Enhancing Biomass and Lipid Production in Messastrum gracile Using Inorganic Carbon Substrates and Alternative Solvents for Lipid Extraction
Abstract
1. Introduction
2. Materials and Methods
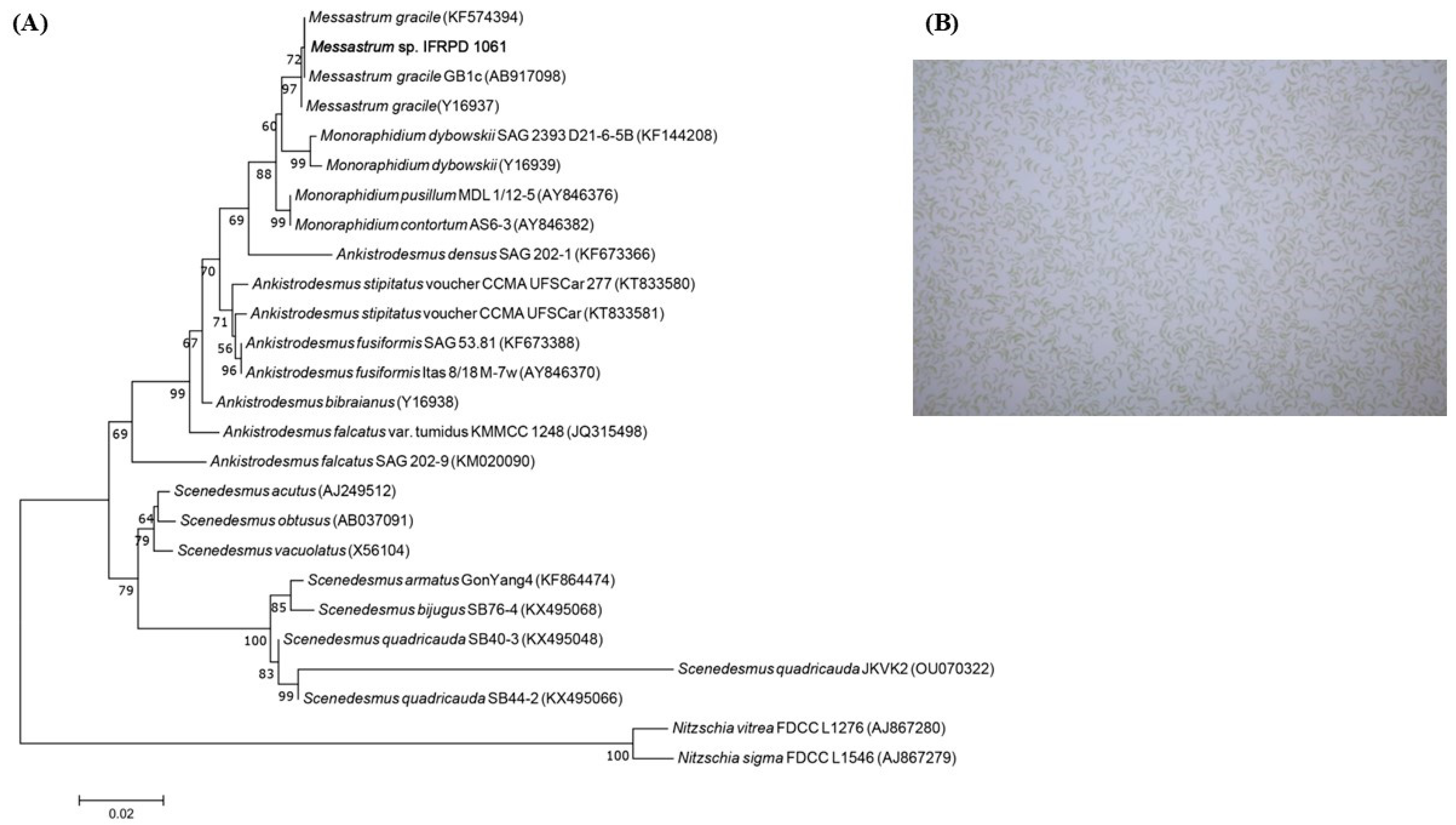
2.1. Microalgae Identification
2.2. Microalga Inoculum Preparation
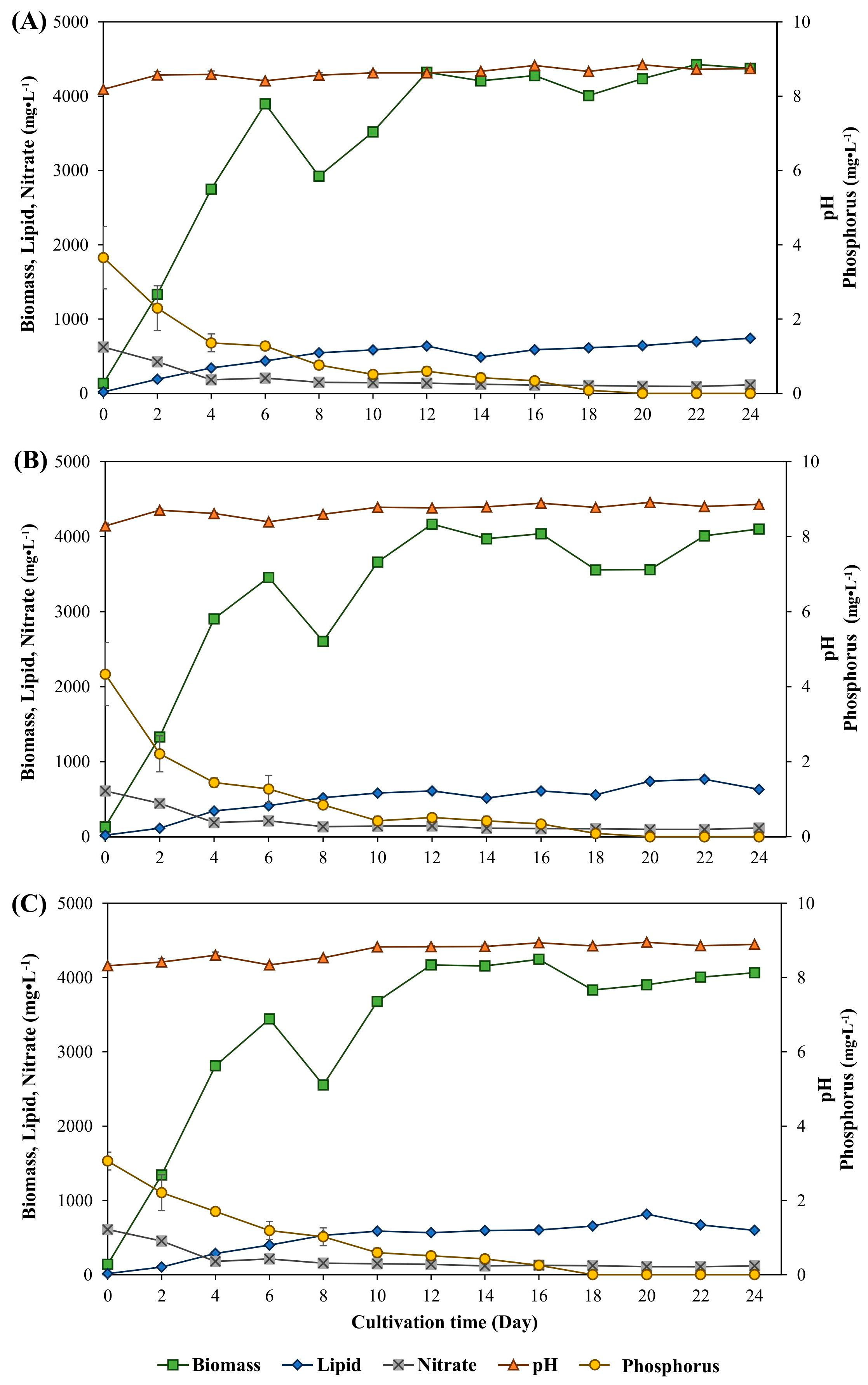
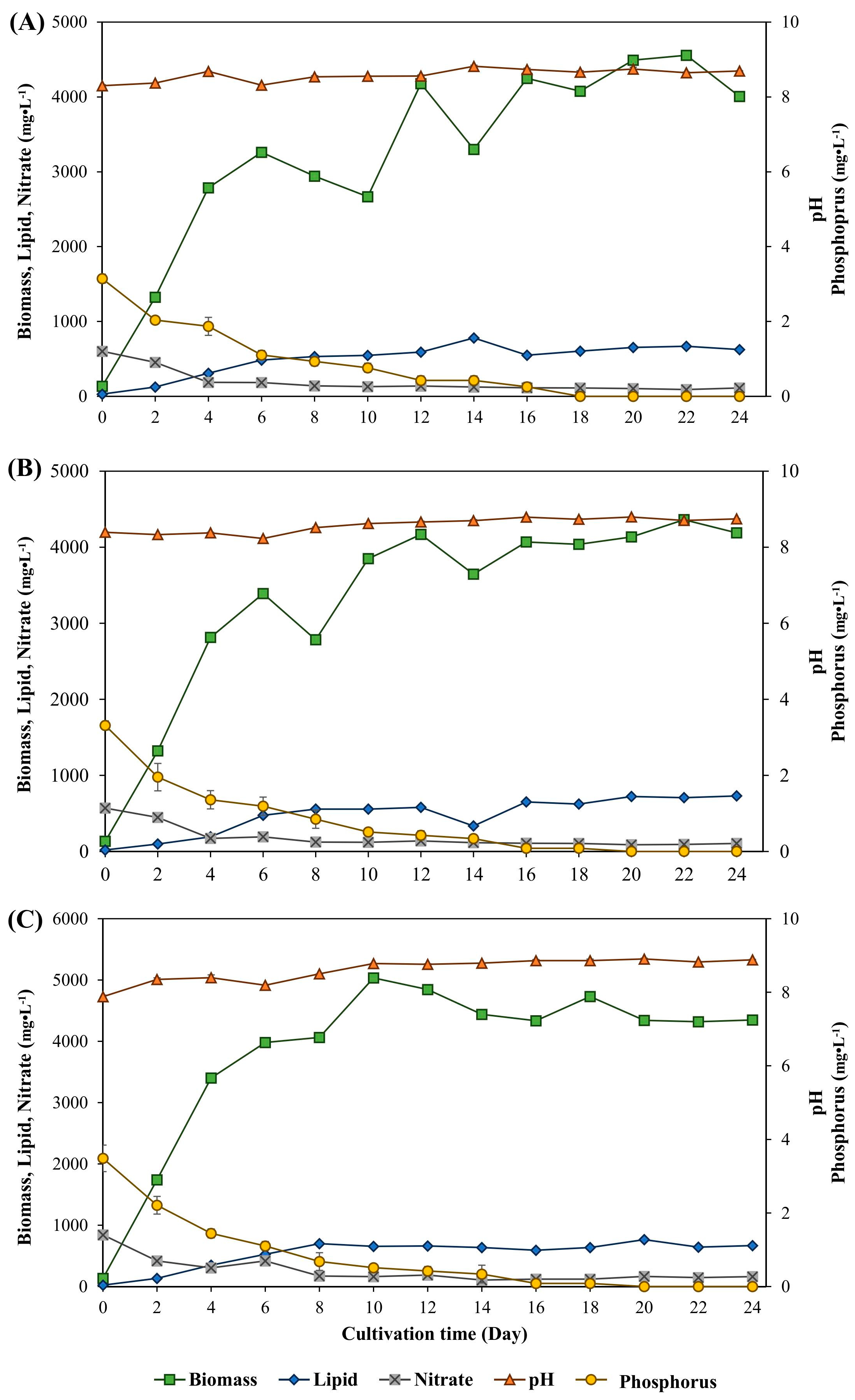
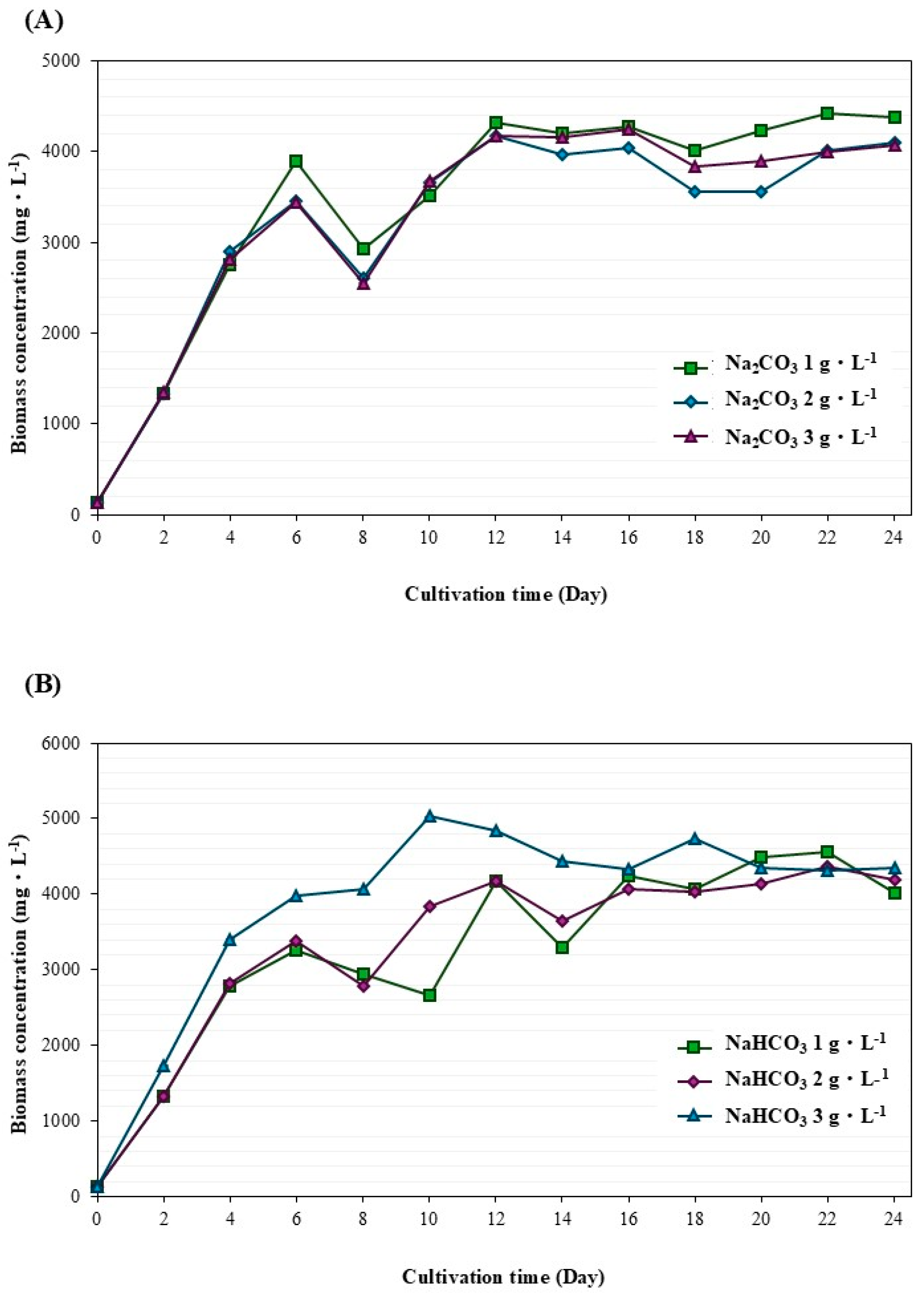
2.3. Biomass and Lipid Accumulation Using Inorganic Supplementation
2.4. Determination of Biomass
2.5. Determination of Lipid Content
2.6. Determination of pH, Nitrate, and Phosphorus
2.7. Kinetic Parameters
2.8. Comparison of Lipid–Solvent Extraction Methods
2.9. Statistical Analysis
3. Results
3.1. Biomass and Lipid Accumulation of Ankistrodesmus gracilis Microalga
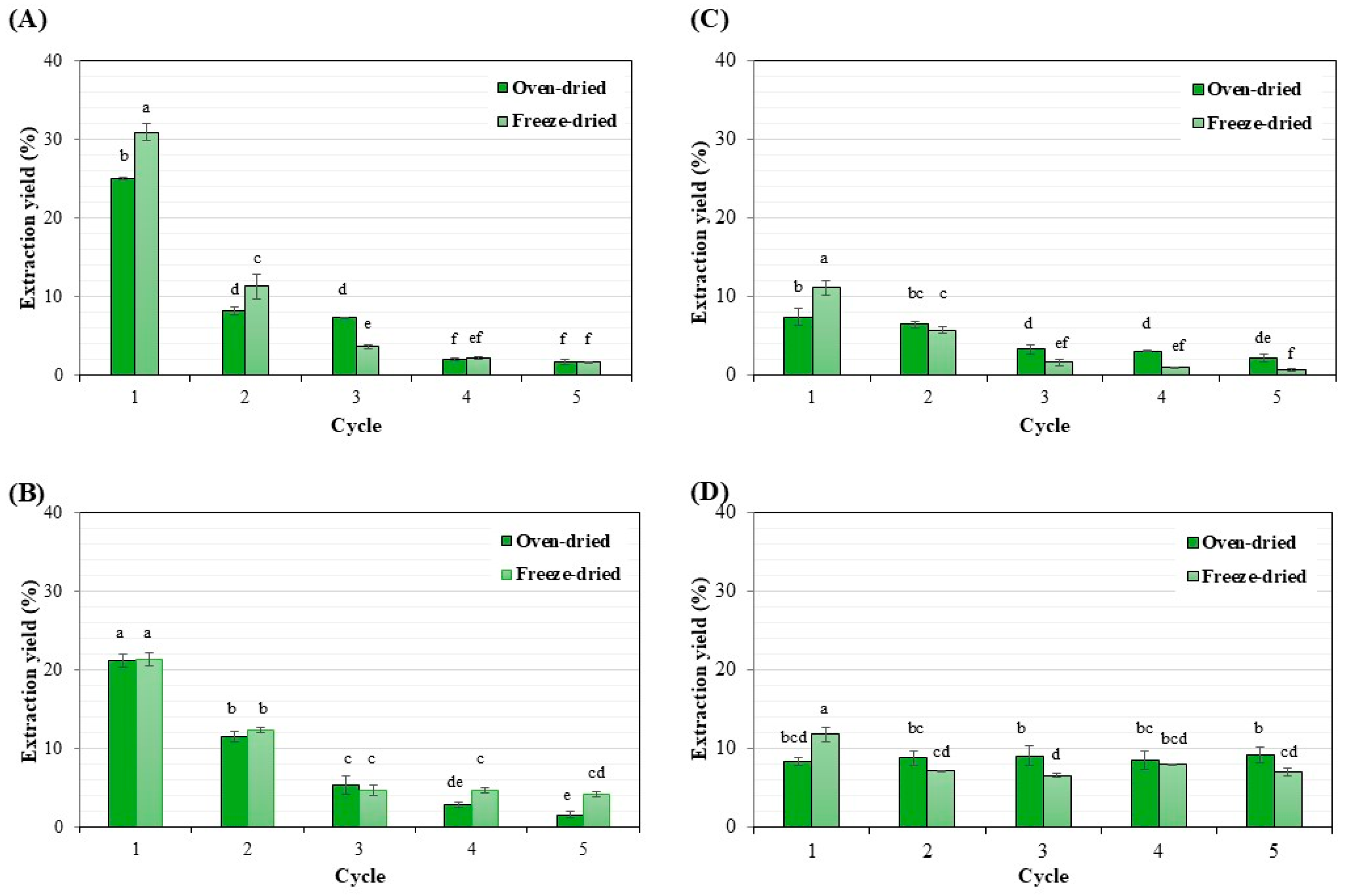
3.2. Comparison of Solvents for Lipid Extraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Maximum specific growth rate (d−1) | |
| Volumetric productivity of biomass production (mg·L−1·d−1) | |
| Volumetric productivity of lipid production (mg·L−1·d−1) |
References
- Marangon, B.B.; Magalhães, I.B.; Pereira, A.S.A.P.; Silva, T.A.; Gama, R.C.N.; Ferreira, J.; Castro, J.S.; Assis, L.R.; Lorentz, J.F.; Calijuri, M.L. Emerging microalgae-based biofuels: Technology, life-cycle and scale-up. Chemosphere 2023, 326, 138447. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, T.; Rajendran, S.; Gnanasekaran, L.; Rachmadona, N.; Jiang, J.J.; Khoo, K.S.; Show, P.L. Bioengineering strategies of microalgae biomass for biofuel production: Recent advancement and insight. Bioengineered 2023, 14, 2252228. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef] [PubMed]
- Sarıtaş, S.; Kalkan, A.E.; Yılmaz, K.; Gurdal, S.; Göksan, T.; Witkowska, A.M.; Lombardo, M.; Karav, S. Biological and nutritional applications of microalgae. Nutrients 2025, 17, 93. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Tomar, S.; Agarwal, S.; Singh, H.; Kumar, R.; Qureshi, K.A.; Jaremko, M.; Emwas, A.-H.; Rai, P.K. Microalgae: A promising source for biofuel production. Biocatal. Agric. Biotechnol. 2023, 53, 102877. [Google Scholar] [CrossRef]
