Diabetes Differentially Affects Vascular Reactivity in Isolated Human Arterial and Venous Bypass Grafts
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Declaration
2.2. Patient Population
2.3. Chemicals
2.4. Statistical Analysis
3. Results
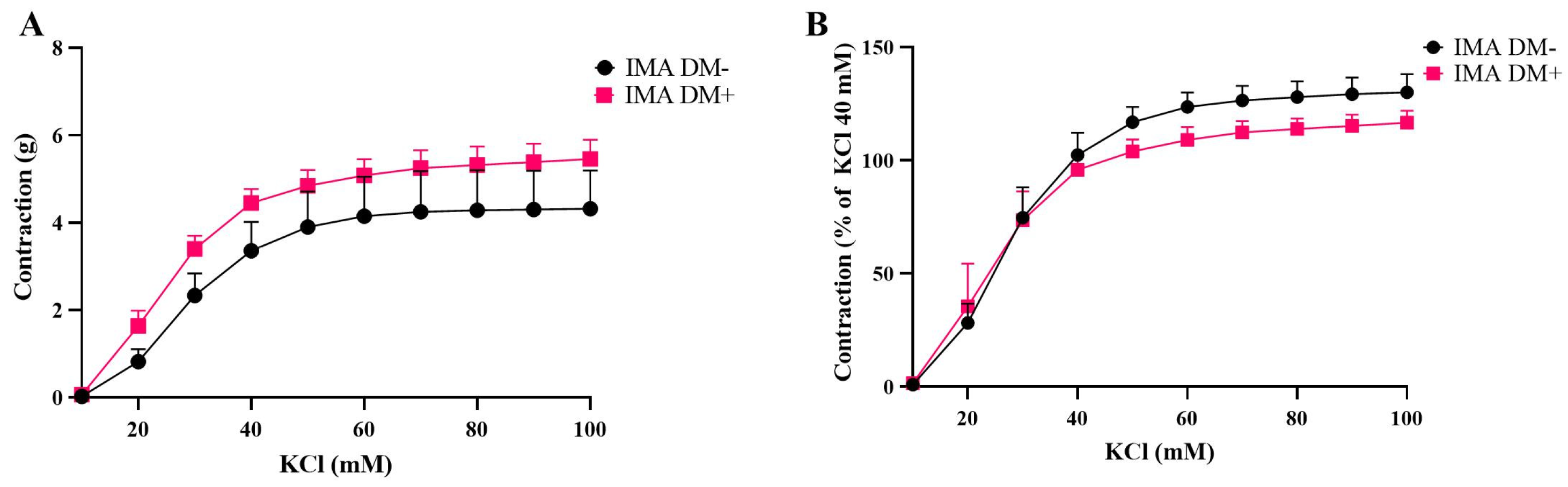
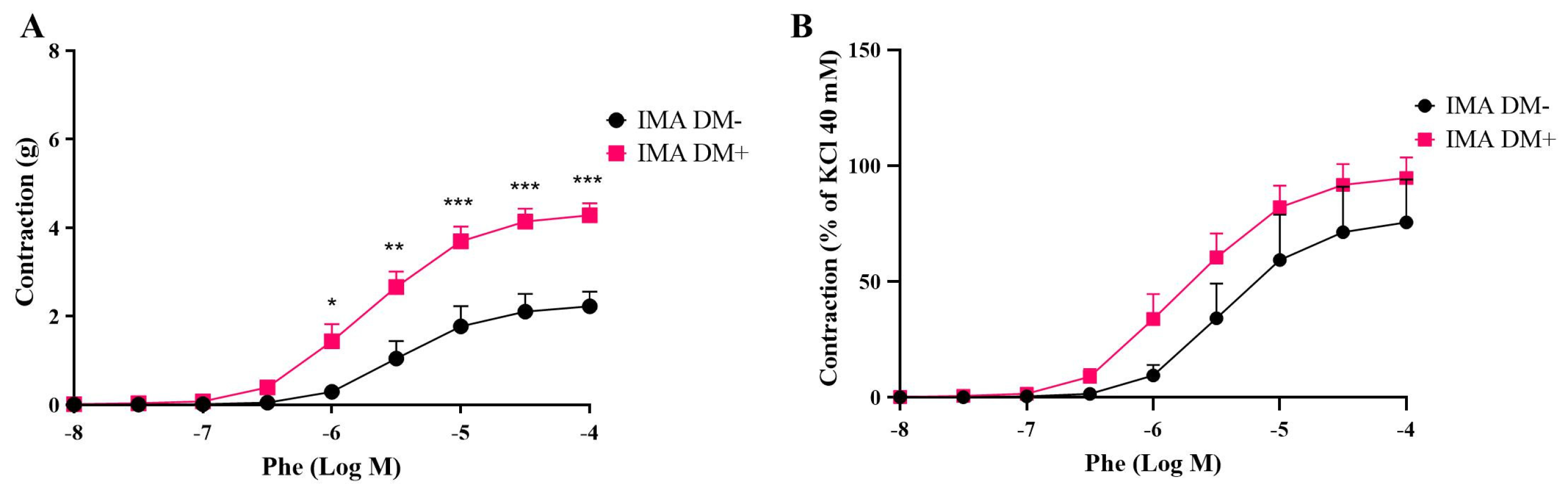
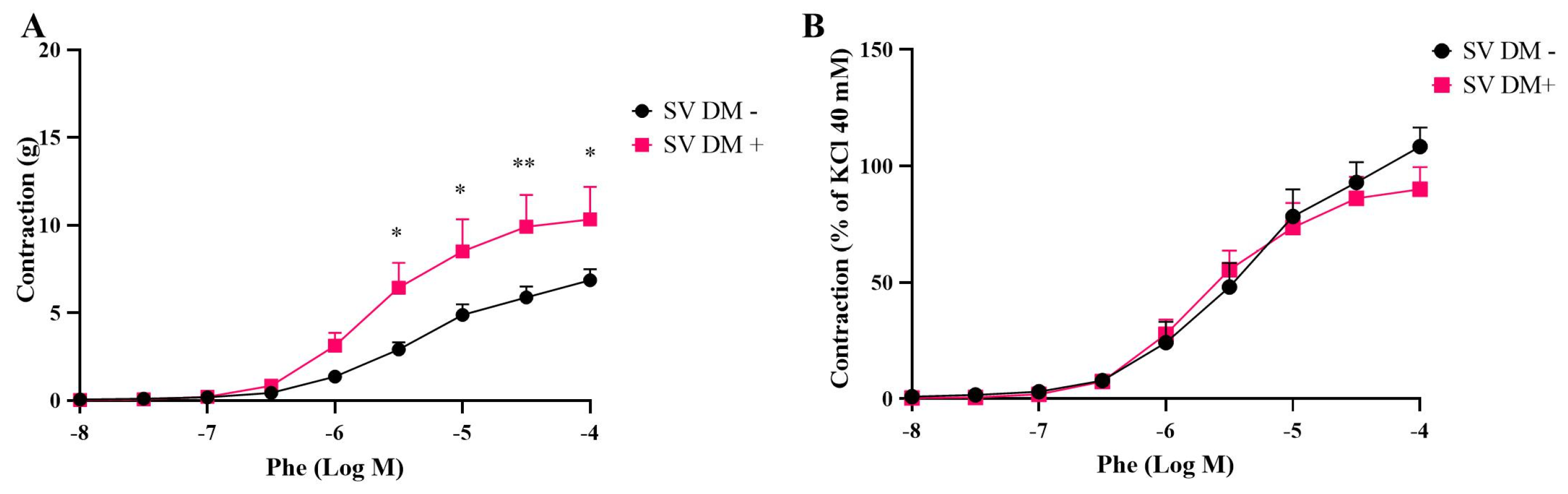
3.1. Contractile Responses of Internal Mammary Arteries (IMAs) and Saphenous Veins (SVs) Isolated from Diabetic (DM+) and Non-Diabetic (DM−) Patients
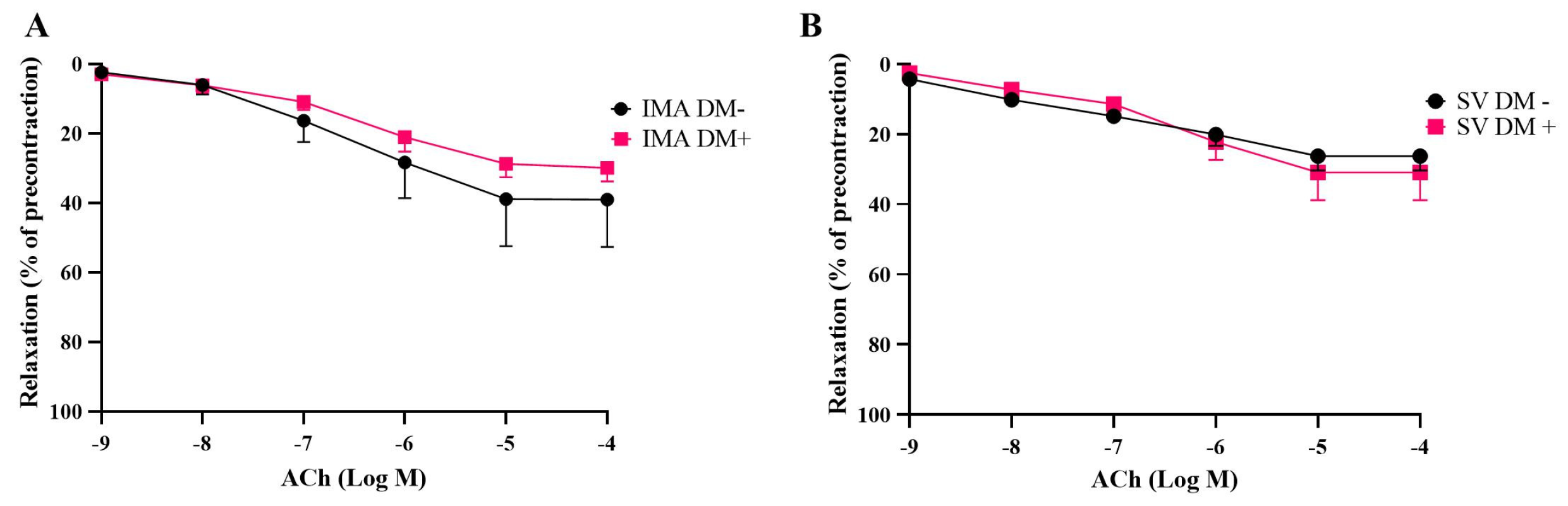
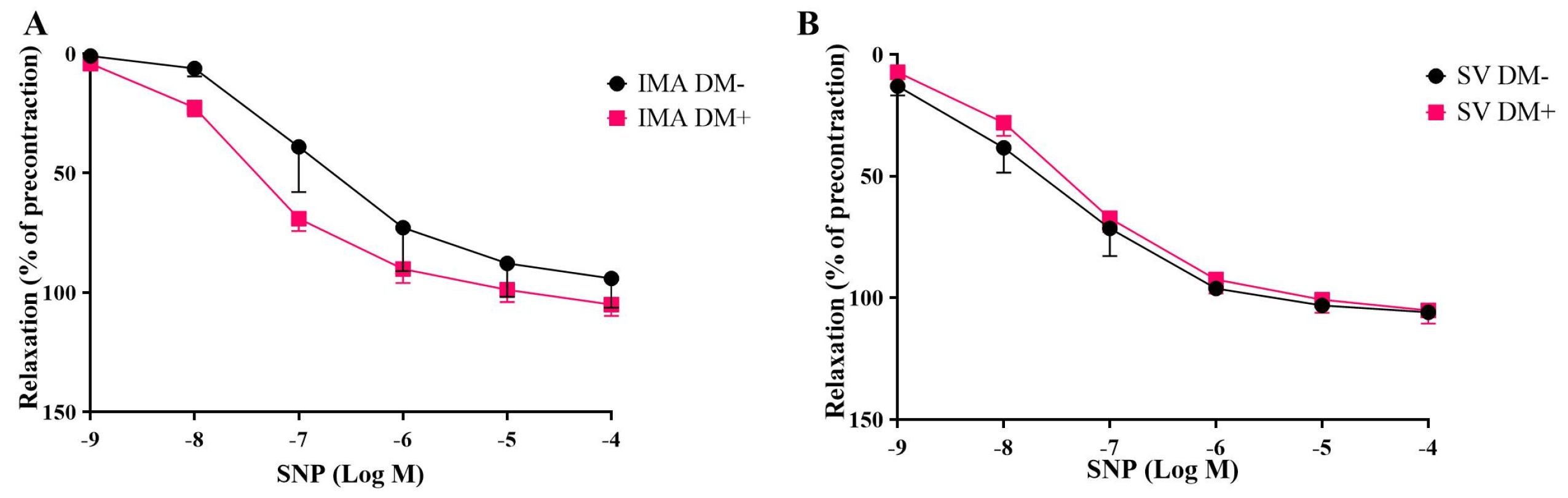
3.2. Endothelium-Dependent and Endothelium-Independent Relaxant Responses of Internal Mammary Arteries (IMAs) and Saphenous Veins (SVs) Isolated from Diabetic (DM+) and Non-Diabetic (DM−) Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG | coronary artery bypass grafting |
| IMA | internal mammary artery |
| SV | saphenous vein |
| DM | diabetes mellitus |
| DM+ | diabetic |
| DM− | non-diabetic |
| KCl | potassium chloride |
| Phe | phenylephrine |
| ACh | acetylcholine |
| SNP | sodium nitroprusside |
| HbA1c | hemoglobin A1c |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| TG | triglyceride |
| HT | hypertension |
| ACE | angiotensin-converting enzyme |
| ARBs | angiotensin receptor blockers |
| S.E.M. | standard error of the mean |
| Emax | maximum effect |
| pEC50 | Negative Logarithm of the EC50 |
| RhoA | Ras Homolog Family Member A |
| NO | nitric oxide |
References
- Chen, Y.; Zhang, H.; Hou, X.; Li, X.; Qian, X.; Feng, X.; Liu, S.; Shi, N.; Zhao, W.; Hu, S.; et al. Glycemic control and risk factors for in-hospital mortality and vascular complications after coronary artery bypass grafting in patients with and without preexisting diabetes. J. Diabetes 2021, 13, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Tanaka, N.; Kuwabara, M.; Yamashita, A.; Matsuo, Y.; Kanai, T.; Onitsuka, T.; Asada, Y.; Hisa, H.; Yamamoto, R. Relative Contributions of 5-Hydroxytryptamine (5-HT) Receptor Subtypes in 5-HT-Induced Vasoconstriction of the Distended Human Saphenous Vein as a Coronary Artery Bypass Graft. Biol. Pharm. Bull. 2011, 34, 82–86. