Engineered Chlamydomonas reinhardtii Strains for Enhanced Astaxanthin Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Cultivation and Strain Maintenance
2.2. Expression Cassette, Transformation and Screening
2.3. Pigment Analyses
2.4. Growth Analysis
3. Results
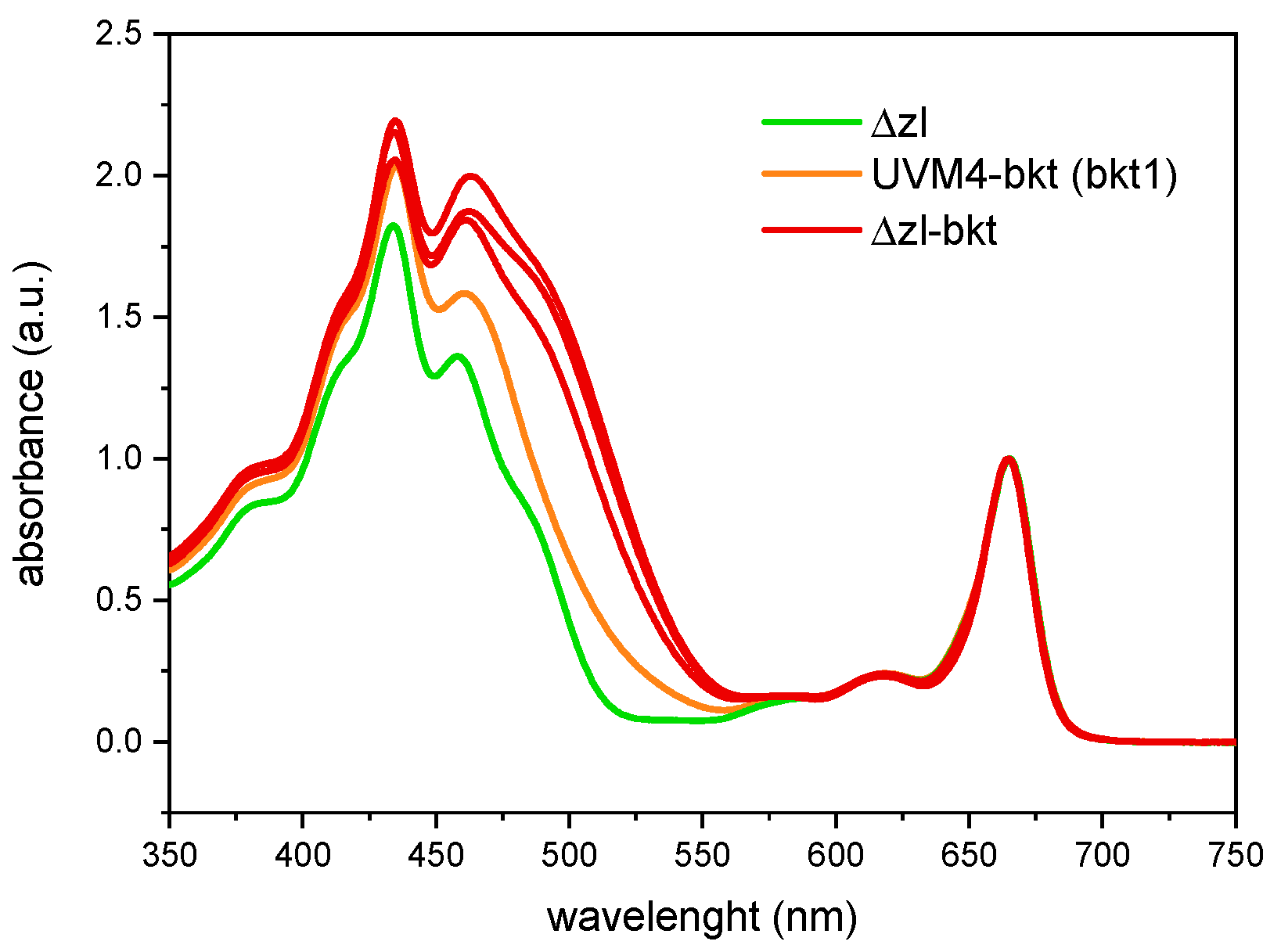
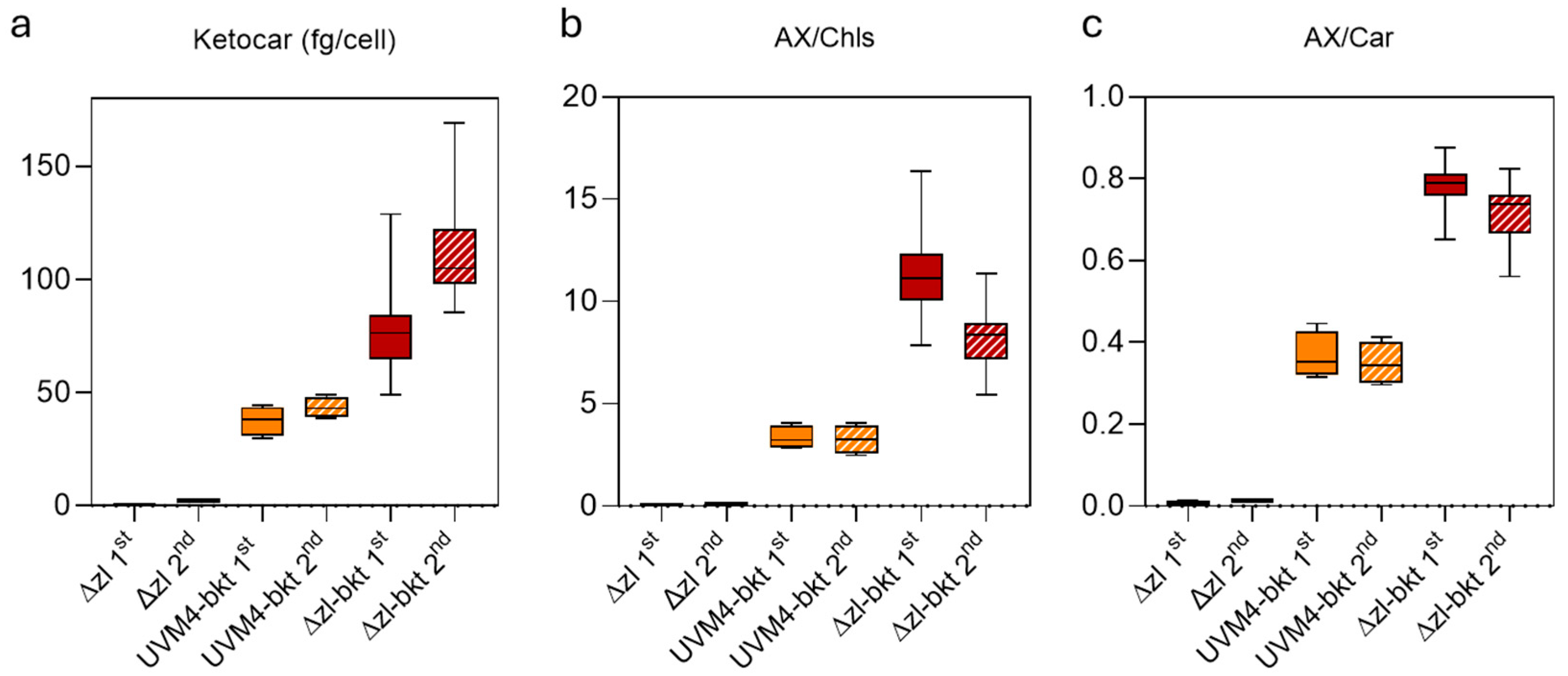
3.1. Overexpression of bkt in Δzl Strains Resulted in Astaxanthin Accumulation
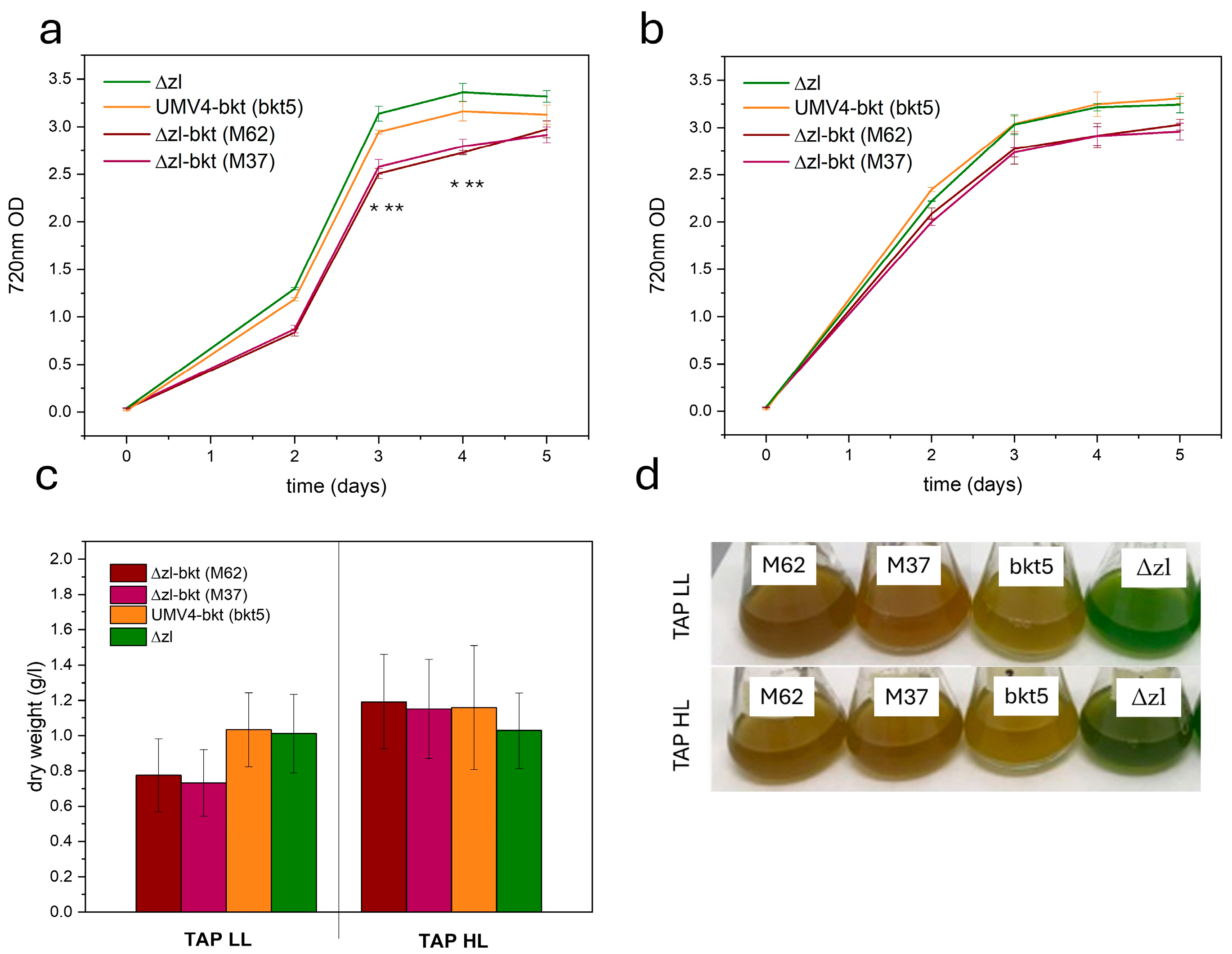
3.2. Algal Growth Is Not Perturbed by Ketocarotenoids Presence
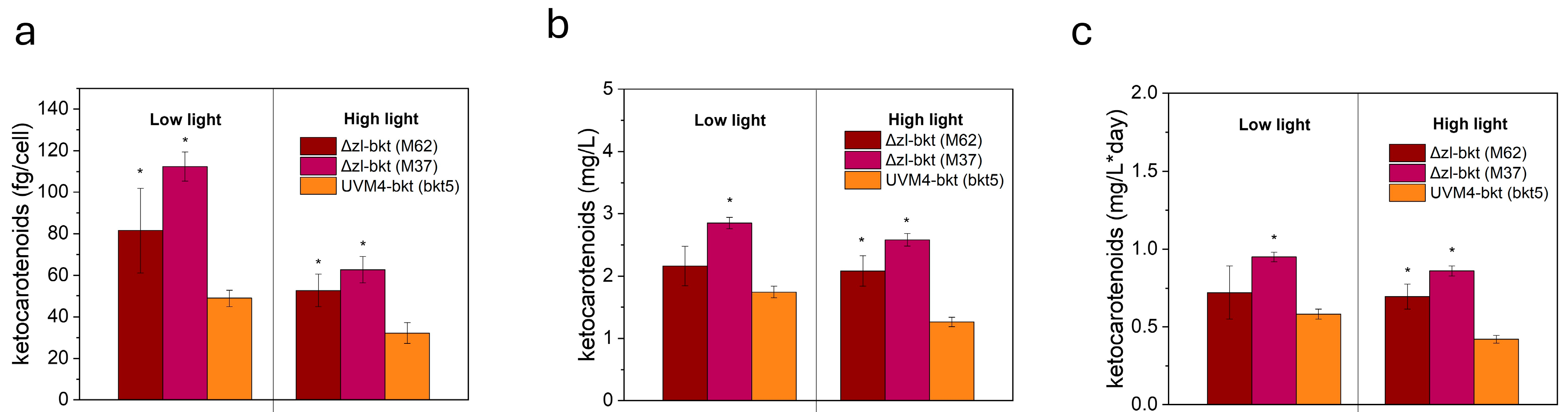
