2020, 25, 4615. [Google Scholar] [CrossRef]
- Sukarni; Sudjito; Hamidi, N.; Yanuhar, U.; Wardana, I.N.G. Potential and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. Int. J. Energy Environ. Eng. 2014, 5, 279–290. [Google Scholar] [CrossRef]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef]
- Mitra, M.; Patidar, S.K.; George, B.; Shah, F.; Mishra, S. A euryhaline Nannochloropsis gaditana with potential for nutraceutical (EPA) and biodiesel production. Algal Res. 2015, 8, 161–167. [Google Scholar] [CrossRef]
- Riveros, K.; Sepulveda, C.; Bazaes, J.; Marticorena, P.; Riquelme, C.; Acién, G. Overall development of a bioprocess for the outdoor production of Nannochloropsis gaditana for aquaculture. Aquac. Res. 2018, 49, 165–176. [Google Scholar] [CrossRef]
- Lock, E.-J.; Waagbø, R.; Bonga, S.W.; Flik, G. The significance of vitamin D for fish: A review. Aquac. Nutr. 2010, 16, 100–116. [Google Scholar] [CrossRef]
- Fernández, I.; Gavaia, P.; Darias, M.J.; Gisbert, E. Fat-Soluble Vitamins in Fish: A Transcriptional Tissue-Specific Crosstalk that Remains to be Unveiled and Characterized. In Emerging Issues in Fish Larvae Research; Yúfera, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 159–208. ISBN 978-3-319-73244-2. [Google Scholar]
- Korf, H.; Decallonne, B.; Mathieu, C. Vitamin D for infections. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Gombart, A. The Antibiotic Effects of Vitamin D. Endocr. Metab. Immune Disord.-Drug Targets 2014, 14, 255–266. [Google Scholar] [CrossRef]
- Golpour, A.; Bereswill, S.; Heimesaat, M.M. Antimicrobial and immune-modulatory effects of vitamin D provide promising antibiotics-independent approaches to tackle bacterial infections—Lessons learnt from a literature survey. Eur. J. Microbiol. Immunol. 2019, 9, 80–87. [Google Scholar] [CrossRef]
- Analysis of Official Analytical Chemists International. AOAC Official Method 954.02 Acid Hydrolysis, Baked Goods & Pet Food. 2006. Available online: https://www.aoac.org (accessed on 3 January 2025).
- Analysis of Official Analytical Chemists International. AOAC Official Method 920.39 Crude Fat in Animal Feed. 2000. Available online: https://www.aoac.org (accessed on 3 January 2025).
- Analysis of Official Analytical Chemists International. AOAC Official Method 996.06 Analysis of Methyl esters by Capillary GLC. 2012. Available online: https://www.aoac.org (accessed on 3 January 2025).
- Analysis of Official Analytical Chemists International. Protein (crude) in animal feed and pet food 984.13. Off. Methods Anal. Off. Anal. Chem. Int. 1995, 1, 30–31. [Google Scholar]
- Analysis of Official Analytical Chemists International. AOAC Official Method 942.05 Determinaton of Ash in Animal feed. 2012, 1392–1397. Available online: https://www.aoac.org (accessed on 3 January 2025).
- Analysis of Official Analytical Chemists International. AOAC Official Method 934.01 Loss on Drying (Moisture) at 95–100 °C for Feeds 2000. Available online: https://www.aoac.org (accessed on 3 January 2025).
- EN 12821 EN 12821:2009; Foodstuffs—Determination of Vitamin D by High Performance Liquid Chromatography. Measurement of Cholecalciferol (D3) or Ergocalciferol (D2). National Standards Authority of Ireland: Dublin, Ireland, 2009.
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Reid, D.P.; Szanto, A.; Glebe, B.; Danzmann, R.G.; Ferguson, M.M. QTL for body weight and condition factor in Atlantic salmon (Salmo salar): Comparative analysis with rainbow trout (Oncorhynchus mykiss) and Arctic charr (Salvelinus alpinus). Heredity 2005, 94, 166–172. [Google Scholar] [CrossRef]
- Topic Popovic, N.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Persin Berakovic, A.; Sauerborn Klobucar, R. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef]
- Directiva 210/63. Directiva 2010/63/UE del parlamento europeo y del consejo de 22 de septiembre de 2010 relativa a la protección de los animales utilizados para fines científicos (Texto pertinente a efectos del EEE). D. Of. Comunidades Eur. 2010, 20, 33–79.