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.R.; Quax, P.H.A. Inflammation in Vein Graft Disease. Front. Cardiovasc. Med. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; Stewart, R.W.; Goormastic, M.; Williams, G.W.; Golding, L.A.; Gill, C.C.; Taylor, P.C.; Sheldon, W.C.; et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 1986, 314, 1–6. [Google Scholar] [CrossRef]
- Gorter, P.M.; Olijhoek, J.K.; Van Der Graaf, Y.; Algra, A.; Rabelink, T.J.; Visseren, F.L.J. Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm. Atherosclerosis 2004, 173, 363–369. [Google Scholar] [CrossRef]
- Gür, D.Ö.; Gür, Ö.; Gürkan, S.; Cömez, S.; Gönültaş, A.; Yılmaz, M. Comparison of endothelial function of coronary artery bypass grafts in diabetic and nondiabetic patients: Which graft offers the best? Anatol. J. Cardiol. 2015, 15, 657–662. [Google Scholar]
- Hoogwerf, B.J.; Waness, A.; Cressman, M.; Canner, J.; Campeau, L.; Domanski, M.; Geller, N.; Herd, A.; Hickey, A.; Hunninghake, D.B.; et al. Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes: The Post Coronary Artery Bypass Graft trial. Diabetes 1999, 48, 1289–1294. [Google Scholar] [CrossRef]
- Markwirth, T.; Hennen, B.; Scheller, B.; Schäfers, N.J.; Wendler, O. Complete Arterial Revascularization Using T-Graft Technique in Diabetics with Coronary Three-Vessel Disease. Thorac. Cardiovasc. Surg. 2000, 48, 269–273. [Google Scholar] [CrossRef]
- Ayan, M.; Saurav, A.; Kabach, A.; Smer, A.; Salih, M.; Abuzaid, A.; Azzouz, M.S.; Akinapelli, A.; Aggarwal, S.; Khashab, M.E.; et al. TCT-280 Impact of Diabetes Mellitus on Graft Patency following Coronary Artery Bypass Graft Surgery: A Propensity Score Analysis. J. Am. Coll. Cardiol. 2015, 66, 110. [Google Scholar] [CrossRef]
