Insights into the Regulatory Effect of Danggui Buxue Tang in Postpartum Dairy Cows Through an Integrated Analysis of Multi-Omics and Network Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. DBT Preparation and Animals Management
2.2. Sample Collection and Serum Markers Analysis
2.3. Sample Pretreatment
2.4. Serum Pharmacochemistry and Metabolomics Profiling Analyses
2.5. Network Pharmacology and Molecule Docking
2.6. Fecal 16S rRNA Amplicon Sequencing and Data Pretreatment
2.7. Statistical Analysis
3. Results
3.1. Effect of DBT on Metabolic Biomarkers in Dairy Cow After Calving
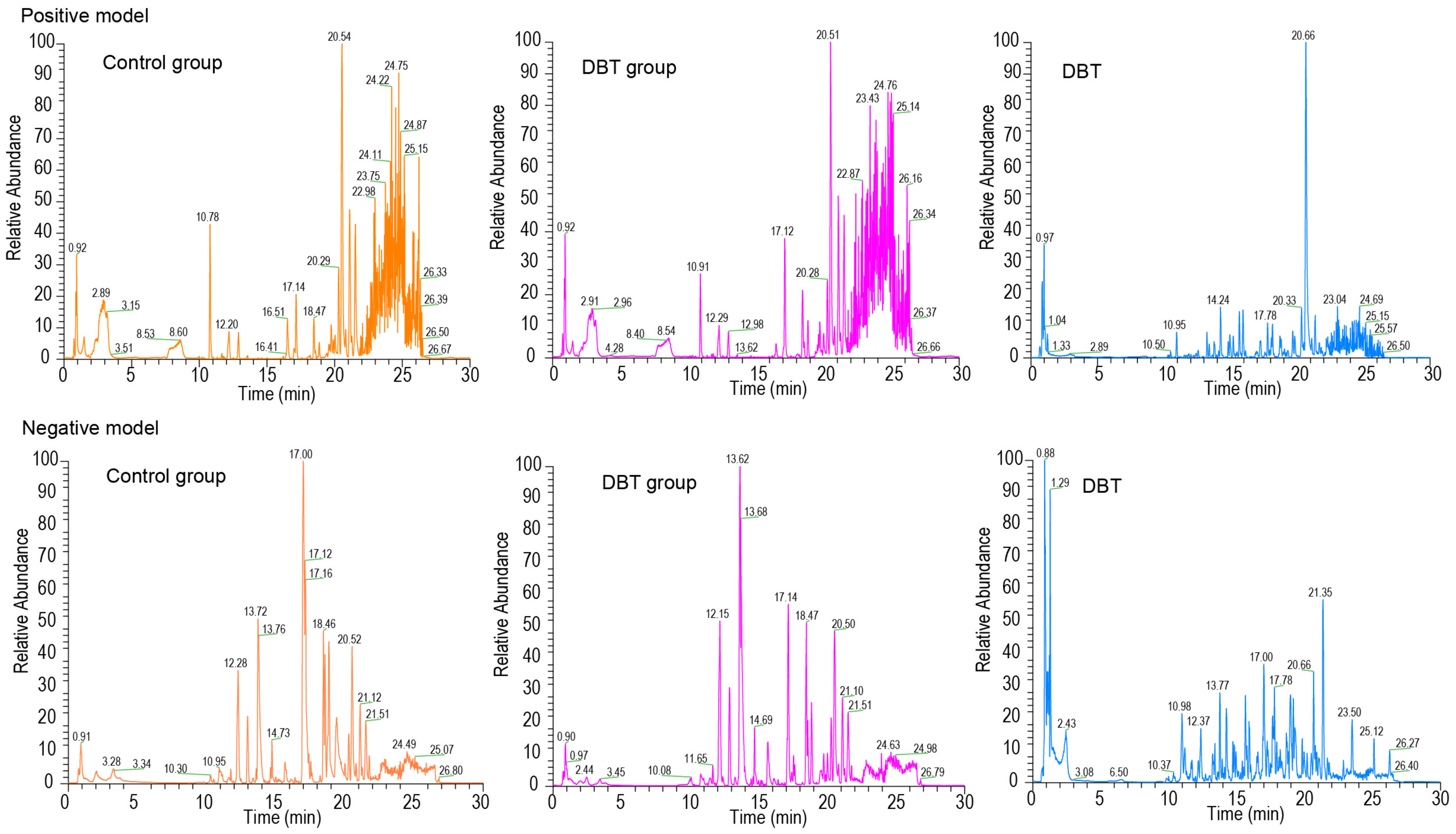
3.2. Identification of Absorbed Prototype Metabolites in DBT
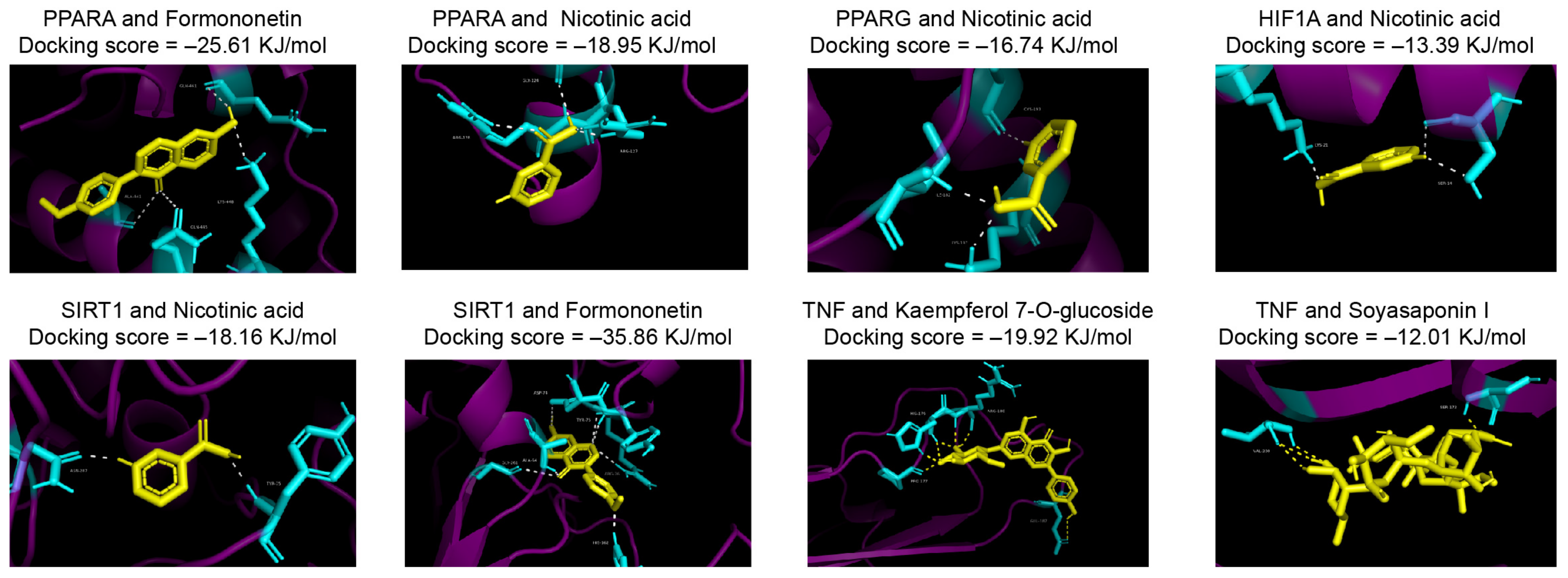
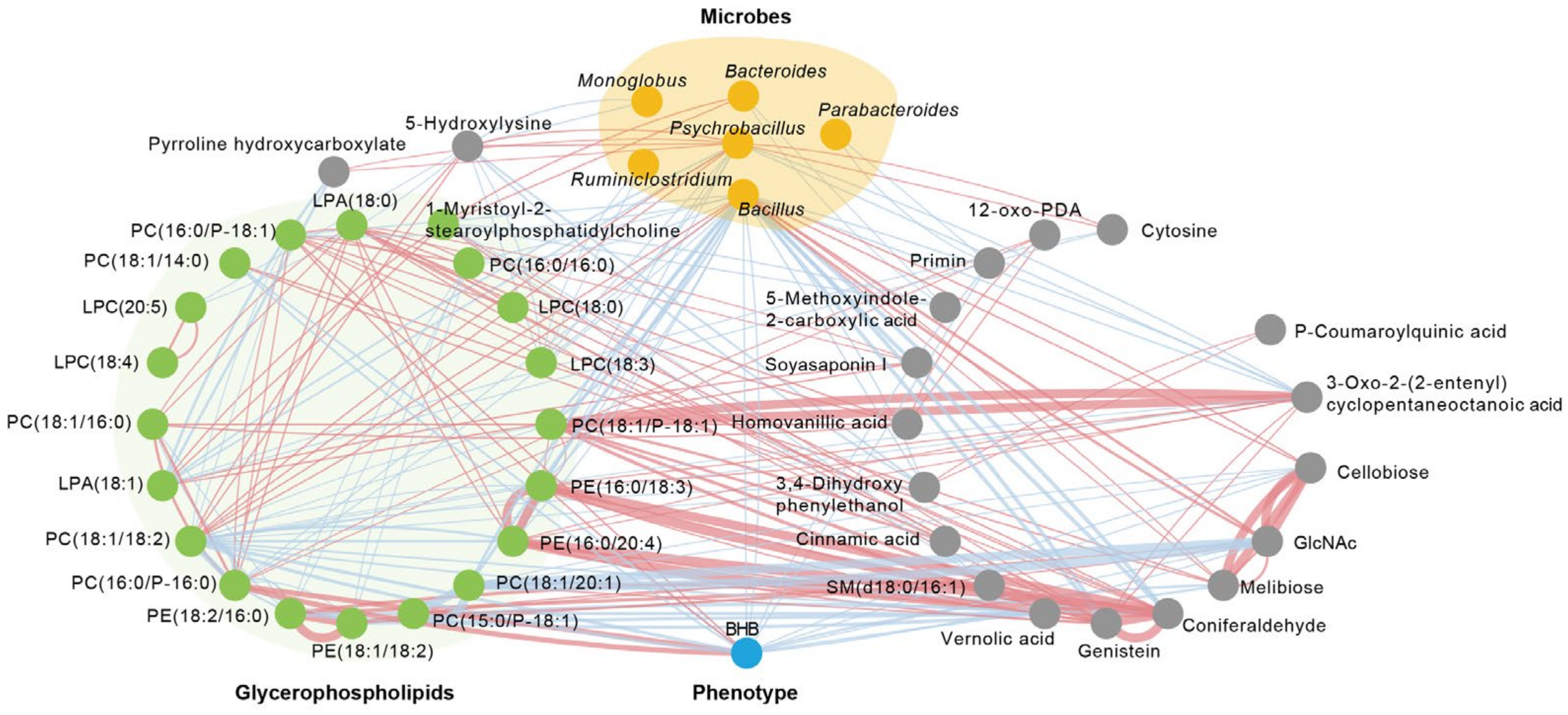
3.3. Network Analysis of Prototype Metabolites in DBT
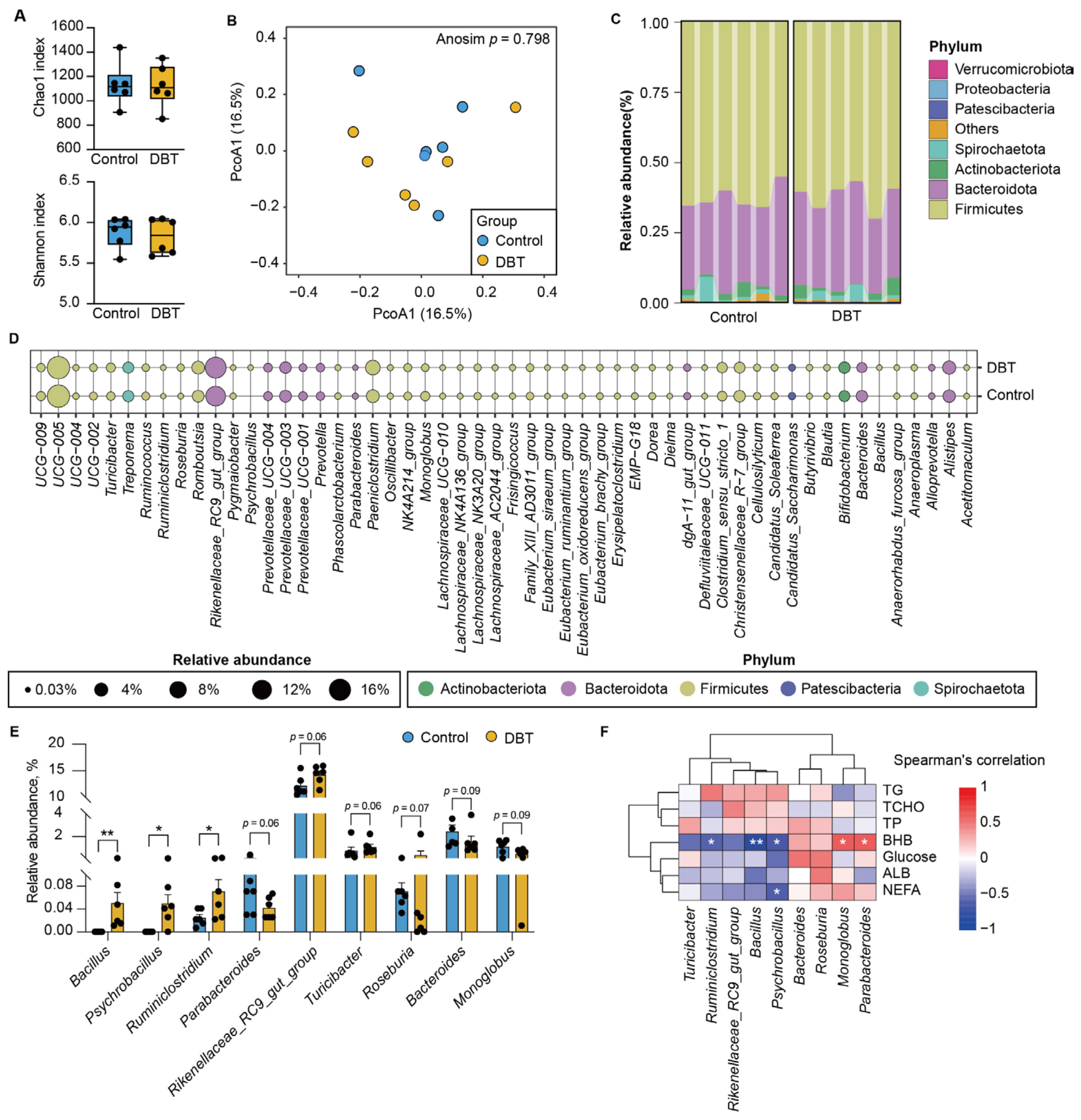
3.4. DBT Intervention Alters the Composition of Fecal Microbiota
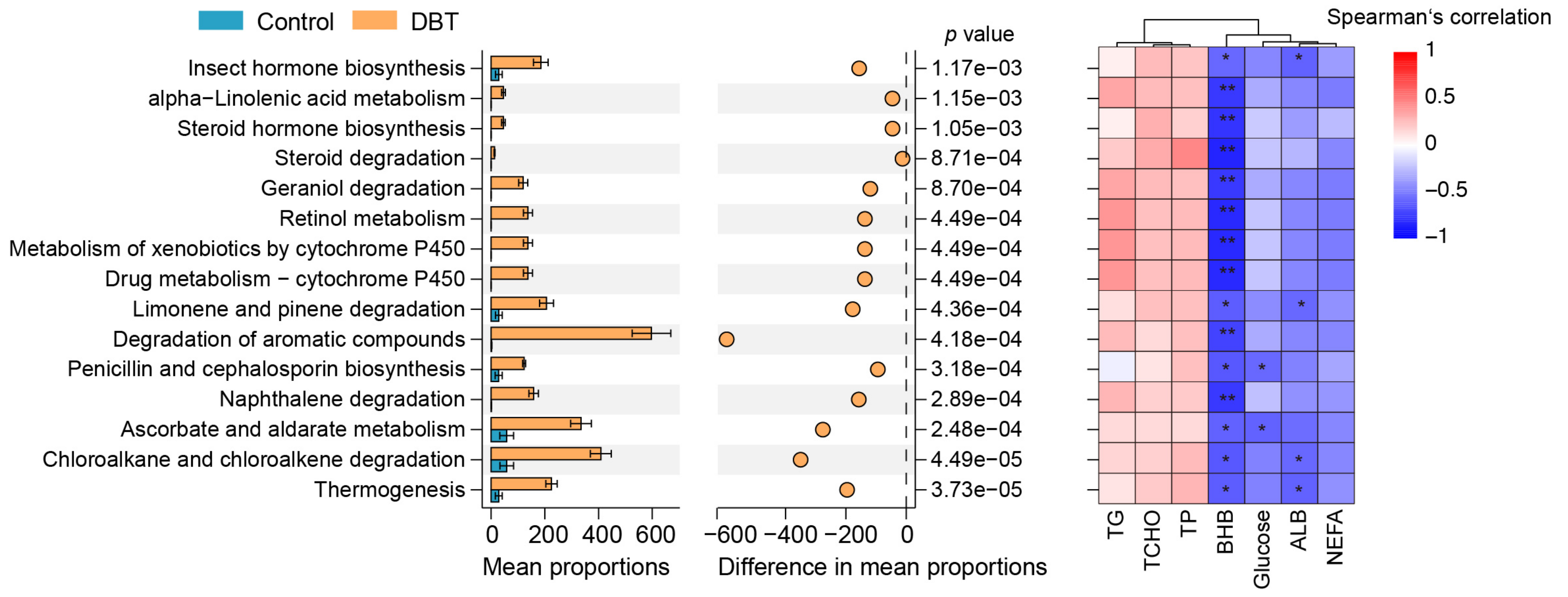
3.5. DBT Alters the Serum Metabolic Profiling of DAIRY Cows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drackley, J.K. Biology of Dairy Cows During the Transition Period: The Final Frontier? J. Dairy. Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Tufarelli, V.; Puvaca, N.; Glamocic, D.; Pugliese, G.; Colonna, M.A. The Most Important Metabolic Diseases in Dairy Cattle during the Transition Period. Animals 2024, 14, 816. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Lopreiato, V.; Mezzetti, M.; Cattaneo, L.; Ferronato, G.; Minuti, A.; Trevisi, E. Role of nutraceuticals during the transition period of dairy cows: A review. J. Anim. Sci. Biotechnol. 2020, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, X.-B.; Peng, Z.-C.; Fu, S.-P.; Zhao, C.-X.; Du, X.-L.; Fang, Z.-Y.; Wang, Z.; Liu, G.-W.; Li, X.-W. Berberine Protects against NEFA-Induced Impairment of Mitochondrial Respiratory Chain Function and Insulin Signaling in Bovine Hepatocytes. Int. J. Mol. Sci. 2018, 19, 1691. [Google Scholar] [CrossRef]
- Wigger, D.; Schumacher, F.; Schneider-Schaulies, S.; Kleuser, B. Sphingosine 1-phosphate metabolism and insulin signaling. Cell Signal 2021, 82, 109959. [Google Scholar] [CrossRef]
- Van Saun, R.J. Indicators of dairy cow transition risks: Metabolic profiling revisited. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2016, 44, 118–126. [Google Scholar] [CrossRef]