- Azeez, N.A.; Oyelami, S.; Adekanmi, A.A.; Ologunye, O.B.; Adedigba, S.A.; Akinola, O.J.; Adeduntan, A.S. Biodiesel potentials of microalgal strains isolated from fresh water environment. Environ. Chall. 2021, 5, 100367. [Google Scholar] [CrossRef]
- Maliha, A.; Abu-Hijleh, B. A review on the current status and post-pandemic prospects of third-generation biofuels. Energy Syst. 2023, 14, 1185–1216. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, J.; Fa, Y.; Liu, X.; Lindblad, P. Enhancing microalgal lipid accumulation for biofuel production. Front. Microbiol. 2022, 13, 1024441. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yang, S.; Tan, X. Photosynthetic conversion of carbon dioxide to oleochemicals by cyanobacteria: Recent advances and future perspectives. Front. Microbiol. 2020, 11, 634. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Z.; Qin, L.; Wang, Z.; Hiltunen, E.; Li, Z. Oil production from pilot-scale microalgae cultivation: An economics evaluation. Energy Sources Part B Econ. Plan. Policy 2016, 11, 11–17. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Factories 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Saranya, D.; Shanthakumar, S. Insights into the influence of CO2 supplement on phycoremediation and lipid accumulation potential of microalgae: An exploration for biodiesel production. Environ. Technol. Innov. 2021, 23, 101596. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Alam, M.A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef]
- Vargas-Estrada, L.; Torres-Arellano, S.; Longoria, A.; Arias, D.M.; Okoye, P.U.; Sebastian, P. Role of nanoparticles on microalgal cultivation: A review. Fuel 2020, 280, 118598. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Verma, P. Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum sp. Cultured under the Mixotrophic Condition. Int. J. Environ. Res. Public Health 2022, 19, 3674. [Google Scholar] [CrossRef]
- Abdur Razzak, S.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae cultivation in photobioreactors: Sustainable solutions for a greener future. Green Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Daneshvar, E.; Sik Ok, Y.