- Deb, S.; Singh, S.K.; Moussa, F.; Tsubota, H.; Une, D.; Kiss, A.; Tomlinson, G.; Afshar, M.; Sless, R.; Cohen, E.A.; et al. The long-term impact of diabetes on graft patency after coronary artery bypass grafting surgery: A substudy of the multicenter Radial Artery Patency Study. J. Thorac. Cardiovasc. Surg. 2014, 148, 1246–1253. [Google Scholar] [CrossRef][Green Version]
- Novella, S.; Martínez, A.C.; Pagán, R.M.; Hernández, M.; García-Sacristán, A.; González-Pinto, A.; González-Santos, J.M.; Benedito, S. Plasma levels and vascular effects of vasopressin in patients undergoing coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2007, 32, 69–76. [Google Scholar] [CrossRef] [PubMed]
- He, G.W. Arterial grafts: Clinical classification and pharmacological management. Ann. Cardiothorac. Surg. 2013, 2, 507–518. [Google Scholar] [PubMed]
- Yokota, A.; Gamoh, S.; Tanaka-Totoribe, N.; Shiba, T.; Kuwabara, M.; Nakamura, E.; Hayase, T.; Hisa, H.; Nakamura, K.; Yamamoto, R. Angiotensin II, as well as 5-hydroxytriptamine, is a potent vasospasm inducer of saphenous vein graft for coronary artery bypass grafting in patients with diabetes mellitus. Biochem. Biophys. Rep. 2016, 6, 82–87. [Google Scholar] [CrossRef][Green Version]
- Chaudhuri, A. Vascular Reactivity in Diabetes Mellitus. Curr. Diabetes Rep. 2002, 2, 305–310. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kuwabara, M.; Tanaka-Totoribe, N.; Kanai, T.; Nakamura, E.; Gamoh, S.; Suzuki, A.; Asada, Y.; Hisa, H.; Yamamoto, R. The defective protein level of myosin light chain phosphatase (MLCP) in the isolated saphenous vein, as a vascular conduit in coronary artery bypass grafting (CABG), harvested from patients with diabetes mellitus (DM). Biochem. Biophys. Res. Commun. 2011, 412, 323–327. [Google Scholar] [CrossRef]