- Wang, W.K.; Fan, L.; Ge, F.; Li, Z.; Zhu, J.; Yin, K.; Xia, J.; Xue, M. Effects of Danggui Buxue decoction on host gut microbiota and metabolism in GK rats with type 2 diabetes. Front. Microbiol. 2022, 13, 1029409. [Google Scholar] [CrossRef]
- Du, R.; Bei, H.; Jia, L.; Huang, C.; Chen, Q.; Tao, C.; Chen, J.; Bo, H. Danggui Buxue Tang restores antibiotic-induced metabolic disorders by remodeling the gut microbiota. J. Ethnopharmacol. 2020, 259, 112953. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Liu, W.; Zhang, N.; Gao, Q.; Shangguan, J.; Li, N.; Zhao, Y.; Jia, Y. Danggui Buxue decoction alleviates cyclophosphamide-induced myelosuppression by regulating beta-hydroxybutyric acid metabolism and suppressing oxidative stress. Pharm. Biol. 2023, 61, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gao, Y.; Wang, X.; Cheng, F.; Haq, S.U.; Liang, Y.; Yang, Z.; Li, B.; Liu, Y.; Wang, Y.; et al. Regulation and analysis of the diversity of intestinal microbiota in SD rats by Danggui Buxue Tang (DBT) fermented with Bacillus subtilis. Ann. Microbiol. 2020, 70, 31. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhu, S.; Hou, J.; Tang, Y.; Liu, J.X.; Xu, Q.; Sun, H.Z. The hindgut microbiome contributes to host oxidative stress in postpartum dairy cows by affecting glutathione synthesis process. Microbiome 2023, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhu, S.; Tang, Y.; Liu, X.; Jia, M.; Malmuthuge, N.; Valencak, T.G.; McFadden, J.W.; Liu, J.X.; Sun, H.Z. Gut microbiome is linked to functions of peripheral immune cells in transition cows during excessive lipolysis. Microbiome 2023, 11, 40. [Google Scholar] [CrossRef]
- Luo, Z.; Du, Z.; Huang, Y.; Zhou, T.; Wu, D.; Yao, X.; Shen, L.; Yu, S.; Yong, K.; Wang, B.; et al. Alterations in the gut microbiota and its metabolites contribute to metabolic maladaptation in dairy cows during the development of hyperketonemia. mSystems 2024, 9, e0002324. [Google Scholar] [CrossRef]
- Ma, C.-C.; Jiang, Y.-H.; Wang, Y.; Xu, R.-R. The Latest Research Advances of Danggui Buxue Tang as an Effective Prescription for Various Diseases: A Comprehensive Review. Curr. Med. Sci. 2022, 42, 913–924. [Google Scholar] [CrossRef]