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and Morphological Changes Triggered by Nitrogen Stress in the Oleaginous Microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Giarikos, D.G.; Brown, J.; Razeghifard, R.; Vo, D.; Castillo, A.; Nagabandi, N.; Gaffney, J.; Zelden, M.; Antakshinova, A.; Rodriguez, S. Effects of nitrogen depletion on the biosorption capacities of Neochloris minuta and Neochloris alveolaris for five heavy metals. Appl. Water Sci. 2021, 11, 39. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.-F.; Kong, F.; Ren, N.-Q.; Ren, H.-Y. Overview on stress-induced strategies for enhanced microalgae lipid production: Application, mechanisms and challenges. Resour. Conserv. Recycl. 2022, 183, 106355. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Lan, C.Q.; Liao, D. Effects of sodium bicarbonate on cell growth, lipid accumulation, and morphology of Chlorella vulgaris. Microb. Cell Fact 2018, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Cordoba-Perez, M.; de Lasa, H. CO2-Derived Carbon Capture Using Microalgae and Sodium Bicarbonate in a PhotoBioCREC Unit: Kinetic Modeling. Processes 2021, 9, 1296. [Google Scholar] [CrossRef]
- Li, Y.; Ghasemi Naghdi, F.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A comparative study: The impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Factories 2014, 13, 14. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Shim, S.-H.; Ryu, Y.-J.; Yang, J.-H.; Lim, S.-M.; Lee, C.-G. Lipid Extraction from Tetraselmis sp. Microalgae for Biodiesel Production Using Hexane-based Solvent Mixtures. Biotechnol. Bioprocess Eng. 2018, 23, 16–22. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Jiménez Callejón, M.J.; Robles Medina, A.; Macías Sánchez, M.D.; Esteban Cerdán, L.; González Moreno, P.A.; Navarro López, E.; Hita Peña, E.; Grima, E.M. Obtaining highly pure EPA-rich lipids from dry and wet Nannochloropsis gaditana microalgal biomass using ethanol, hexane and acetone. Algal Res. 2020, 45, 101729. [Google Scholar] [CrossRef]
- Koçer, A.T.; Özçimen, D. A comprehensive study on extracellular green synthesis, antibacterial activity and process design of metallic nanoparticles from Botryococcus braunii microalga. JOM 2023, 75, 5591–5605. [Google Scholar] [CrossRef]
- Pan-utai, W.; Srinophakun, P.; Inrung, W. Nutrients formulation to maximize Ankistrodesmus sp. microalgal cell biomass and lipid productivities. J. Biol. Res. Boll. Della Soc. Ital. Biol. Sper. 2019, 92. [Google Scholar] [CrossRef]
