Surviving a Dark Age: The Oldest Baleen-Bearing Whales (Cetacea: Chaeomysticeti) of Pacific South America (Lower Miocene, Peru)
Abstract
1. Introduction
2. Materials and Methods
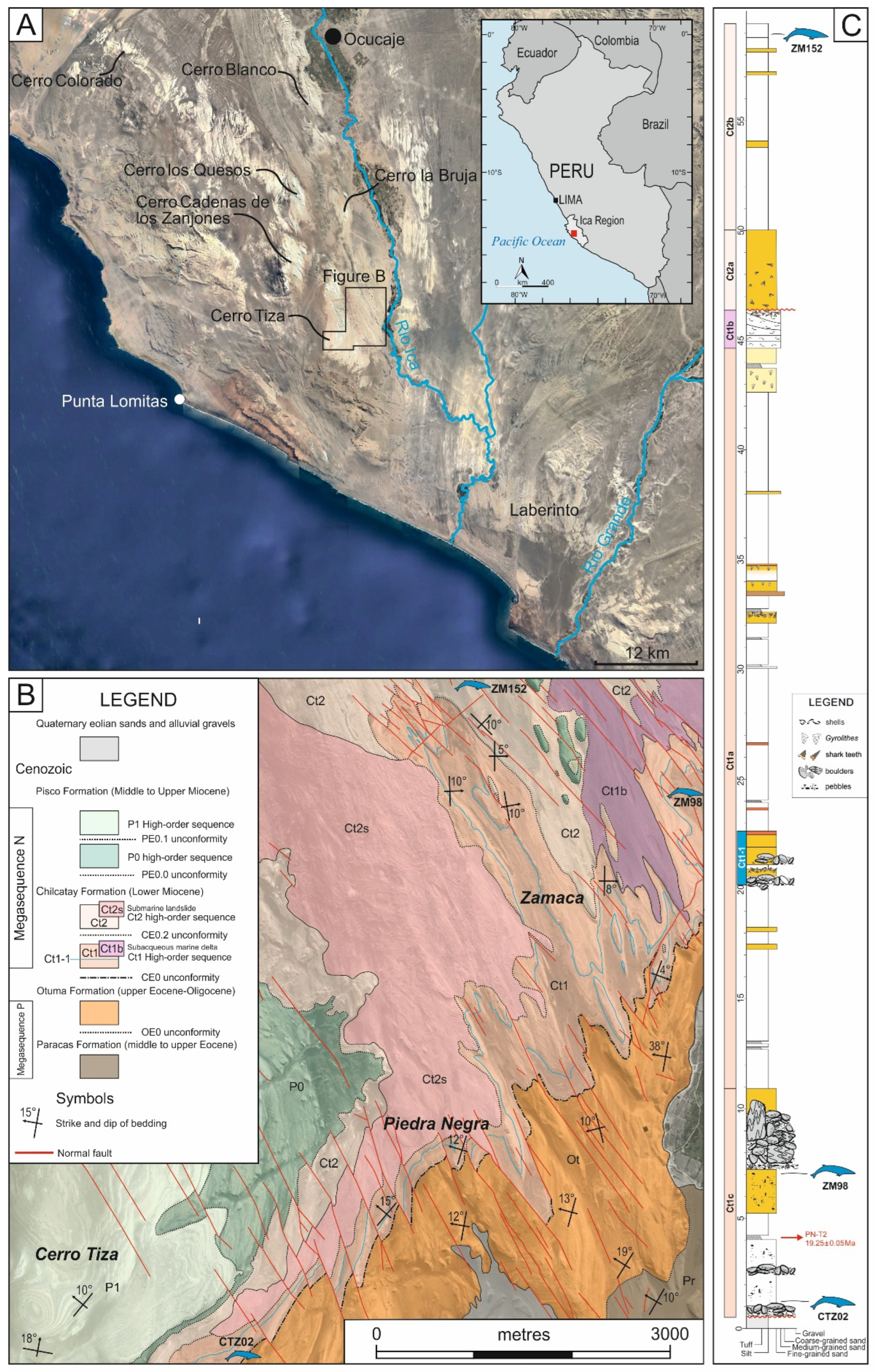
3. Geological Setting
4. The New Mysticete Skull from Cerro Tiza
5. Other Baleen Whales from the Chilcatay Formation
5.1. ZM 98
5.2. ZM 152
6. Systematic Affinities of the Chilcatay Mysticetes
6.1. Preliminary Remarks
6.2. Affinities of CTZ 02
6.3. Affinities of ZM 98
6.4. Affinities of ZM 152
7. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marx, F.G.; Fordyce, R.E. Baleen Boom and Bust: A synthesis of Mysticete phylogeny, diversity and disparity. R. Soc. Open Sci. 2015, 2, 140434. [Google Scholar] [CrossRef] [PubMed]
- Marx, F.G.; Lambert, O.; Uhen, M.D. Cetacean Paleobiology, 1st ed.; John Wiley & Sons: Cichester, UK, 2016. [Google Scholar]
- Bisconti, M.; Pellegrino, L.; Carnevale, G. The chronology of Mysticete diversification (Mammalia, Cetacea, Mysticeti): Body size, morphological evolution and global change. Earth-Science Rev. 2023, 239, 104373. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Richards, M.D. A review of New Zealand Eomysticetidae (Mammalia, Cetacea) and implications for the evolution of baleen whales: New specimens, functional anatomy, and phylogeny. J. R. Soc. New Zeal. 2024, 54, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Marx, F.G.; Coste, A.; Richards, M.D.; Palin, J.M.; Fordyce, R.E. Strontium isotopes reveal a globally unique assemblage of Early Miocene baleen whales. J. R. Soc. New Zeal. 2024, 54, 711–721. [Google Scholar] [CrossRef]
- Tsai, C.H. In search of the origin of crown Mysticeti. J. R. Soc. New Zeal. 2024, 54, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Marx, F.G.; Fitzgerald, E.M.G.; Fordyce, R.E. Like phoenix from the ashes: How modern baleen whales arose from a fossil “Dark age”. Acta Palaeontol. Pol. 2019, 64, 231–238. [Google Scholar] [CrossRef]
- Bianucci, G.; Collareta, A.; Bosio, G.; Landini, W.; Gariboldi, K.; Gioncada, A.; Lambert, O.; Malinverno, E.; de Muizon, C.; Varas-Malca, R.; et al. Taphonomy and palaeoecology of the Lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, Southern Peru). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 511, 256–279. [Google Scholar] [CrossRef]
- Bianucci, G.; Lambert, O.; Urbina, M.; Merella, M.; Collareta, A.; Bennion, R.; Salas-Gismondi, R.; Benites-Palomino, A.; Post, K.; de Muizon, C.; et al. A heavyweight early whale pushes the boundaries of vertebrate morphology. Nature 2023, 620, 824–829. [Google Scholar] [CrossRef]
- DeVries, T.J. Fossil Cenozoic crassatelline bivalves from Peru: New species and generic insights. Acta Palaeontol. Pol. 2016, 61, 661–688. [Google Scholar] [CrossRef]
- Bosio, G.; Collareta, A.; Di Celma, C.; Lambert, O.; Marx, F.G.; De Muizon, C.; Gioncada, A.; Gariboldi, K.; Malinverno, E.; Malca, R.V.; et al. Taphonomy of marine vertebrates of the Pisco Formation (Miocene, Peru): Insights into the origin of an outstanding fossil-lagerstätte. PLoS ONE 2021, 16, e0254395. [Google Scholar] [CrossRef]