3.3. Yield of Ketocarotenoids in Different Growth Conditions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kull, O.; Pfander, H. List of new carotenoids. In Carotenoids: Isolation and Analysis; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkauser Publishing: Basel, Switzerland, 1995; pp. 295–317. [Google Scholar]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Fraser, P.D.; Miura, Y.; Misawa, N. In Vitro Characterization of Astaxanthin Biosynthetic Enzymes. J. Biol. Chem. 1997, 272, 6128–6135. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Bendich, A. Physiological role of antioxidants in the immune system. J. Dairy Sci. 1993, 76, 2789–2794. [Google Scholar] [CrossRef]
- Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef]
- Xue, Y.; Qu, Z.; Fu, J.; Zhen, J.; Wang, W.; Cai, Y.; Wang, W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bull. 2017, 131, 221–228. [Google Scholar] [CrossRef]
- Bubrick, P. Production of Astaxanthin from Haematococcus. Bioresour. Technol. 1991, 38, 237–239. [Google Scholar] [CrossRef]
- Mascia, F.; Girolomoni, L.; Alcocer, M.J.P.; Bargigia, I.; Perozeni, F.; Cazzaniga, S.; Cerullo, G.; D’Andrea, C.; Ballottari, M. Functional analysis of photosynthetic pigment binding complexes in the green alga Haematococcus pluvialis reveals distribution of astaxanthin in Photosystems. Sci. Rep. 2017, 7, 16319. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis1. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Fan, F.; Wan, M.; Huang, J.; Wang, W.; Bai, W.; He, M.; Li, Y. Modeling of astaxanthin production in the two-stage cultivation of Haematococcus pluvialis and its application on the optimization of vertical multi-column airlift photobioreactor. Algal Res. 2021, 58, 102301. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: A Comparative Case of Synthetic vs. Natural Production. 2013. Available online: http://trace.tennessee.edu/utk_chembiopubs/94 (accessed on 14 May 2025).