- Al-Sabti, H.A.; Al Kindi, A.; Al-Rasadi, K.; Banerjee, Y.; Al-Hashmi, K.; Al-Hinai, A. Saphenous vein graft vs. radial artery graft searching for the best second coronary artery bypass graft. J. Saudi Heart Assoc. 2013, 25, 247–254. [Google Scholar] [CrossRef]
- Lorusso, R.; Pentiricci, S.; Raddino, R.; Scarabelli, T.M.; Zambelli, C.; Villanacci, V.; Burattin, A.; Romanelli, G.; Casari, S.; Scelsi, R.; et al. Influence of Type 2 Diabetes on Functional and Structural Properties of Coronary Artery Bypass Conduits. Diabetes 2003, 52, 2814–2820. [Google Scholar] [CrossRef]
- Wendler, O.; Landwehr, P.; Bandner-Risch, D.; Gerorg, T.; Schafers, H.-J. Vasoreactivity of arterial grafts in the patient with diabetes mellitus:investigations on internal thoracic artery and radial artery conduits. Eur. J. Cardio-Thorac. Surg. 2001, 20, 305–311. [Google Scholar] [CrossRef]
- Grapow, M.T.R.; Reineke, D.C.; Kern, T.; Müller-Schweinitzer, E.; Carrel, T.; Eckstein, F.S. Human internal thoracic arteries from diabetic patients are resistant to endothelial dysfunction. Fundam. Clin. Pharmacol. 2009, 23, 567–572. [Google Scholar] [CrossRef]
- Irat, A.M.; Aslamaci, S.; Karasu, C.; Ari, N. Alteration of vascular reactivity in diabetic human mammary artery and the effects of thiazolidinediones. J. Pharm. Pharmacol. 2010, 58, 1647–1653. [Google Scholar] [CrossRef]
- Okon, E.B.; Chung, A.W.Y.; Rauniyar, P.; Padilla, E.; Tejerina, T.; McManus, B.M.; Luo, H.; Breemen, C.V. Compromised Arterial Function in Human Type 2 Diabetic Patients. Diabetes 2005, 54, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, R.J.; Webb, R.C. Diabetes and reactivity of isolated human saphenous vein. Clin. Physiol. 1984, 4, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.M.; Moat, N.E.; Chong, C.F.; Collins, P. Vascular reactivity and flow characteristics of radial artery and long saphenous vein coronary bypass grafts: A 5-year follow-up. Circulation 2010, 122, 861–867. [Google Scholar] [CrossRef]