- Collareta, A.; Lambert, O.; Marx, F.G.; de Muizon, C.; Varas-Malca, R.; Landini, W.; Bosio, G.; Malinverno, E.; Gariboldi, K.; Gioncada, A.; et al. Vertebrate palaeoecology of the Pisco Formation (Miocene, Peru): Glimpses into the ancient Humboldt current ecosystem. J. Mar. Sci. Eng. 2021, 9, 1188. [Google Scholar] [CrossRef]
- Bianucci, G.; Collareta, A. An Overview of the fossil record of cetaceans from the East Pisco Basin (Peru). Boll. Soc. Paleontol. Ital. 2022, 61, 19–60. [Google Scholar] [CrossRef]
- Di Celma, C.; Pierantoni, P.P.; Malinverno, E.; Collareta, A.; Lambert, O.; Landini, W.; Bosio, G.; Gariboldi, K.; Gioncada, A.; de Muizon, C.; et al. Allostratigraphy and paleontology of the Lower Miocene Chilcatay Formation in the Zamaca Area, East Pisco Basin, Southern Peru. J. Maps 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Lydekker, R. Contributions to a knowledge of the fossil vertebrates of Argentina. 2-Cetacean skulls from Patagonia. Ann. Mus. Plata 1894, 2, 1–13 + 6 Pl. [Google Scholar]
- Cabrera, A. Cetáceos fósiles del Museo de La Plata. Rev. Mus. Plata 1926, 29, 363–411. [Google Scholar]
- Packard, E.L.; Kellogg, R. A New Cetothere from the Miocene Astoria Formation of Newport, Oregon. In Marine Mammals; Carnegie Institution of Washington: Washington, DC, USA, 1934; p. 136. [Google Scholar]
- Benham, W.B. Fossil Cetacea of New Zealand II-On Lophocephalus, a new genus of zeuglodont Cetacea. Trans. R. Soc. New Zealand 1937, 67, 1–7. [Google Scholar]
- Benham, W.B. Mauicetus: A fossil whale. Nature 1939, 143, 765. [Google Scholar] [CrossRef]
- Benham, W.B. Fossil Cetacea of New Zealand. V. Mauicetus, a generic name substituted for Lophocephalus Benham. Trans. Roy. Soc. N.Z. 1942, 71, 260–270. [Google Scholar]
- Marples, B.J. Cetotheres (Cetacea) from the Oligocene of New Zealand. Proc. ZooL. Soc. London 1956, 126, 565–580. [Google Scholar] [CrossRef]
- Kellogg, R. Fossil Marine Mammals from the Miocene Calvert Formation of Maryland and Virginia-Parts 1 & 2. United States Natl. Museum Bull. 1965, 247, 1–201. [Google Scholar]
- Kellogg, R. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia Parts 5–8 (End of Volume). United States Natl. Museum Bull. 1968, 247, 103–201. [Google Scholar]
- Czyżewska, T.; Ryziewicz, Z. Pinocetus polonicus gen.n., sp.n. (Cetacea) from the Miocene Limestones of Pińczów, Poland. Acta Palaeontol. Pol. 1976, 21, 259–291. [Google Scholar]
- Barnes, L.G.; Kimura, M.; Furusawa, H.; Sawamura, H. Classification and distribution of Oligocene Aetiocetidae (Mammalia; Cetacea; Mysticeti) from Western North America and Japan. Isl. Arc 1994, 3, 392–431. [Google Scholar] [CrossRef]
- Kimura, T.; Sakamoto, O.; Hasegawa, Y. A Cetothere from the Miocene Chichibumachi Group, Saitama prefecture, Japan. Bull Saitama Mus. Nat. Hist 1998, 16, 1–13. [Google Scholar]
- Kimura, T.; Hasegawa, Y.; Suzuki, T. A new species of baleen whale (Isanacetus-Group) from the Early Miocene, Japan. Paleontol. Res. 2022, 27, 85–101. [Google Scholar] [CrossRef]
- Kimura, T.; Ozawa, T. A new Cetothere (Cetacea: Mysticeti) from the Early Miocene of Japan. J. Vertebr. Paleontol. 2002, 22, 684–702. [Google Scholar] [CrossRef]