- AOAC. AOAC Official Method 948.15, Fat (crude) in Seafood. Acid Hydrolysis Method 1948. Available online: https://www.aoac.org (accessed on 3 January 2025).
- AOAC. AOAC Official Method 938.08, Ash of Seafood 1938. Available online: https://www.aoac.org (accessed on 3 January 2025).
- Yañez, A.; Valenzuela, K.; Silva, H.; Retamales, J.; Romero, A.; Enriquez, R.; Figueroa, J.; Claude, A.; Gonzalez, J.; Avendaño-Herrera, R.; et al. Broth medium for the successful culture of the fish pathogen Piscirickettsia salmonis. Dis. Aquat. Organ. 2012, 97, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Yañez, A.J.; Silva, H.; Valenzuela, K.; Pontigo, J.P.; Godoy, M.; Troncoso, J.; Romero, A.; Figueroa, J.; Carcamo, J.G.; Avendaño-Herrera, R. Two novel blood-free solid media for the culture of the salmonid pathogen Piscirickettsia salmonis. J Fish Dis 2013, 36, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.T.; Speck, P.; Doroudi, M.; Smith, B.; Benkendorff, K. Variation in the antiviral and antibacterial activity of abalone Haliotis laevigata, H. rubra and their hybrid in South Australia. Aquaculture 2011, 315, 242–249. [Google Scholar] [CrossRef]
- Alves, S.P.; Mendonça, S.H.; Silva, J.L.; Bessa, R.J.B. Nannochloropsis oceanica, a novel natural source of rumen-protected eicosapentaenoic acid (EPA) for ruminants. Sci. Rep. 2018, 8, 10269. [Google Scholar] [CrossRef]
- Gong, Y.; Sørensen, S.L.; Dahle, D.; Nadanasabesan, N.; Dias, J.; Valente, L.M.P.; Sørensen, M.; Kiron, V. Approaches to improve utilization of Nannochloropsis oceanica in plant-based feeds for Atlantic salmon. Aquaculture 2020, 522, 735122. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef]
- Sørensen, S.L.; Ghirmay, A.; Gong, Y.; Dahle, D.; Vasanth, G.; Sørensen, M.; Kiron, V. Growth, Chemical Composition, Histology and Antioxidant Genes of Atlantic Salmon (Salmo salar) Fed Whole or Pre-Processed Nannochloropsis oceanica and Tetraselmis sp. Fishes 2021, 6, 23. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy 2019, 15, 707–725. [Google Scholar] [CrossRef]
- Almoudi, M.M.M.; Hussein, A.S.; Abu Hassan, M.I.; Al Talib, H.; Khan, H.B.S.G.; Nazli, S.A.B.; Effandy, N.A.E.B. The antibacterial effects of vitamin D3 against mutans streptococci: An in vitro study. Eur. Oral Res. 2021, 55, 8–15. [Google Scholar] [CrossRef]
- Liu, J.; Shao, R.; Lan, Y.; Liao, X.; Zhang, J.; Mai, K.; Ai, Q.; Wan, M. Vitamin D3 protects turbot (Scophthalmus maximus L.) from bacterial infection. Fish Shellfish Immunol. 2021, 118, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega- 3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Bastías, J.M.; Balladares, P.; Acuña, S.; Quevedo, R.; Muñoz, O. Determining the effect of different cooking methods on the nutritional composition of salmon (Salmo salar) and chilean jack mackerel (Trachurus murphyi) fillets. PLoS ONE 2017, 12, e0180993. [Google Scholar] [CrossRef] [PubMed]
- Toshkova-Yotova, T.; Georgieva, A.; Iliev, I.; Alexandrov, S.; Ivanova, A.; Pilarski, P.; Toshkova, R. Antitumor and antimicrobial activity of fatty acids from green microalga Coelastrella sp. BGV. S. Afr. J. Bot. 2022, 151, 394–402. [Google Scholar] [CrossRef]
- Domb, A.J.; Kunduru, K.R.; Farah, S. Antimicrobial Materials for Biomedical Applications; Royal Society of Chemistry: London, UK, 2019; ISBN 978-1-78801-854-8. [Google Scholar]