- Zhou, Z.; Yong, K.; Luo, Z.; Du, Z.; Zhou, T.; Li, X.; Yao, X.; Shen, L.; Yu, S.; Huang, Y.; et al. The Positive Regulatory Effect of DBT on Lipid Metabolism in Postpartum Dairy Cows. Metabolites 2025, 15, 58. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Zhu, Z.; Feng, Y.-D.; Zou, Y.-L.; Xiao, Y.-H.; Wu, J.-J.; Yang, Y.-R.; Jiang, X.-X.; Wang, L.; Xu, W. Integrating serum pharmacochemistry, network pharmacology and untargeted metabolomics strategies to reveal the material basis and mechanism of action of Feining keli in the treatment of chronic bronchitis. J. Ethnopharmacol. 2024, 335, 118643. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, S.; Liu, R.; Yu, C.; Zhang, L.; Li, X.-L.; Yan, G.; Zheng, M.; Zhe Min, J. Comprehensive characterization of the chemical composition of Lurong dabu decoction and its absorbed prototypes and metabolites in rat plasma using UHPLC–Q Exactive Orbitrap–HRMS. Food Res. Int. 2022, 161, 111852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, X.; Sun, J.; Bi, W.; Yu, Y. Active components and mechanisms of total flavonoids from Rhizoma Drynariae in enhancing cranial bone regeneration: An investigation employing serum pharmacochemistry and network pharmacology approaches. J. Ethnopharmacol. 2024, 319, 117253. [Google Scholar] [CrossRef]
- Kia, S.; Mohri, M.; Seifi, H.A. Association of precalving serum NEFA concentrations with postpartum diseases and reproductive performance in multiparous Holstein cows: Cut-off values. Vet. Med. Sci. 2023, 9, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.H.; Sadri, H.; Schuh, K.; Dusel, G.; Prehn, C.; Adamski, J.; Koch, C.; Sauerwein, H. Alterations of the acylcarnitine profiles in blood serum and in muscle from periparturient cows with normal or elevated body condition. J. Dairy. Sci. 2020, 103, 4777–4794. [Google Scholar] [CrossRef]
- Shen, T.; Li, X.; Loor, J.J.; Zhu, Y.; Du, X.; Wang, X.; Xing, D.; Shi, Z.; Fang, Z.; Li, X.; et al. Hepatic nuclear factor kappa B signaling pathway and NLR family pyrin domain containing 3 inflammasome is over-activated in ketotic dairy cows. J. Dairy. Sci. 2019, 102, 10554–10563. [Google Scholar] [CrossRef]
- Xu, C.; Shu, S.; Xia, C.; Wang, B.; Zhang, H.; Jun, B. Investigation on the Relationship of Insulin Resistance and Ketosis in Dairy Cows. J. Vet. Sci. Technol. 2014, 5, 162. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, J.; Ma, S.; Huang, X.; Huang, Y. Effect of Chinese herbal medicine treatment on plasma lipid profile and hepatic lipid metabolism in Hetian broiler. Poult. Sci. 2017, 96, 1918–1924. [Google Scholar] [CrossRef]