- Sanders, A.E.; Barnes, L.G. Paleontology of the Late Oligocene Ashley and Chandler Bridge Formations of South Carolina, 3: Eomysticetidae, a new family of primitive Mysticetes (Mammalia: Cetacea). In Cenozoic Mammals of Land and Sea: Tributes to the Career of Clayton E. Ray; Smithsonian Institution Press: Washington, DC, USA, 2002; pp. 313–356. [Google Scholar]
- Sanders, A.E.; Barnes, L.G. Paleontology of the Late Oligocene Ashley and Chandler Bridge Formations of South Carolina, 2: Micromysticetus rothauseni, a Primitive Cetotheriid Mysticete (Mammalia: Cetacea). In Cenozoic Mammals of Land and Sea: Tributes to the Career of Clayton E. Ray; Smithsonian Institution Press: Washington, DC, USA, 2002; pp. 271–293. [Google Scholar]
- Geisler, J.H.; Sanders, A.E. Morphological Evidence for the phylogeny of Cetacea BT. J. Mamm. Evol. 2003, 10, 23–129. [Google Scholar] [CrossRef]
- Steeman, M.E. Description of Uranocetus gramensis gen. et sp. nov. (Cetacea, Mysticeti) from the Late Miocene Gram Formation, Denmark, revision of Mysticete classification and of extinct Belgian Mysticetes. Ph.D. Thesis, The University of Copenhagen, Copenhagen, Denmark, 2004. [Google Scholar]
- Bisconti, M. Titanocetus, a New Baleen Whale from the Middle Miocene of northern Italy (Mammalia, Cetacea, Mysticeti). J. Vertebr. Paleontol. 2006, 26, 344–354. [Google Scholar] [CrossRef]
- Bouetel, V.; De Muizon, C. The anatomy and relationships of Piscobalaena nana (Cetacea, Mysticeti), a Cetotheriidae s.s. from the Early Pliocene of Peru. Geodiversitas 2006, 28, 319–395. [Google Scholar]
- Otsuka, H.; Ota, Y. Cetotheres from the Early Middle Miocene Bihoku Group in Shobara district, Hiroshima prefecture, West Japan. Misc. Rep. Hiwa Museum Nat. Hist. 2008, 49, 1–66. [Google Scholar]
- Kimura, T.; Hasegawa, Y. A new baleen Whale (Mysticeti: Cetotheriidae) from the Earliest Late Miocene of Japan and a reconsideration of the phylogeny of Cetotheres. J. Vertebr. Paleontol. 2010, 30, 577–591. [Google Scholar] [CrossRef]
- Okazaki, Y. A new Mysticete from the Upper Oligocene Ashiya Group, Kyushu, Japan and its significance to Mysticete evolution. Nat. Hist. Hum. Hist., Ser. A 2012, 10, 129–152. [Google Scholar]
- Tarasenko, K.K.; Lopatin, A.V. New baleen whale genera (Cetacea, Mammalia) from the Miocene of the Northern Caucasus and Ciscaucasia: 1. Kurdalagonus gen. nov. from the Middle-Late Sarmatian of Adygea. Paleontol. J. 2012, 46, 531–542. [Google Scholar] [CrossRef]
- Bisconti, M.; Lambert, O.; Bosselaers, M. Taxonomic revision of Isocetus depauwi (Mammalia, Cetacea, Mysticeti) and the phylogenetic relationships of archaic “Cetothere” Mysticetes. Palaeontology 2013, 56, 95–127. [Google Scholar] [CrossRef]
- Bisconti, M.; Damarco, P.; Marengo, L.; Macagno, M.; Daniello, R.; Pavia, M.; Carnevale, G. Anatomy and relationships of a new gray whale from the Pliocene of Piedmont, Northwestern Italy. Diversity 2024, 16, 547. [Google Scholar] [CrossRef]