- Nieves-Cintron, M.; Flores-Tamez, V.A.; Le, T.; Baudel, M.M.; Navedo, M.F. Cellular and molecular effects of hyperglycemia on ion channels in vascular smooth muscle. Cell. Mol. Life Sci. 2021, 78, 31–61. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Nieves-Cintron, M.; Patriarchi, T.; Buonarati, O.R.; Prada, M.P.; Morotti, S.; Grandi, E.; Fernandes, J.D.; Forbush, K.; Hofmann, F.; et al. Ser1928 phosphorylation by PKA stimulates the L-type Ca2+ channel CaV1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci. Signal. 2017, 10, eaaf9647. [Google Scholar] [CrossRef]
- Prada, M.P.; Syed, A.U.; Buonarati, O.R.; Reddy, G.R.; Nystoriak, M.A.; Ghosh, D.; Simo, S.; Sato, D.; Sasse, K.C.; Ward, S.M.; et al. A G(s)-coupled purinergic receptor boosts Ca2+ influx and vascular contractility during diabetic hyperglycemia. eLife 2019, 8, e42214. [Google Scholar] [CrossRef]
- Syed, A.U.; Reddy, G.R.; Ghosh, D.; Prada, M.P.; Nystoriak, M.A.; Morotti, S.; Grandi, E.; Sirish, P.; Chiamvimonvat, N.; Hell, J.W.; et al. Adenylyl cyclase 5-generated cAMP controls cerebral vascular reactivity during diabetic hyperglycemia. J. Clin. Investig. 2019, 129, 3140–3152. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Y.; Tang, G.; Wu, L.; Hanna, S.T. Altered L-type Ca2+ channel currents in vascular smooth muscle cells from experimental diabetic rats. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, 714–722. [Google Scholar] [CrossRef]
- Bieger, D.; Ford, C.A.; Mong, K.; Tabrizchi, R. Transmural pressure and membrane potential in human saphenous vein. Eur. J. Cardiothorac. Surg. 2008, 34, 109–112. [Google Scholar] [CrossRef][Green Version]
- Razga, Z.; Kovacs, G.; Bódi, N.; Talapka, P. Upregulation of the L-type Calcium Channel in Renin-Positive Smooth Muscle Cells of Arterioles in the Kidneys of Rats with Streptozotocin-Induced Diabetes. Anal. Quant. Cytol. Histol. 2015, 37, 214–220. [Google Scholar]