- Zeng, H.; Xi, Y.; Li, Y.; Wang, Z.; Zhang, L.; Han, Z. Analysis of Astragalus Polysaccharide Intervention in Heat-Stressed Dairy Cows’ Serum Metabolomics. Animals 2020, 10, 574. [Google Scholar] [CrossRef]
- Tian, M.; Li, K.; Liu, R.; Du, J.; Zou, D.; Ma, Y. Angelica polysaccharide attenuates LPS-induced inflammation response of primary dairy cow claw dermal cells via NF-kappaB and MAPK signaling pathways. BMC Vet. Res. 2021, 17, 248. [Google Scholar] [CrossRef]
- Yin, D.; Zhong, Y.; Liu, H.; Hu, J. Lipid metabolism regulation by dietary polysaccharides with different structural properties. Int. J. Biol. Macromol. 2024, 270, 132253. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Q.; Yue, S.J.; Tang, Y.P.; Chen, Y.Y.; Zhou, G.S.; Zhang, J.; Zhu, Z.H.; Liu, P.; Duan, J.A. A network pharmacology approach to investigate the blood enriching mechanism of Danggui buxue Decoction. J. Ethnopharmacol. 2019, 235, 227–242. [Google Scholar] [CrossRef]
- Yang, S.; Wei, L.; Xia, R.; Liu, L.; Chen, Y.; Zhang, W.; Li, Q.; Feng, K.; Yu, M.; Zhang, W.; et al. Formononetin ameliorates cholestasis by regulating hepatic SIRT1 and PPARα. Biochem. Biophys. Res. Commun. 2019, 512, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xie, X.; Wang, N.; Wang, Y.; Zhao, J.; Chen, F.; Qu, F.; Wen, W.; Miao, J.; Cui, H. Formononetin promotes fatty acid β-oxidation to treat non-alcoholic steatohepatitis through SIRT1/PGC-1α/PPARα pathway. Phytomedicine 2024, 124, 155285. [Google Scholar] [CrossRef]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in Animals: Metabolism and Effects in Livestock and Occurrence in Feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Lundh, T.J.O.; Pettersson, H.I.; Martinsson, K.A. Comparative levels of free and conjugated plant estrogens in blood plasma of sheep and cattle fed estrogenic silage. J. Agric. Food Chem. 1990, 38, 1530–1534. [Google Scholar] [CrossRef]
- Tienken, R.; Kersten, S.; Hüther, L.; Frahm, J.; Meyer, U.; Dänicke, S. Relative Bioavailability of Niacin Supplements for Dairy Cows: Effects of Rumen Protection and of Feed Processing. Vet. Sci. 2015, 2, 440–455. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.; Dong, G. Niacin nutrition and rumen-protected niacin supplementation in dairy cows: An updated review. Br. J. Nutr. 2019, 122, 1103–1112. [Google Scholar] [CrossRef]