- Gol’din, P.; Startsev, D. Brandtocetus, a new genus of baleen whales (Cetacea, Cetotheriidae) from the Late Miocene of Crimea, Ukraine. J. Vertebr. Paleontol. 2014, 34, 419–433. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. Anatomy, feeding ecology, and ontogeny of a transitional baleen whale: A new genus and species of Eomysticetidae (Mammalia: Cetacea) from the Oligocene of New Zealand. PeerJ 2015, 2015, e1129. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. A new Eomysticetid from the Oligocene Kokoamu Greensand of New Zealand and a review of the Eomysticetidae (Mammalia, Cetacea). J. Syst. Palaeontol. 2017, 15, 429–469. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Fordyce, R.E. Cosmopolitanism and Miocene survival of Eomysticetidae (Cetacea: Mysticeti) revealed by new fossils from New Zealand. New Zeal. J. Geol. Geophys. 2017, 60, 145–157. [Google Scholar] [CrossRef]
- Gol’din, P.; Steeman, M.E. From problem taxa to problem solver: A new Miocene family, Tranatocetidae, brings perspective on baleen whale evolution. PLoS ONE 2015, 10, e0135500. [Google Scholar] [CrossRef][Green Version]
- Tsai, C.H.; Fordyce, R.E. The earliest gulp-feeding Mysticete (Cetacea: Mysticeti) from the Oligocene of New Zealand. J. Mamm. Evol. 2015, 22, 535–560. [Google Scholar] [CrossRef]
- Tsai, C.H.; Fordyce, R.E. Archaic baleen whale from the Kokoamu Greensand: Earbones distinguish a new Late Oligocene Mysticete (Cetacea: Mysticeti) from New Zealand. J. R. Soc. New Zeal. 2016, 46, 117–138. [Google Scholar] [CrossRef]
- Tsai, C.H.; Fordyce, R.E. A new archaic baleen whale Toipahautea waitaki (Early Late Oligocene, New Zealand) and the origins of crown Mysticeti. R. Soc. Open Sci. 2018, 5, 172453. [Google Scholar] [CrossRef]
- Peredo, C.M.; Uhen, M.D. A new basal Chaeomysticete (Mammalia: Cetacea) from the Late Oligocene Pysht Formation of Washington, USA. Pap. Palaeontol. 2016, 2, 533–554. [Google Scholar] [CrossRef]
- Buono, M.R.; Fernández, M.S.; Cozzuol, M.A.; Cuitiño, J.I.; Fitzgerald, E.M.G. The Early Miocene balaenid Morenocetus parvus from Patagonia (Argentina) and the evolution of right whales. PeerJ 2017, 2017, e4148. [Google Scholar] [CrossRef]
- Hernández Cisneros, A.E.; Barba, G.G.; Fordyce, R.E. Oligocene Cetaceans from Baja California Sur, Mexico. Bol. Soc. Geol. Mex. 2017, 69, 149–173. [Google Scholar] [CrossRef]
- Hernández-Cisneros, A.E.; Schwennicke, T.; Rochín-Bañaga, H.; Tsai, C.H. Echericetus novellus n. gen. n. sp. (Cetacea, Mysticeti, Eomysticetidae), an Oligocene baleen whale from Baja California Sur, Mexico. J. Paleontol. 2023, 97, 1309–1328. [Google Scholar] [CrossRef]
- Peredo, C.M.; Pyenson, N.D.; Marshall, C.D.; Uhen, M.D. Tooth loss precedes the origin of baleen in whales. Curr. Biol. 2018, 28, 3992–4000.e2. [Google Scholar] [CrossRef]
- Duboys de Lavigerie, G.; Bosselaers, M.; Goolaerts, S.; Park, T.; Lambert, O.; Marx, F.G. New Pliocene right whale from Belgium informs balaenid phylogeny and function. J. Syst. Palaeontol. 2020, 18, 1141–1166. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohara, M.; Kimura, T. A new specimen of cf. Isanacetus laticephalus (baleen whale) from the Oi Formation, Ichishi Group (Late Early Miocene) in Japan. Paleontol. Res. 2023, 28, 26–36. [Google Scholar] [CrossRef]
- Tsai, C.H.; Goedert, J.L.; Boessenecker, R.W. The oldest Mysticete in the Northern Hemisphere. Curr. Biol. 2024, 34, 1794–1800.e3. [Google Scholar] [CrossRef] [PubMed]
- Bown, P.R.; Young, J.R. Introduction. In Calcareous Nannofossil Biostratigraphy; Bown, P.R., Ed.; Chapman & Hall: London, UK, 1998; pp. 1–15. [Google Scholar]