- Kawachi, M.; Tanoi, T.; Demura, M.; Kaya, K.; Watanabe, M.M. Relationship between hydrocarbons and molecular phylogeny of Botryococcus braunii. Algal Res. 2012, 1, 114–119. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef]
- Mitra, R.; Das Gupta, A.; Kumar, R.R.; Sen, R. A cleaner and smarter way to achieve high microalgal biomass density coupled with facilitated self-flocculation by utilizing bicarbonate as a source of dissolved carbon dioxide. J. Clean. Prod. 2023, 391, 136217. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Pan-utai, W.; Poopat, N.; Parakulsuksatid, P. Photoautotrophic Cultivation of Arthrospira maxima for Protein Accumulation under Minimum Nutrient Availability. Appl. Food Biotechnol. 2020, 7, 225–234. [Google Scholar] [CrossRef]
- Bekirogullari, M.; Pittman, J.K.; Theodoropoulos, C. Multi-factor kinetic modelling of microalgal biomass cultivation for optimised lipid production. Bioresour. Technol. 2018, 269, 417–425. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. J. King Saud Univ. Sci. 2019, 31, 1535–1542. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef]
- Sündermann, A.; Eggers, L.F.; Schwudke, D. Liquid Extraction: Bligh and Dyer. In Encyclopedia of Lipidomics; Wenk, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–4. [Google Scholar]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef]
- Shi, T.Q.; Wang, L.R.; Zhang, Z.X.; Sun, X.M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Sundaram, S.; Kishor, K. Carbon-Concentrating Mechanism of Microalgae. In Photosynthetic Microorganisms: Mechanism For Carbon Concentration; Singh, S.K., Sundaram, S., Kishor, K., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 63–81. [Google Scholar]
- Hwangbo, K.; Lim, J.-M.; Jeong, S.-W.; Vikramathithan, J.; Park, Y.-I.; Jeong, W.-J. Elevated inorganic carbon concentrating mechanism confers tolerance to high light in an arctic Chlorella sp. ArM0029B. Front. Plant Sci. 2018, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.; Feike, D.; Mackinder, L.C.; Meyer, M.T.; Griffiths, H.; Jonikas, M.C.; Smith, A.M.; McCormick, A.J. Introducing an algal carbon-concentrating mechanism into higher plants: Location and incorporation of key components. Plant Biotechnol. J. 2016, 14, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 282, 245–253. [Google Scholar] [CrossRef]