- Henke, N.A.; Heider, S.A.; Peters-Wendisch, P.; Wendisch, V.F. Production of the Marine Carotenoid Astaxanthin by Metabolically Engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124. [Google Scholar] [CrossRef]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Torres-Haro, A.; Verdín, J.; Kirchmayr, M.R.; Arellano-Plaza, M. Metabolic engineering for high yield synthesis of astaxanthin in Xanthophyllomyces dendrorhous. Microb. Cell Factories 2021, 20, 175. [Google Scholar] [CrossRef]
- Kildegaard, K.R.; Adiego-Perez, B.; Domenech Belda, D.; Khangura, J.K.; Holkenbrink, C.; Borodina, I. Engineering of Yarrowia lipolytica for production of astaxanthin. Synth. Syst. Biotechnol. 2017, 2, 287–294. [Google Scholar] [CrossRef]
- Miura, Y.; Kondo, K.; Saito, T.; Shimada, H.; Fraser, P.D.; Misawa, N. Production of the carotenoids lycopene, beta-carotene, and astaxanthin in the food yeast Candida utilis. Appl. Environ. Microbiol. 1998, 64, 1226–1229. [Google Scholar] [CrossRef]
- Harada, H.; Maoka, T.; Osawa, A.; Hattan, J.; Kanamoto, H.; Shindo, K.; Otomatsu, T.; Misawa, N. Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res. 2014, 23, 303–315. [Google Scholar] [CrossRef]
- Nogueira, M.; Enfissi, E.M.A.; Martinez Valenzuela, M.E.; Menard, G.N.; Driller, R.L.; Eastmond, P.J.; Schuch, W.; Sandmann, G.; Fraser, P.D. Engineering of tomato for the sustainable production of ketocarotenoids and its evaluation in aquaculture feed. Proc. Natl. Acad. Sci. USA 2017, 114, 10876–10881. [Google Scholar] [CrossRef]
- Jayaraj, J.; Devlin, R.; Punja, Z. Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res. 2008, 17, 489–501. [Google Scholar] [CrossRef]
- Harker, M.; Hirschberg, J. Biosynthesis of ketocarotenoids in transgenic cyanobacteria expressing the algal gene for beta-C-4-oxygenase, crtO. FEBS Lett. 1997, 404, 129–134. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Y.; Chen, J.; Qin, S.; Chen, G. Metabolic engineering of Synechocystis sp. PCC6803 to produce astaxanthin. Algal Res. 2019, 44, 101679. [Google Scholar] [CrossRef]
- Amendola, S.; Kneip, J.S.; Meyer, F.; Perozeni, F.; Cazzaniga, S.; Lauersen, K.J.; Ballottari, M.; Baier, T. Metabolic Engineering for Efficient Ketocarotenoid Accumulation in the Green Microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 2023, 12, 820–831. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Perozeni, F.; Baier, T.; Ballottari, M. Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnol. Biofuels Bioprod. 2022, 15, 77. [Google Scholar] [CrossRef]
- Perozeni, F.; Cazzaniga, S.; Baier, T.; Zanoni, F.; Zoccatelli, G.; Lauersen, K.J.; Wobbe, L.; Ballottari, M. Turning a green alga red: Engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020, 18, 2053–2067. [Google Scholar] [CrossRef]
- Seger, M.; Mammadova, F.; Villegas-Valencia, M.; Bastos de Freitas, B.; Chang, C.; Isachsen, I.; Hemstreet, H.; Abualsaud, F.; Boring, M.; Lammers, P.J.; et al. Engineered ketocarotenoid biosynthesis in the polyextremophilic red microalga Cyanidioschyzon merolae 10D. Metab. Eng. Commun. 2023, 17, e00226. [Google Scholar] [CrossRef]