- Ibrahim, D.; Abd El-Hamid, M.I.; Al-Zaban, M.I.; ElHady, M.; El-Azzouny, M.M.; ElFeky, T.M.; Al Sadik, G.M.; Samy, O.M.; Hamed, T.A.; Albalwe, F.M.; et al. Impacts of Fortifying Nile Tilapia (Oreochromis niloticus) Diet with Different Strains of Microalgae on Its Performance, Fillet Quality and Disease Resistance to Aeromonas hydrophila Considering the Interplay between Antioxidant and Inflammatory Response. Antioxidants 2022, 11, 2181. [Google Scholar] [CrossRef]
- Guzmán, F.; Wong, G.; Román, T.; Cárdenas, C.; Alvárez, C.; Schmitt, P.; Albericio, F.; Rojas, V. Identification of Antimicrobial Peptides from the Microalgae Tetraselmis suecica (Kylin) Butcher and Bactericidal Activity Improvement. Mar. Drugs 2019, 17, 453. [Google Scholar] [CrossRef]
- Bahi, A.; Ramos-Vega, A.; Angulo, C.; Monreal-Escalante, E.; Guardiola, F.A. Microalgae with immunomodulatory effects on fish. Rev. Aquac. 2023, 15, 1522–1539. [Google Scholar] [CrossRef]
- Tülay Çağatay, I.; Özbaş, M.; Yilmaz, H.E.; Ali, N. Determination of Antibacterial Effect of Nannochloropsis oculata Against Some Rainbow Trout Pathogens. Nat. Eng. Sci. 2021, 6, 87–95. [Google Scholar] [CrossRef]
- Putra, Y.; Mustikasari, I.; Pangestuti, R.; Rahmadi, P.; Siahaan, E.A. Fatty acid profiles and biological activity of Nannochloropsis oculata and Isochrysis galbana, clone t-ISO. IOP Conf. Ser. Earth Environ. Sci. 2022, 1083, 012079. [Google Scholar] [CrossRef]
- Martinez-Rubio, L.; Morais, S.; Evensen, Ø.; Wadsworth, S.; Vecino, J.G.; Ruohonen, K.; Bell, J.G.; Tocher, D.R. Effect of functional feeds on fatty acid and eicosanoid metabolism in liver and head kidney of Atlantic salmon (Salmo salar L.) with experimentally induced Heart and Skeletal Muscle Inflammation. Fish Shellfish Immunol. 2013, 34, 1533–1545. [Google Scholar] [CrossRef]
- Fritsche, K.L. The Science of Fatty Acids and Inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T.R.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Nierenberg, A.A.; Felger, J.C.; Maddipati, K.R.; et al. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2021, 164, 102219. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Kopra, E.; Pietiäinen, M.; Lehto, M.; Zaric, S.; Paju, S.; Salminen, A. Periodontitis and cardiometabolic disorders: The role of lipopolysaccharide and endotoxemia. Periodontology 2000 2022, 89, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Severín, J.; Travisany, D.; Maass, A.; Cambiazo, V.; Chávez, F.P. Global Proteomic Profiling of Piscirickettsia salmonis and Salmon Macrophage-Like Cells during Intracellular Infection. Microorganisms 2020, 8, 1845. [Google Scholar] [CrossRef] [PubMed]
- Marín-Palma, D.; Taborda, N.A.; Urcuqui-Inchima, S.; Hernandez, J.C. Inflamación y respuesta inmune innata: Participación de las lipoproteínas de alta densidad. Iatreia 2017, 30, 423–435. [Google Scholar]
- Panpetch, W.; Sawaswong, V.; Chanchaem, P.; Ondee, T.; Dang, C.P.; Payungporn, S.; Tumwasorn, S.; Leelahavanichkul, A. Candida Administration Worsens Cecal Ligation and Puncture-Induced Sepsis in Obese Mice Through Gut Dysbiosis Enhanced Systemic Inflammation, Impact of Pathogen-Associated Molecules From Gut Translocation and Saturated Fatty Acid. Front. Immunol. 2020, 11, 561652. [Google Scholar]
- Keller, R.; Fischer, W.; Keist, R.; Bassetti, S. Macrophage response to bacteria: Induction of marked secretory and cellular activities by lipoteichoic acids. Infect. Immun. 1992, 60, 3664–3672. [Google Scholar] [CrossRef]
- Namgaladze, D.; Brüne, B. Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 1796–1807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).