- Lazarus, D.; Barron, J.; Renaudie, J.; Diver, P.; Turke, A. Cenozoic planktonic marine diatom diversity and correlation to climate change. PLoS ONE 2014, 9, e84857. [Google Scholar] [CrossRef] [PubMed]
- Gradstein, F.M.; Ogg, J.G. The Chronostratigraphic Scale. In Geologic Time Scale; Elsevier BV: Amsterdam, The Netherlands, 2020; ISBN 9780128243602. [Google Scholar]
- Thornburg, T.M.; Kulm, L.D. Sedimentary basins of the Peru continental margin: Structure, stratigraphy, and Cenozoic tectonics from 6°S to 16°S Latitude. In Nazca Plate: Crustal Formation and Andean Convergence. Geol. Soc. Am. Mem. 1981, 154, 393–422. [Google Scholar]
- Dunbar, R.B.; Marty, R.C.; Baker, P.A. Cenozoic marine sedimentation in the Sechura and Pisco basins, Peru. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 77, 235–261. [Google Scholar] [CrossRef]
- Di Celma, C.; Pierantoni, P.P.; Volatili, T.; Molli, G.; Mazzoli, S.; Sarti, G.; Ciattoni, S.; Bosio, G.; Malinverno, E.; Collareta, A.; et al. Towards deciphering the Cenozoic evolution of the East Pisco Basin (Southern Peru). J. Maps 2022, 18, 397–412. [Google Scholar] [CrossRef]
- Coletti, G.; Bosio, G.; Collareta, A.; Malinverno, E.; Bracchi, V.A.; Di Celma, C.; Basso, D.; Stainbank, S.; Spezzaferri, S.; Cannings, T.; et al. Biostratigraphic, evolutionary, and paleoenvironmental significance of the southernmost Lepidocyclinids of the Pacific Coast of South America (East Pisco Basin, Southern Peru). J. S. Am. Earth Sci. 2019, 96, 102372. [Google Scholar] [CrossRef]
- Malinverno, E.; Bosio, G.; Di Celma, C.; Gariboldi, K.; Gioncada, A.; Pierantoni, P.P.; Collareta, A.; Molli, G.; Bagnoli, G.; Sarti, G.; et al. (Bio)Stratigraphic overview and paleoclimatic- paleoceanographic implications of the Middle-Upper Eocene deposits from the Ica River Valley(East Pisco Basin, Peru). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 578, 11056. [Google Scholar] [CrossRef]
- DeVries, T.J. Oligocene deposition and Cenozoic sequence boundaries in the Pisco Basin (Peru). J. S. Am. Earth Sci. 1998, 11, 217–231. [Google Scholar] [CrossRef]
- DeVries, T.J.; Barron, J.A.; Ochoa, D.; McDougall, K. Chronology and paleoenvironment of the Tunga Formation, a new lowermost Miocene sequence in the East Pisco Basin of Southern Peru. Stratigraphy 2024, 21, 189–224. [Google Scholar] [CrossRef]
- DeVries, T.J.; Jud, N.A. Lithofacies patterns and paleogeography of the Miocene Chilcatay and Lower Pisco depositional sequences (East Pisco Basin, Peru). Bol. Soc. Geol. Perú 2018, 8, 124–167. [Google Scholar]
- Di Celma, C.; Malinverno, E.; Collareta, A.; Bosio, G.; Gariboldi, K.; Lambert, O.; Landini, W.; Pierantoni, P.P.; Gioncad, A.; Villa, I.M.; et al. Facies analysis, stratigraphy and marine vertebrate assemblage of the Lower Miocene Chilcatay Formation at Ullujaya (Pisco Basin, Peru). J. Maps 2018, 14, 257–268. [Google Scholar] [CrossRef]
- Malinverno, E.; Bosio, G.; Gastaldello, M.E.; Pellegrino, L.; Bianucci, G.; Collareta, A.; Gariboldi, K.; Urbina, M.; Villa, I.M.; Di Celma, C. The early depositional history of the Pisco Formation (Middle to Upper Miocene, Peru). Newsl. Stratigr. 2025. [Google Scholar] [CrossRef]