- Ratz, P.H.; Berg, K.M.; Urban, N.H.; Miner, A.S. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am. J. Physiol. Cell Physiol. 2005, 288, C769–C783. [Google Scholar] [CrossRef] [PubMed]
- Riches, K.; Warburton, P.; O’Regan, D.J.; Turner, N.A.; Porter, K.E. Type 2 diabetes impairs venous, but not arterial smooth muscle cell function: Possible role of differential RhoA activity. Cardiovasc. Revasc. Med. 2014, 15, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kun, X.; Lefeng, W.; Rongjing, D.; Xincun, Y. RhoA/ROK pathway related to the mechanism of higher susceptibility to spasm in RA than in IMA. J. Card. Surg. 2009, 24, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Hu, D.Y.; Xia, K.; Yang, X.C. Increased RhoA/ROK mRNA expression and function in diabetic vein grafts compared with nondiabetic vein grafts and diabetic arterial grafts. J. Thorac. Cardiovasc. Surg. 2010, 58, 148–153. [Google Scholar] [CrossRef]
- Hien, T.T.; Turczynska, K.M.; Dahan, D.; Ekman, M.; Grossi, M.; Sjogren, J.; Nilsson, J.; Braun, T.; Boettger, T.; Garcia-Vaz, E.; et al. Elevated Glucose Levels Promote Contractile and Cytoskeletal Gene Expression in Vascular Smooth Muscle via Rho/Protein Kinase C and Actin Polymerization. J. Biol. Chem. 2016, 291, 3552–3568. [Google Scholar] [CrossRef]
- Creager, M.A.; Lüscher, T.F.; Cosentino, F.; Beckman, J.A. Diabetes and vascular disease. Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003, 108, 1527–1532. [Google Scholar] [CrossRef]
- Pompilio, G.; Rossoni, G.; Alamanni, F.; Tartara, P.; Barajon, I.; Rumio, C.; Manfredi, B.; Biglioli, P. Comparison of Endothelium-Dependent Vasoactivity of Internal Mammary Arteries From Hypertensive, Hypercholesterolemic, and Diabetic Patients. Ann. Thorac. Cardiovasc. Surg. 2001, 72, 1290–1297. [Google Scholar] [CrossRef]