- Ryu, Y.-K.; Lee, W.-K.; Park, G.-H.; Kim, T.; Lee, Y.; Koh, E.-J.; Heo, S.-J.; Choi, W.-Y.; Oh, C. Preliminary assessment of astaxanthin production in a new Chlamydomonas strain. Algal Res. 2024, 82, 103629. [Google Scholar] [CrossRef]
- Leon, R.; Couso, I.; Fernandez, E. Metabolic engineering of ketocarotenoids biosynthesis in the unicelullar microalga Chlamydomonas reinhardtii. J. Biotechnol. 2007, 130, 143–152. [Google Scholar] [CrossRef]
- Tan, C.-P.; Zhao, F.-Q.; Su, Z.-L.; Liang, C.-W.; Qin, S. Expression of β-carotene hydroxylase gene (crtR-B) from the green alga Haematococcus pluvialis in chloroplasts of Chlamydomonas reinhardtii. J. Appl. Phycol. 2007, 19, 347–355. [Google Scholar] [CrossRef]
- Liu, M.; Yu, L.; Zheng, J.; Shao, S.; Pan, Y.; Hu, H.; Shen, L.; Wang, W.; Zhou, W.; Liu, J. Turning the industrially relevant marine alga Nannochloropsis red: One move for multifaceted benefits. New Phytol. 2024, 244, 1467–1481. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, J.; Yu, L.; Shao, S.; Zhou, W.; Liu, J. Engineering Nannochloropsis oceanica for concurrent production of canthaxanthin and eicosapentaenoic acid. Bioresour. Technol. 2024, 413, 131525. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, C.; Xiao, M.; Chen, J.; Li, J.; Hu, Z. Expression of bkt and bch genes from Haematococcus pluvialis in transgenic Chlamydomonas. Sci. China Life Sci. 2014, 57, 1028–1033. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Grossman, A.R.; Lohr, M.; Im, C.S. Chlamydomonas reinhardtii in the landscape of pigments. Annu. Rev. Genet. 2004, 38, 119–173. [Google Scholar] [CrossRef]
- Lohr, M.; Im, C.S.; Grossman, A.R. Genome-based examination of chlorophyll and carotenoid biosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 2005, 138, 490–515. [Google Scholar] [CrossRef]
- Li, Z.; Peers, G.; Dent, R.M.; Bai, Y.; Yang, S.Y.; Apel, W.; Leonelli, L.; Niyogi, K.K. Evolution of an atypical de-epoxidase for photoprotection in the green lineage. Nat. Plants 2016, 2, 16140. [Google Scholar] [CrossRef]
- Girolomoni, L.; Bellamoli, F.; Valbuena, G.; Perozeni, F.; D’Andrea, C.; Cerullo, G.; Cazzaniga, S.; Ballottari, M. Evolutionary divergence of photoprotection in the green algal lineage: A plant-like violaxanthin de-epoxidase enzyme activates the xanthophyll cycle in the green algaChlorella vulgarismodulating photoprotection. New Phytol. 2020, 228, 136–150. [Google Scholar] [CrossRef]
- Kneip, J.S.; Kniepkamp, N.; Jang, J.; Mortaro, M.G.; Jin, E.; Kruse, O.; Baier, T. CRISPR/Cas9-Mediated Knockout of the Lycopene ε-Cyclase for Efficient Astaxanthin Production in the Green Microalga Chlamydomonas reinhardtii. Plants 2024, 13, 1393. [Google Scholar] [CrossRef]
- Song, I.; Kim, J.; Baek, K.; Choi, Y.; Shin, B.; Jin, E. The generation of metabolic changes for the production of high-purity zeaxanthin mediated by CRISPR-Cas9 in Chlamydomonas reinhardtii. Microb. Cell Factories 2020, 19, 220. [Google Scholar] [CrossRef]
- Kropat, J.; Hong-Hermesdorf, A.; Casero, D.; Ent, P.; Castruita, M.; Pellegrini, M.; Merchant, S.; Malasarn, D. A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 2011, 66, 770–780. [Google Scholar] [CrossRef]
- Harris, E.H. Introduction to Chlamydomonas and Its Laboratory Use; Academic Press: San Diego, CA, USA, 2008; Volume 1. [Google Scholar]