- DeVries, T.J.; Barron, J.A.; Urbina-Schmitt, M.; Ochoa, D.; Esperante, R.; Snee, L.W. The Miocene stratigraphy of the Laberinto Area (Río Ica Valley) and its bearing on the geological history of the East Pisco Basin (South-Central Peru). J. S. Am. Earth Sci. 2021, 111, 103458. [Google Scholar] [CrossRef]
- Bosio, G.; Bianucci, G.; Collareta, A.; Landini, W.; Urbina, M.; Di Celma, C. Ultrastructure, composition, and 87Sr/86Sr dating of shark teeth from Lower Miocene sediments of Southwestern Peru. J. S. Am. Earth Sci. 2022, 118, 103909. [Google Scholar] [CrossRef]
- Bosio, G.; Malinverno, E.; Collareta, A.; Di Celma, C.; Gioncada, A.; Parente, M.; Berra, F.; Marx, F.G.; Vertino, A.; Urbina, M.; et al. Strontium isotope stratigraphy and the thermophilic fossil fauna from the Middle Miocene of the East Pisco Basin (Peru). J. S. Am. Earth Sci. 2020, 97, 102399. [Google Scholar] [CrossRef]
- Bosio, G.; Malinverno, E.; Villa, I.M.; Di Celma, C.; Gariboldi, K.; Gioncada, A.; Barberini, V.; Urbina, M.; Bianucci, G. Tephrochronology and chronostratigraphy of the Miocene Chilcatay and Pisco formations (East Pisco Basin, Peru). Newsletters Stratigr. 2020, 53, 213–247. [Google Scholar] [CrossRef]
- Bukry, D. Synthesis of silicoflagellate stratigraphy for Maastrichtian to Quaternary marine sediment. SEPM Spec. Publ. 1981, 32, 433–444. [Google Scholar]
- Lambert, O.; Martínez-Cáceres, M.; Bianucci, G.; Di Celma, C.; Salas-Gismondi, R.; Steurbaut, E.; Urbina, M.; de Muizon, C. Earliest Mysticete from the Late Eocene of Peru sheds new light on the origin of baleen whales. Curr. Biol. 2017, 27, 1535–1541.e2. [Google Scholar] [CrossRef]
- Sawamura, K.; Yanagisawa, Y. Fossil silicoflagellates in the Lower to Middle Miocene sequence in the Southern Boso Peninsula, central Japan. Open-File Rep. Geol. Surv. Jpn. AIST 2012, 547, 1–23. [Google Scholar]
- Tsai, C.H.; Fordyce, R.E. Disparate heterochronic processes in baleen whale evolution. Evol. Biol. 2014, 41, 299–307. [Google Scholar] [CrossRef]
- Roston, R.A.; Boessenecker, R.W.; Geisler, J.H. Evolution and development of the cetacean skull roof: A case study in novelty and homology. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220086. [Google Scholar] [CrossRef] [PubMed]
- Ekdale, E.G.; Deméré, T.A.; Berta, A. Vascularization of the gray whale palate (Cetacea, Mysticeti, Eschrichtius robustus): Soft tissue evidence for an alveolar source of blood to baleen. Anat. Rec. 2015, 298, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Bisconti, M.; Carnevale, G. Skeletal transformations and the origin of baleen whales (Mammalia, Cetacea, Mysticeti): A study on evolutionary patterns. Diversity 2022, 14, 221. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Beatty, B.L.; Geisler, J.H. New specimens and species of the Oligocene toothed baleen whale Coronodon from South Carolina and the origin of Neoceti. PeerJ 2023, 11, e14795. [Google Scholar] [CrossRef]
- Gatesy, J.; McGowen, M.R. Higher level phylogeny of baleen whales. In The Bowhead Whale; Academic Press: Cambridge, MA, USA, 2021; pp. 3–10. [Google Scholar]
- Fordyce, R.E.; Marx, F.G. The pygmy right whale Caperea marginata: The last of the Cetotheres. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122645. [Google Scholar] [CrossRef]