- Abebe, W.; Harris, K.H.; MacLeod, K.M. Enhanced contractile responses of arteries from diabetic rats to alpha 1-adrenoceptor stimulation in the absence and presence of extracellular calcium. J. Cardiovasc. Pharmacol. 1990, 16, 239–248. [Google Scholar] [CrossRef]
- Ungvari, Z.; Pacher, P.; Kecskemeti, V.; Papp, G.; Szollár, L.; Koller, A. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc. Res. 1999, 43, 1018–1028. [Google Scholar] [CrossRef]
- White, R.E.; Carrier, G.O. Enhanced vascular alpha-adrenergic neuroeffector system in diabetes: Importance of calcium. Am. J. Physiol. 1988, 255, H1036–H1042. [Google Scholar] [CrossRef]

| DM+ | DM− | p-Value | |
|---|---|---|---|
| Number of patients | 12 | 10 | |
| Sex | |||
| Male/Female | 10/2 | 10/0 | |
| Age (years) | 63.13 ± 2.5 | 58.38 ± 2.25 | 0.1801 |
| IMA grafts | 65.00 ± 3.29 | 55.25 ± 3.28 | 0.0805 |
| SV grafts | 61.25 ± 4.00 | 61.50 ± 2.53 | 0.9597 |
| Pre-operative laboratory results | |||
| Fasting blood glucose (mg/dL) | 202.5 ± 20.70 *** | 106.0 ± 4.31 | 0.0004 |
| HbA1c % | 8.87 ± 0.51 ** | 5.500 ± 0.37 | 0.0014 |
| HDL (mg/dL) | 44.50 ± 5.48 | 38.86 ± 3.68 | 0.3988 |
| LDL (mg/dL) | 100.5 ± 6.88 | 118.0 ± 15.33 | 0.3472 |
| TG (mg/dL) | 121.2 ± 11.24 | 175.6 ± 40.82 | 0.2575 |
| Total cholesterol (mg/dL) | 170.5 ± 6.80 | 178.9 ± 9.45 | 0.5013 |
| Mean perioperative plasma glucose level (mg/dL) | 200.3 ± 12.80 *** | 128.4 ± 7.05 | 0.0002 |
| HT | 56% | 50% | |
| Medications | |||
| Aspirin | 16% | 26% | |
| ACE inhibitors | 40% | 26% | |
| ARBs | 16% | 16% | |
| Ca+2 channel blockers | 4% | 13% | |
| β-Blockers | 20% | 21% | |
| Statins | 8% | 3% | |
| Clopidogrel | 12% | 3% | |
| Insulin | 40% | ||
| Metformin | 52% | ||
| Gliclazide | 24% | ||
| Dapagliflozin | 4% | 3% | |
| Empagliflozin | 4% | ||
| Sitagliptin | 4% |
| Emax (g) | Emax (% of KCl 40 mM) | pEC50 | n | |
|---|---|---|---|---|
| KCl | ||||
| SV DM+ | 12.98 ± 1.83 | 113.8 ± 7.48 * | 15.13 ± 7.94 | 5 |
| SV DM− | 7.92 ± 0.66 | 124.3 ± 4.17 | 11.17 ± 2.28 | 5 |