- Kindle, K.L.; Schnell, R.A.; Fernández, E.; Lefebvre, P.A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 1989, 109, 2589–2601. [Google Scholar] [CrossRef]
- Croce, R.; Canino, G.; Ros, F.; Bassi, R. Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry 2002, 41, 7334–7343. [Google Scholar] [CrossRef]
- Lagarde, D.; Beuf, L.; Vermaas, W. Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2000, 66, 64–72. [Google Scholar] [CrossRef]
- Kim, M.; Cazzaniga, S.; Jang, J.; Pivato, M.; Kim, G.; Ballottari, M.; Jin, E. Photoautotrophic cultivation of a Chlamydomonas reinhardtii mutant with zeaxanthin as the sole xanthophyll. Biotechnol. Biofuels Bioprod. 2024, 17, 41. [Google Scholar] [CrossRef]
- Baier, T.; Wichmann, J.; Kruse, O.; Lauersen, K.J. Intron-containing algal transgenes mediate efficient recombinant gene expression in the green microalga Chlamydomonas reinhardtii. Nucleic Acids Res. 2018, 46, 6909–6919. [Google Scholar] [CrossRef]
- Eskling, M.; Arvidsson, P.O.; Akerlund, H.E. The xanthophyll cycle, its regulation and components. Physiol. Plant. 1997, 100, 806–816. [Google Scholar] [CrossRef]
- Masi, A.; Leonelli, F.; Scognamiglio, V.; Gasperuzzo, G.; Antonacci, A.; Terzidis, M.A. Chlamydomonas reinhardtii: A Factory of Nutraceutical and Food Supplements for Human Health. Molecules 2023, 28, 1185. [Google Scholar] [CrossRef]
- Perozeni, F.; Baier, T. Current Nuclear Engineering Strategies in the Green Microalga Chlamydomonas reinhardtii. Life 2023, 13, 1566. [Google Scholar] [CrossRef]


| Δzl-bkt (M62) | UVM4-bkt (bkt5) | Δzl | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAP LL | pg Chl/cell | 0.43 | ± | 0.04 | 0.70 | ± | 0.04 | 0.88 | ± | 0.06 |
| Chla/Chlb | 4.55 | ± | 0.48 | 2.32 | ± | 0.08 | 2.61 | ± | 0.10 | |
| Chl/Car | 2.09 | ± | 0.16 | 2.65 | ± | 0.07 | 3.2 | ± | 0.33 | |
| TAP HL | pg Chl/cell | 0.19 | ± | 0.01 | 0.24 | ± | 0.05 | 0.54 | ± | 0.08 |
| Chla/Chlb | 3.72 | ± | 0.09 | 2.55 | ± | 0.33 | 2.83 | ± | 0.19 | |
| Chl/Car | 1.24 | ± | 0.01 | 1.93 | ± | 0.01 | 2.18 | ± | 0.09 | |
| Δzl-bkt (M62) | Δzl-bkt (M37) | UVM4-bkt (bkt5) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TAP LL | 60% | ± | 2% | 71% | ± | 3% | 29% | ± | 1.5% |
| TAP HL | 65% | ± | 1.5% | 66% | ± | 2.5% | 40% | ± | 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perozeni, F.; Angelini, M.; Ballottari, M.; Cazzaniga, S. Engineered Chlamydomonas reinhardtii Strains for Enhanced Astaxanthin Production. Life 2025, 15, 813. https://doi.org/10.3390/life15050813
Perozeni F, Angelini M, Ballottari M, Cazzaniga S. Engineered Chlamydomonas reinhardtii Strains for Enhanced Astaxanthin Production. Life. 2025; 15(5):813. https://doi.org/10.3390/life15050813
Chicago/Turabian StylePerozeni, Federico, Margherita Angelini, Matteo Ballottari, and Stefano Cazzaniga. 2025. "Engineered Chlamydomonas reinhardtii Strains for Enhanced Astaxanthin Production" Life 15, no. 5: 813. https://doi.org/10.3390/life15050813
APA StylePerozeni, F., Angelini, M., Ballottari, M., & Cazzaniga, S. (2025). Engineered Chlamydomonas reinhardtii Strains for Enhanced Astaxanthin Production. Life, 15(5), 813. https://doi.org/10.3390/life15050813