- Bisconti, M. Comparative osteology and phylogenetic relationships of Miocaperea pulchra, the first fossil pygmy right whale genus and species (Cetacea, Mysticeti, Neobalaenidae). Zool. J. Linn. Soc. 2012, 166, 876–911. [Google Scholar] [CrossRef]
- Geisler, J.H.; McGowen, M.R.; Yang, G.; Gatesy, J. A Supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evol. Biol. 2011, 11, 112. [Google Scholar] [CrossRef]
- Hernández-Cisneros, A.E.; Nava-Sanchez, E.H. Oligocene dawn baleen whales in Mexico (Cetacea, Eomysticetidae) and palaeobiogeographic notes [Ballenas del alba en el Oligoceno de México (Cetacea, Eomysticetidae) y notas paleobiogeográficas]. Paleontol. Mex. 2022, 11, 1–12. [Google Scholar]
- Ekdale, E.G.; .Deméré, T.A. Neurovascular evidence for a co-occurrence of teeth and baleen in an Oligocene mysticete and the transition to filter-feeding in baleen whales. Zool. J. Lin. Soc. 2022, 194, 395–415. [Google Scholar] [CrossRef]
- Bisconti, M.; Bosselaers, M. On Plesiocetus Van Beneden, 1859 (Mammalia, Cetacea, Mysticeti). Riv. Ital. Paleontol. Stratigr. 2021, 127, 231–274. [Google Scholar]
- Cuitiño, J.I.; Bilmes, A.; Buono, M.R.; Bordese, S.; Herazo, L.; Scasso, R.A. Stratigraphy, provenance, and timing of Neogene sedimentation in the Western Valdés Basin, Patagonia. accurate paleogeographic reconstructions as a key piece for Andean-passive margin integration. J. S. Am. Earth Sci. 2023, 124, 104278. [Google Scholar] [CrossRef]
- Lambert, O.; de Muizon, C.; Varas-Malca, R.M.; Urbina, M.; Bianucci, G. Eurhinodelphinids from the Early Miocene of Peru: First unambiguous records of these hyper-longirostrine dolphins outside the North Atlantic realm. Riv. Ital. Paleontol. Stratigr. 2021, 127, 17–32. [Google Scholar]
- Cohen, K.M.; Finney, S.C.; Gibbard, P.L.; Fan, J.-X. International Chronostratigraphic Chart. ICS Int. Chronostratigr. Chart 2021, 36, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Dal Piaz, G. Gli Odontoceti del Miocene bellunese. Mem. Ist. Geol. R. Univ. Padova, allegato al Vol. 4 1916, l, VIII+25+3 Pl. [Google Scholar]
- Dal Piaz, G. Gli Odontoceti del Miocene bellunese. Parte quinta-decima. Cyrtodelphis-Acrodelphis-Protodelphinus-Ziphiodelphis-Scaldicetus-Conclusioni generali e considerazioni filogenetiche. Mem. Ist. Geol. R. Univ. Padova, allegato al Vol. 4 1977, 4, 1–127. [Google Scholar]
- Pilleri, G. The Miocene Cetacea of the Belluno Sandstones (Eastern Southern Alps). Mem. Sci. Geol. 1985, 36, 1–250. [Google Scholar]
- Costa, V.; Doglioni, C.; Grandesso, P.; Masetti, D.; Pellegrini, G.B.; Tracanella, E. Note illustrative Della Carta Geologica d’Italia Alla Scala 1:50.000 “Foglio 063-Belluno”; Servizio Geologico d’Italia; ISPRA: Roma, Italy, 1996; p. 74. [Google Scholar]
- Fornasiero, M.; Del Favero, L. I Cetacei del Museo di Zoologia dell’Università di Padova. Museol. Sci. Mem. 2014, 13, 62–69. [Google Scholar]
- Addicott, W.O. Early Miocene age of the Clallam Formation, Western Washington. U.S. Geol. Surv. Bull. 1975, 1405, A26. [Google Scholar]
- Ray, C.E. Fossil marine mammals of Oregon. Syst. Zool 1976, 25, 420–436. [Google Scholar] [CrossRef]
- Muizon, C. de A new Ziphiidae (Cetacea) from the Early Miocene of Washington State (USA) and phylogenetic analysis of the major groups of Odontocetes. Bull. Mus. Hist. Nat. Paris 1991, 12, 279–326. [Google Scholar]