- Standish, R.B.; Wright, A.D.; Whitehouse, N.L.; Erickson, P.S. Effect of nicotinic acid supplementation on digestion, metabolism, microbiome, and production in late-lactation Holstein cows. J. Dairy. Sci. 2024, 107, 7786–7797. [Google Scholar] [CrossRef]
- Kholif, A.E. A Review of Effect of Saponins on Ruminal Fermentation, Health and Performance of Ruminants. Vet. Sci. 2023, 10, 450. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Khajuria, V.; Gupta, S.; Sharma, N.; Tiwari, H.; Bhardwaj, S.; Dutt, P.; Satti, N.; Nargotra, A.; Bhagat, A.; Ahmed, Z. Kaempferol-3-o-β-d-glucuronate exhibit potential anti-inflammatory effect in LPS stimulated RAW 264.7 cells and mice model. Int. Immunopharmacol. 2018, 57, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy. Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Brodzki, P.; Marczuk, J.; Lisiecka, U.; Szczubial, M.; Brodzki, A.; Gorzkos, H.; Kulpa, K. Comparative evaluation of cytokine and acute-phase protein concentrations in sera of dairy cows with subclinical and clinical ketosis as a different view of the causes of the disease. Vet. World 2021, 14, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.M.; El-Bahr, S.M. Biomarkers of ketosis in dairy cows at postparturient period: Acute phase proteins and pro-inflammatory cytokines. Vet. Arh. 2017, 87, 431–440. [Google Scholar] [CrossRef]
- Luo, Z.; Yong, K.; Du, Z.; Huang, Y.; Zhou, T.; Ma, L.; Yao, X.; Shen, L.; Yu, S.; Yan, Z.; et al. Association between Tryptophan Metabolism and Inflammatory Biomarkers in Dairy Cows with Ketosis. Metabolites 2023, 13, 333. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Jia, W. The influence of gut microbiota on drug metabolism and toxicity. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 31–40. [Google Scholar] [CrossRef]
- Chen, J.; Du, R.; Huang, C.; Jia, L.; Tie, D.; Fan, Z.; Zhou, C.; Chen, Q.; Bo, H. Gut microbiota affects the efficacy of Danggui Buxue Tang by affecting plasma concentration of active ingredients. J. Ethnopharmacol. 2021, 270, 113835. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Cappellozza, B.I.; Sousa, D.O.; Alveblad, C.; Queiroz, O.; Joergensen, J.N.; Rustas, B.-O. Effects of supplementing a Bacillus-based direct-fed microbial on performance, nutrient digestibility, rumen fermentation characteristics, and metabolic responses of lactating dairy cows. JDS Commun. 2024, 5, 107–112. [Google Scholar] [CrossRef]