- Manhaeghe, D.; Blomme, T.; Van Hulle, S.; Rousseau, D. Experimental assessment and mathematical modelling of the growth of Chlorella vulgaris under photoautotrophic, heterotrophic and mixotrophic conditions. Water Res. 2020, 184, 116152. [Google Scholar] [CrossRef]
- Mokashi, K.; Shetty, V.; George, S.A.; Sibi, G. Sodium bicarbonate as inorganic carbon source for higher biomass and lipid production integrated carbon capture in Chlorella vulgaris. Achiev. Life Sci. 2016, 10, 111–117. [Google Scholar] [CrossRef]
- Gao, P.; Guo, L.; Gao, M.; Zhao, Y.; Jin, C.; She, Z. Regulation of carbon source metabolism in mixotrophic microalgae cultivation in response to light intensity variation. J. Environ. Manag. 2022, 302, 114095. [Google Scholar] [CrossRef]
- Bhandari, M.; Kharkwal, S.; Prajapati, S.K. Bicarbonate-assisted microalgae cultivation for drinking water RO reject recycling and bioproduct generation. Bioresour. Technol. Rep. 2024, 25, 101756. [Google Scholar] [CrossRef]
- Roy, U.K.; Wagner, J.; Radu, T. Production of Metabolites in Microalgae Under Alkali Halophilic Growth Medium Using a Dissolved Inorganic Carbon Source. Waste Biomass Valorization 2023, 14, 3339–3354. [Google Scholar] [CrossRef]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus–A potential strain for bio-fuel production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef]
- Shubert, L.E. 7-Nonmotile Coccoid and Colonial Green Algae. In Freshwater Algae of North America; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: Burlington, VT, USA, 2003; pp. 253–309. [Google Scholar]
- Brennan, G.; Collins, S. Growth responses of a green alga to multiple environmental drivers. Nat. Clim. Chang. 2015, 5, 892–897. [Google Scholar] [CrossRef]
- Sampathkumar, S.J.; Gothandam, K.M. Sodium bicarbonate augmentation enhances lutein biosynthesis in green microalgae Chlorella pyrenoidosa. Biocatal. Agric. Biotechnol. 2019, 22, 101406. [Google Scholar] [CrossRef]