- Nelson, M.D.; Uhen, M.D. First occurrence of a Squalodelphinid (Cetacea, Odontoceti) from the Early Miocene of Washington State. J. Vertebr. Paleontol. 2018, 38, e1428197. [Google Scholar] [CrossRef]
- Snavely, P.D., Jr.; Rau, W.W.; Wagner, H.C. Miocene stratigraphy of the Yaquina Bay area, Newport, Oregon. Ore Bin 1964, 26, 133–152. [Google Scholar]
- Prothero, D.R.; Bitboul, C.Z.; Moore, G.W.; Niem, A.R. Magnetic stratigraphy and tectonic rotation of the Oligocene Alsea, Yaquina, and Nye Formations, Lincoln County, Oregon. In Magnetic Stratigraphy of the Pacific Coast Cenozoic; Society for Sedimentary Geology Pacific Section; SEPM, Society for Sedimentary Geology: Los Angeles, CA, USA, 2001; pp. 184–194. [Google Scholar]
- Lambert, O.; Godfrey, S.J.; Fitzgerald, E.M.G. Yaquinacetus meadi, a new latest Oligocene–Early Miocene dolphin (Cetacea, Odontoceti, Squaloziphiidae, fam. nov.) from the Nye Mudstone (Oregon, U.S.A.). J. Vertebr. Paleontol. 2019, 38, e1559174. [Google Scholar] [CrossRef]
- Nelson, M.D.; Uhen, M.D. A new platanistoid, Perditicetus yaconensis gen. et sp. nov. (Cetacea, Odontoceti), from the Chattian–Aquitanian Nye Formation of Oregon. J. Syst. Palaeontol. 2020, 18, 1497–1517. [Google Scholar] [CrossRef]
- Nesbitt, E.A. Cenozoic, marine formations of Washington and Oregon: An annotated catalogue. PaleoBios 2018, 35, 1–20. [Google Scholar] [CrossRef]
- Boessenecker, R.W. Oligocene-Miocene marine mammals from Belgrade Quarry, North Carolina. Geobios 2022, 74, 1–19. [Google Scholar] [CrossRef]
- Boessenecker, R.W.; Geisler, J.H. New skeletons of the ancient dolphin Xenorophus sloanii and earliest Odontoceti. Diversity 2023, 15, 1154. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobile, F.; Lambert, O.; Bianucci, G.; Amson, E.; Bosselaers, M.; Bosio, G.; Pellegrino, L.; Malinverno, E.; Di Celma, C.; Urbina, M.; et al. Surviving a Dark Age: The Oldest Baleen-Bearing Whales (Cetacea: Chaeomysticeti) of Pacific South America (Lower Miocene, Peru). Life 2025, 15, 452. https://doi.org/10.3390/life15030452
Nobile F, Lambert O, Bianucci G, Amson E, Bosselaers M, Bosio G, Pellegrino L, Malinverno E, Di Celma C, Urbina M, et al. Surviving a Dark Age: The Oldest Baleen-Bearing Whales (Cetacea: Chaeomysticeti) of Pacific South America (Lower Miocene, Peru). Life. 2025; 15(3):452. https://doi.org/10.3390/life15030452
Chicago/Turabian StyleNobile, Francesco, Olivier Lambert, Giovanni Bianucci, Eli Amson, Mark Bosselaers, Giulia Bosio, Luca Pellegrino, Elisa Malinverno, Claudio Di Celma, Mario Urbina, and et al. 2025. "Surviving a Dark Age: The Oldest Baleen-Bearing Whales (Cetacea: Chaeomysticeti) of Pacific South America (Lower Miocene, Peru)" Life 15, no. 3: 452. https://doi.org/10.3390/life15030452
APA StyleNobile, F., Lambert, O., Bianucci, G., Amson, E., Bosselaers, M., Bosio, G., Pellegrino, L., Malinverno, E., Di Celma, C., Urbina, M., & Collareta, A. (2025). Surviving a Dark Age: The Oldest Baleen-Bearing Whales (Cetacea: Chaeomysticeti) of Pacific South America (Lower Miocene, Peru). Life, 15(3), 452. https://doi.org/10.3390/life15030452