- Terré, M.; Prat, N.; Sabrià, D.; Queiroz, O.; Joergensen, J.N.; Copani, G.; Cappellozza, B.I. Supplementing a Bacillus-based direct-fed microbial improves feed efficiency in lactating dairy cows. Transl. Anim. Sci. 2024, 8, txae110. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Bellmer, D.; Jadeja, R.; Muriana, P. The Potential of Bacillus Species as Probiotics in the Food Industry: A Review. Foods 2024, 13, 2444. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, D.; Sun, M.; Miao, X.; Jin, X.; Xu, X.; Su, Y.; Xu, H.; Wang, J.; Niu, H. Bacillus natto ameliorates obesity by regulating PI3K/AKT pathways in rats. Biochem. Biophys. Res. Commun. 2022, 603, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Suva, M.; Sureja, V.; Kheni, D. Novel insight on probiotic Bacillus subtilis: Mechanism of action and clinical applications. J. Curr. Res. Sci. Med. 2016, 2, 65–72. [Google Scholar] [CrossRef]
- Chugh, B.; Kamal-Eldin, A. Bioactive compounds produced by probiotics in food products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef]
- Sun, Y.; Nie, Q.; Zhang, S.; He, H.; Zuo, S.; Chen, C.; Yang, J.; Chen, H.; Hu, J.; Li, S.; et al. Parabacteroides distasonis ameliorates insulin resistance via activation of intestinal GPR109a. Nat. Commun. 2023, 14, 7740. [Google Scholar] [CrossRef]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides distasonis: Intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Hendriksen, H.M.A.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2021, 12, 794519. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 41–51. [Google Scholar] [CrossRef]
- Frisardi, V.; Panza, F.; Seripa, D.; Farooqui, T.; Farooqui, A.A. Glycerophospholipids and glycerophospholipid-derived lipid mediators: A complex meshwork in Alzheimer’s disease pathology. Prog. Lipid Res. 2011, 50, 313–330. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy. Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Huang, Y.; Ma, L.; Jiang, J.; Luo, Q.; Yang, Z.; Yong, K.; Shen, L.; Yu, S.; Yao, X.; et al. Untargeted Metabolomics Reveals Metabolic Stress Alleviation by Prepartum Exercise in Transition Dairy Cows. Metabolites 2022, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. A Multi-Platform Metabolomics Approach Identifies Urinary Metabolite Signatures That Differentiate Ketotic from Healthy Dairy Cows. Front. Vet. Sci. 2021, 8, 595983. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, B.; Mauck, J.; Loor, J.J.; Wei, B.; Shen, B.; Wang, Y.; Zhao, C.; Zhu, X.; Wang, J. Plasma and milk metabolomics profiles in dairy cows with subclinical and clinical ketosis. J. Dairy. Sci. 2024, 107, 6340–6357. [Google Scholar] [CrossRef]