- Guo, W.; Cheng, J.; Song, Y.; Kumar, S.; Ali, K.A.; Guo, C.; Qiao, Z. Developing a CO2 bicarbonation absorber for promoting microalgal growth rates with an improved photosynthesis pathway. RSC Adv. 2019, 9, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, S.; Chen, J.P. Carbon capture and utilization by algae with high concentration CO2 or bicarbonate as carbon source. Sci. Total Environ. 2024, 918, 170325. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.-Y.; Lu, T. Effects of dissolved inorganic carbon and mixing on autotrophic growth of Chlorella vulgaris. Biochem. Eng. J. 2014, 82, 34–40. [Google Scholar] [CrossRef]
- Li, Y.; Huang, A.; Gu, W.; Wu, S.; Xie, X.; Wang, G. Effects of inorganic carbon and light on acetate assimilation by Nannochloropsis oceanica (Eustigmatophyceae) in mixotrophic cultivation. Eur. J. Phycol. 2020, 55, 64–75. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Freitas, M.A.V.; Mata, T.M.; Martins, A.A.; Caetano, N. Biochemical characterization of Phaeodactylum tricornutum for microalgae-based biorefinery. Energy Procedia 2018, 153, 466–470. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Jiang, X.; Wang, X.; Liang, W. Lipid accumulation of Chlorella pyrenoidosa under mixotrophic cultivation using acetate and ammonium. Bioresour. Technol. 2018, 262, 342–346. [Google Scholar] [CrossRef]
- Bellido-Pedraza, C.M.; Torres, M.J.; Llamas, A. The microalgae Chlamydomonas for bioremediation and bioproduct production. Cells 2024, 13, 1137. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, X.; Turcotte, F.; Deschênes, J.-S.; Tremblay, R.; Jolicoeur, M. Current lipid extraction methods are significantly enhanced adding a water treatment step in Chlorella protothecoides. Microb. Cell Factories 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae Bioactive Compounds to Topical Applications Products-A Review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef] [PubMed]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2, 61. [Google Scholar] [CrossRef]
- Wang, Q.; Oshita, K.; Takaoka, M. Effective lipid extraction from undewatered microalgae liquid using subcritical dimethyl ether. Biotechnol. Biofuels 2021, 14, 17. [Google Scholar] [CrossRef]