| IMA DM+ | 5.46 ± 0.44 | 116.7 ± 1.97 | 23.83 ± 2.73 | 7 |
| IMA DM− | 4.32 ± 0.88 | 130.1 ± 8.03 | 24.53 ± 3.36 | 5 |
| Phe | ||||
| SV DM+ | 10.33 ± 1.85 | 89.98 ± 9.53 * | 5.69 ± 0.13 | 5 |
| SV DM− | 6.86 ± 0.63 | 108.3 ± 8.14 | 5.32 ± 0.15 | 5 |
| IMA DM+ | 4.28 ± 0.27 ## | 94.75 ± 8.79 ### | 5.70 ± 0.12 | 7 |
| IMA DM− | 2.22 ± 0.33 | 75.61 ± 18.44 | 5.36 ± 0.16 | 5 |
| Emax (%) | pEC50 | n | |
|---|---|---|---|
| ACh | |||
| SV DM+ | 30.9 ± 7.9 | 6.5 ± 0.1 | 5 |
| SV DM− | 26.2 ± 4.1 | 7.9 ± 0.7 | 5 |
| IMA DM+ | 29.8 ± 3.9 | 7.0 ± 0.5 | 7 |
| IMA DM− | 39.0 ± 13.6 | 6.4 ± 0.1 | 5 |
| SNP | |||
| SV DM+ | 105.1 ± 5.4 | 9.2 ± 1.8 | 5 |
| SV DM− | 105.8 ± 1.7 | 7.4 ± 0.3 | 5 |
| IMA DM+ | 105.0 ± 4.7 | 7.3 ± 0.1 | 7 |
| IMA DM− | 94.0 ± 12.2 | 6.7 ± 0.3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidin Şen, A.; Uydeş Doğan, B.S.; Kısa, U.; Koçoğulları, C.U.; Teskin, Ö.; Alp Yıldırım, F.İ. Diabetes Differentially Affects Vascular Reactivity in Isolated Human Arterial and Venous Bypass Grafts. Life 2025, 15, 454. https://doi.org/10.3390/life15030454
Vidin Şen A, Uydeş Doğan BS, Kısa U, Koçoğulları CU, Teskin Ö, Alp Yıldırım Fİ. Diabetes Differentially Affects Vascular Reactivity in Isolated Human Arterial and Venous Bypass Grafts. Life. 2025; 15(3):454. https://doi.org/10.3390/life15030454
Chicago/Turabian StyleVidin Şen, Aylin, Birsel Sönmez Uydeş Doğan, Uğur Kısa, Cevdet Uğur Koçoğulları, Önder Teskin, and Fatoş İlkay Alp Yıldırım. 2025. "Diabetes Differentially Affects Vascular Reactivity in Isolated Human Arterial and Venous Bypass Grafts" Life 15, no. 3: 454. https://doi.org/10.3390/life15030454
APA StyleVidin Şen, A., Uydeş Doğan, B. S., Kısa, U., Koçoğulları, C. U., Teskin, Ö., & Alp Yıldırım, F. İ. (2025). Diabetes Differentially Affects Vascular Reactivity in Isolated Human Arterial and Venous Bypass Grafts. Life, 15(3), 454. https://doi.org/10.3390/life15030454