- Huang, Y.; Kong, Y.; Shen, B.; Li, B.; Loor, J.J.; Tan, P.; Wei, B.; Mei, L.; Zhang, Z.; Zhao, C.; et al. Untargeted metabolomics and lipidomics to assess plasma metabolite changes in dairy goats with subclinical hyperketonemia. J. Dairy. Sci. 2023, 106, 3692–3705. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Li, L.; Tan, J.; Wang, Y.; Liu, M.; Jiang, L. Multi-omics analysis reveals that the metabolite profile of raw milk is associated with dairy cows’ health status. Food Chem. 2023, 428, 136813. [Google Scholar] [CrossRef]
- Zheng, L.; Xie, C.; Zheng, J.; Dong, Q.; Si, T.; Zhang, J.; Hou, S.T. An imbalanced ratio between PC(16:0/16:0) and LPC(16:0) revealed by lipidomics supports the role of the Lands cycle in ischemic brain injury. J. Biol. Chem. 2021, 296, 100151. [Google Scholar] [CrossRef]
- Potts, S.B.; Brady, K.M.; Scholte, C.M.; Moyes, K.M.; Sunny, N.E.; Erdman, R.A. Rumen-protected choline and methionine during the periparturient period affect choline metabolites, amino acids, and hepatic expression of genes associated with one-carbon and lipid metabolism. J. Dairy. Sci. 2023, 106, 4559–4579. [Google Scholar] [CrossRef]
- Nielsen, N.I.; Ingvartsen, K.L. Propylene glycol for dairy cows. Anim. Feed. Sci. Technol. 2004, 115, 191–213. [Google Scholar] [CrossRef]
- Chalmeh, A.; Pourjafar, M.; Badiei, K.; Jalali, M.; Sebdani, M.M. The comparative effects of dietary monensin and propylene glycol on insulin resistance of transition dairy cows. Trop. Anim. Health Prod. 2020, 52, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.S.; Boomhower, S.R.; Marsh, C.M.; Jack, M.M. Considerations for deriving a safe intake of propylene glycol. Food Chem. Toxicol. 2024, 186, 114460. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]


| Items | Day 0 | Day 7 | SEM | Percentage Change at Day 7 | P Time | P Group | PTime × group | ||
|---|---|---|---|---|---|---|---|---|---|
| Control | DBT | Control | DBT | ||||||
| NEFA (mmol/L) | 0.63 | 0.61 | 0.51 | 0.32 # | 0.05 | −37.25% | 0.005 | 0.11 | 0.24 |
| BHB (mmol/L) | 0.37 | 0.33 | 1.07 | 0.73 * | 0.08 | −31.78% | <0.001 | <0.001 | 0.004 |