- Sousa, V.; Pereira, R.N.; Vicente, A.A.; Dias, O.; Geada, P. Microalgae biomass as an alternative source of biocompounds: New insights and future perspectives of extraction methodologies. Food Res. Int. 2023, 173, 113282. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Low power ultrasound treatment for the enhanced production of microalgae biomass and lipid content. Biocatal. Agric. Biotechnol. 2019, 20, 101230. [Google Scholar] [CrossRef]
- Yao, S.; Mettu, S.; Law, S.Q.; Ashokkumar, M.; Martin, G.J. The effect of high-intensity ultrasound on cell disruption and lipid extraction from high-solids viscous slurries of Nannochloropsis sp. biomass. Algal Res. 2018, 35, 341–348. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, R.; Li, T.; Zhou, J.; Cen, K. Biodiesel from wet microalgae: Extraction with hexane after the microwave-assisted transesterification of lipids. Bioresour. Technol. 2014, 170, 69–75. [Google Scholar] [CrossRef]
- Marcheafave, G.G.; Tormena, C.D.; Pauli, E.D.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. Experimental mixture design solvent effects on pigment extraction and antioxidant activity from Coffea arabica L. leaves. Microchem. J. 2019, 146, 713–721. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Bezawada, J.; Kaur, R.; Kuttiraja, M.; Tyagi, R.D. Detergent assisted lipid extraction from wet yeast biomass for biodiesel: A response surface methodology approach. Bioresour. Technol. 2016, 218, 667–673. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Klai, N.; Kaur, R.; Tyagi, R.D.; Surampalli, R.Y. Oleaginous yeast biomass flocculation using bioflocculant produced in wastewater sludge and transesterification using petroleum diesel as a co-solvent. Renew. Energy 2019, 131, 217–228. [Google Scholar] [CrossRef]
- Pan-utai, W.; Parakulsuksatid, P.; Phomkaivon, N. Effect of inducing agents on growth and astaxanthin production in Haematococcus pluvialis: Organic and inorganic. Biocatal. Agric. Biotechnol. 2017, 12, 152–158. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Santoro, I.; Nardi, M.; Benincasa, C.; Costanzo, P.; Giordano, G.; Procopio, A.; Sindona, G. Sustainable and Selective Extraction of Lipids and Bioactive Compounds from Microalgae. Molecules 2019, 24, 4347. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technol. Sustain. 2024, 2, 100060. [Google Scholar] [CrossRef]

| Condition (g·L−1) | (d−1) | (mg·L−1·d−1) | (mg·L−1·d−1) |
|---|---|---|---|
| Sodium carbonate induced | |||
| 1 | 0.54 ± 0.023 a | 195.03 ± 0.006 d | 30.10 ± 0.008 b |
| 2 | 0.53 ± 0.006 a | 336.34 ± 0.013 b | 33.86 ± 0.002 b |
| 3 | 0.52 ± 0.008 a | 256.55 ± 0.001 c | 40.00 ± 0.007 ab |
| Sodium bicarbonate induced | |||
| 1 | 0.51 ± 0.013 a | 201.00 ± 0.001 d | 53.57 ± 0.007 a |
| 2 | 0.52 ± 0.026 a | 192.23 ± 0.009 d | 29.58 ± 0.003 b |
| 3 | 0.54 ± 0.001 a | 392.64 ± 0.015 a | 37.12 ± 0.007 b |
| Condition | Extraction Yield (%) |
|---|---|
| Oven-dried biomass preparation | |
| (1) methanol/chloroform/water | 44.31 ± 0.182 bc |
| (2) methanol/hexane | 42.18 ± 0.153 cd |
| (3) hexane | 21.62 ± 0.048 e |
| (4) methanol/hexane/water | 43.62 ± 0.016 bc |
| Freeze-dried biomass preparation | |
| (1) methanol/chloroform/water | 49.75 ± 0.234 a |
| (2) methanol/hexane | 46.81 ± 0.141 ab |
| (3) hexane | 20.22 ± 0.085 e |
| (4) methanol/hexane/water | 39.71 ± 0.038 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan-utai, W.; Pornpukdeewattana, S.; Inrung, W.; Thurakit, T.; Srinophakun, P. Enhancing Biomass and Lipid Production in Messastrum gracile Using Inorganic Carbon Substrates and Alternative Solvents for Lipid Extraction. Life 2025, 15, 407. https://doi.org/10.3390/life15030407
Pan-utai W, Pornpukdeewattana S, Inrung W, Thurakit T, Srinophakun P. Enhancing Biomass and Lipid Production in Messastrum gracile Using Inorganic Carbon Substrates and Alternative Solvents for Lipid Extraction. Life. 2025; 15(3):407. https://doi.org/10.3390/life15030407
Chicago/Turabian StylePan-utai, Wanida, Soisuda Pornpukdeewattana, Wilasinee Inrung, Theera Thurakit, and Penjit Srinophakun. 2025. "Enhancing Biomass and Lipid Production in Messastrum gracile Using Inorganic Carbon Substrates and Alternative Solvents for Lipid Extraction" Life 15, no. 3: 407. https://doi.org/10.3390/life15030407
APA StylePan-utai, W., Pornpukdeewattana, S., Inrung, W., Thurakit, T., & Srinophakun, P. (2025). Enhancing Biomass and Lipid Production in Messastrum gracile Using Inorganic Carbon Substrates and Alternative Solvents for Lipid Extraction. Life, 15(3), 407. https://doi.org/10.3390/life15030407