| TG (mmol/L) | 0.14 | 0.15 | 0.13 | 0.17 | 0.01 | +30.77% | 0.08 | 0.73 | 0.26 |
| TCHO (mmol/L) | 1.88 | 1.83 | 2.04 | 2.29 | 0.11 | +12.25% | 0.1 | 0.58 | 0.42 |
| Glucose (mmol/L) | 4.44 | 4.78 | 3.58 | 3.3 | 0.2 | −7.82% | <0.0001 | 0.91 | 0.19 |
| ALB (g/L) | 29.42 | 27.41 | 29.31 | 31.36 | 1.78 | +6.99% | 0.54 | 0.99 | 0.52 |
| TP (g/L) | 61.78 | 62.31 | 63.8 | 64.46 | 1.35 | +1.03% | 0.8 | 0.39 | 0.98 |
| Name | Formula | RT (min) | Calculated (m/z) | Adduct | Error (ppm) | MS/MS Spectrum | Origin |
|---|---|---|---|---|---|---|---|
| Amygdalin | C20H27NO11 | 11.915 | 502.15604 | [M + FA-H]- | 1.10 | 59.0120; 71.01202; 89.02302; 101.02272; 113.02249; 179.05492; 221.06511 | DG |
| Formononetin | C16H12O4 | 17.788 | 267.06561 | [M-H]- | 2.62 | 132.0206; 223.03844; 252.04068 | HQ |
| Kaempferol 3-glucuronide | C21H18O12 | 13.185 | 461.07217 | [M-H]- | 0.74 | 59.01207; 85.02802; 113.02233; 175.02414; 285.03918; 327.05161 | HQ |
| Nicotinic acid | C6H5NO2 | 1.437 | 124.03951 | [M + H]+ | 1.85 | 56.05018; 68.05043; 78.03463; 82.06587; 96.04481; 109.05316 | DG, HQ |
| Soyasaponin I | C48H78O18 | 17.571 | 987.51794 | [M + FA-H]- | 0.92 | 85.02788; 143.03345; 205.0695;457.36401; 615.39209; 733.4502; 879.51715; 923.50751; 941.51624 | HQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, K.; Luo, Z.; Zhou, Z.; Huang, Y.; Zhang, C.; Cao, S. Insights into the Regulatory Effect of Danggui Buxue Tang in Postpartum Dairy Cows Through an Integrated Analysis of Multi-Omics and Network Analysis. Life 2025, 15, 408. https://doi.org/10.3390/life15030408
Yong K, Luo Z, Zhou Z, Huang Y, Zhang C, Cao S. Insights into the Regulatory Effect of Danggui Buxue Tang in Postpartum Dairy Cows Through an Integrated Analysis of Multi-Omics and Network Analysis. Life. 2025; 15(3):408. https://doi.org/10.3390/life15030408
Chicago/Turabian StyleYong, Kang, Zhengzhong Luo, Zheng Zhou, Yixin Huang, Chuanshi Zhang, and Suizhong Cao. 2025. "Insights into the Regulatory Effect of Danggui Buxue Tang in Postpartum Dairy Cows Through an Integrated Analysis of Multi-Omics and Network Analysis" Life 15, no. 3: 408. https://doi.org/10.3390/life15030408
APA StyleYong, K., Luo, Z., Zhou, Z., Huang, Y., Zhang, C., & Cao, S. (2025). Insights into the Regulatory Effect of Danggui Buxue Tang in Postpartum Dairy Cows Through an Integrated Analysis of Multi-Omics and Network Analysis. Life, 15(3), 408. https://doi.org/10.3390/life15030408






