Berries as Nature’s Therapeutics: Exploring the Potential of Vaccinium Metabolites in Gastric Cancer Treatment Through Computational Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Screening, and ADMET-Based Filtering of Metabolites in the Vaccinium Genus
2.2. Target Gene Prediction for the Vaccinium Genus
2.3. Collection of RNA Sequence Data Associated with Gastric Cancer
2.4. Compound–Protein and Protein–Protein Interaction Network Construction and Analysis
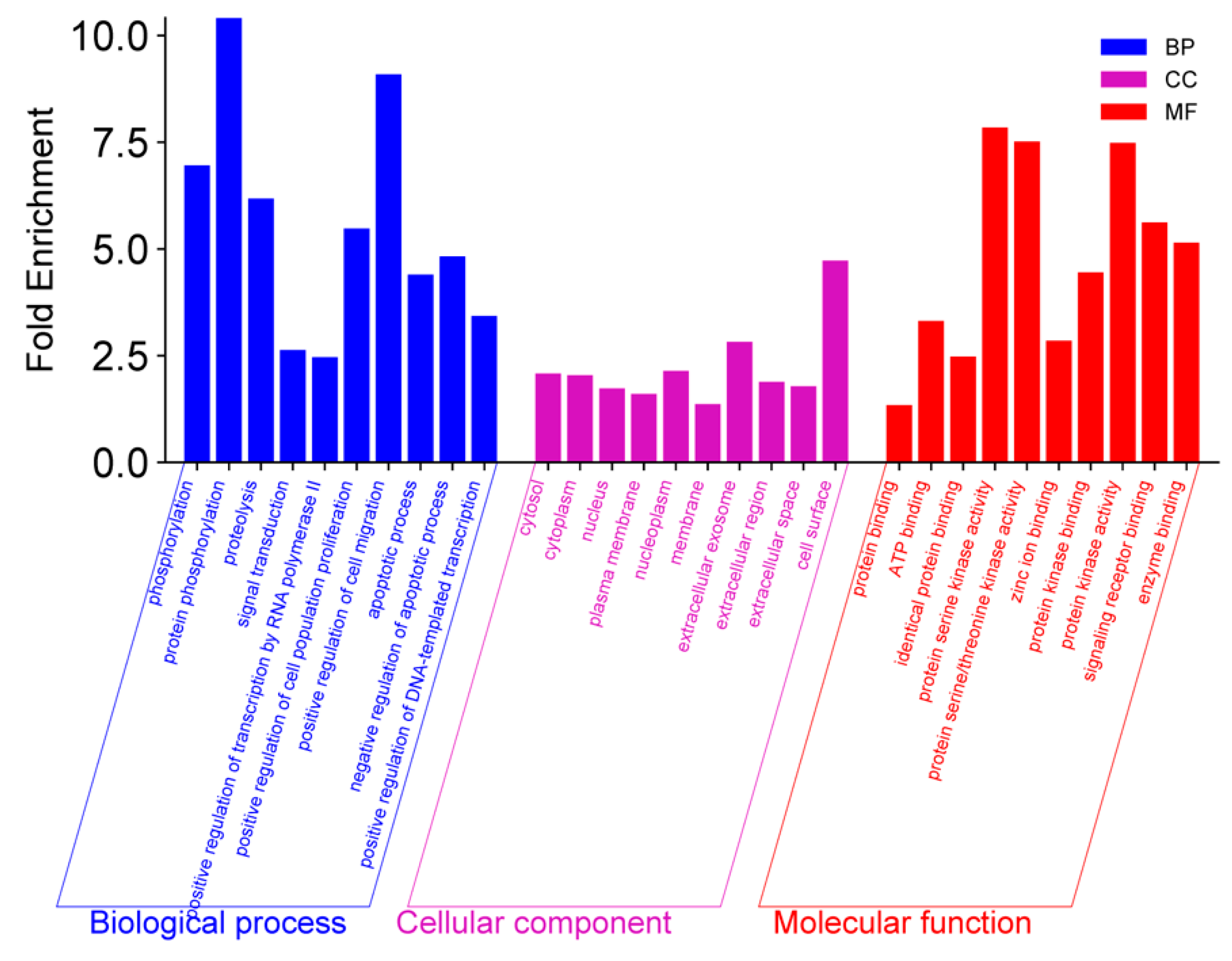
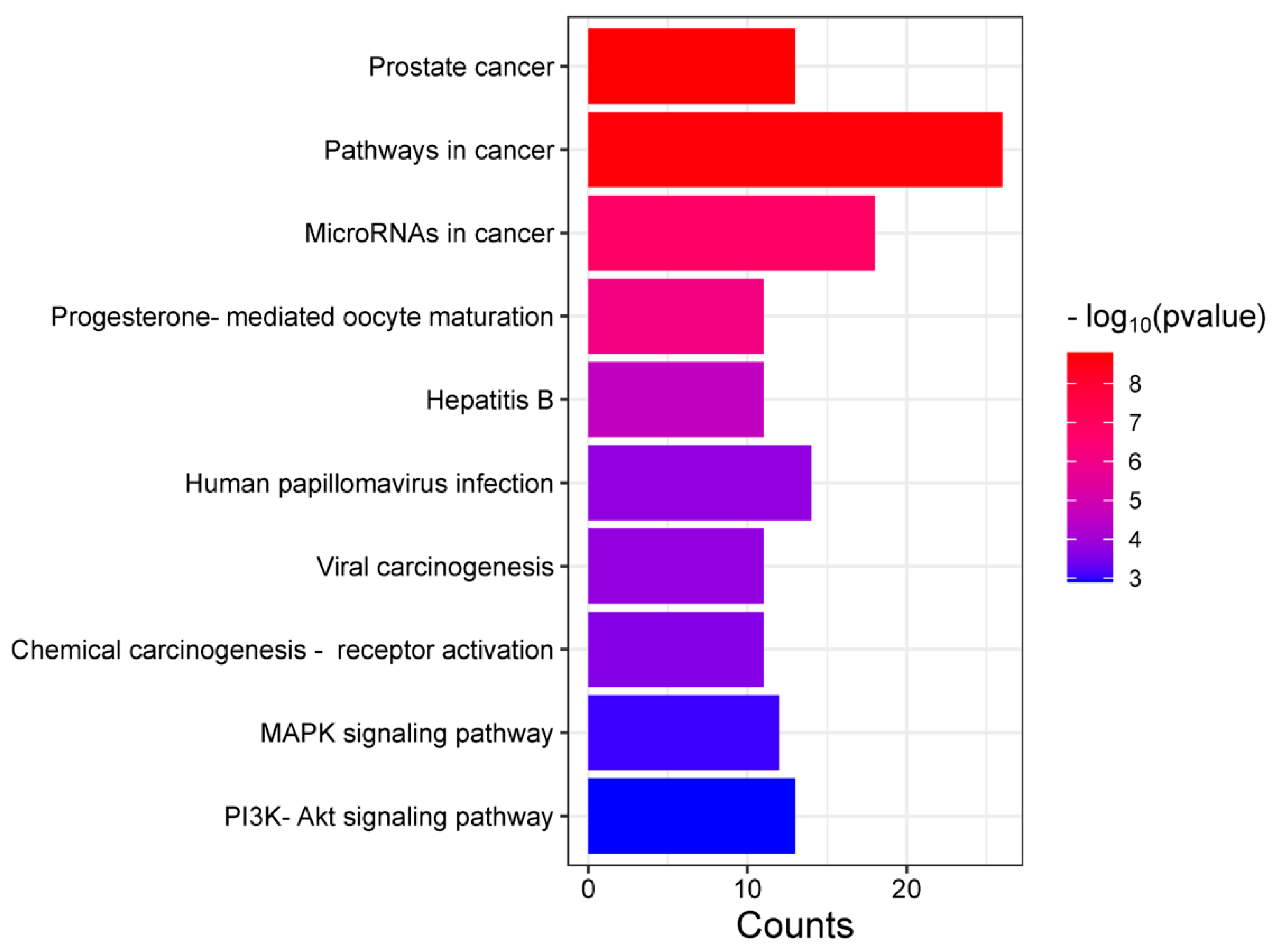
2.5. Gene Ontology and Enrichment Analysis
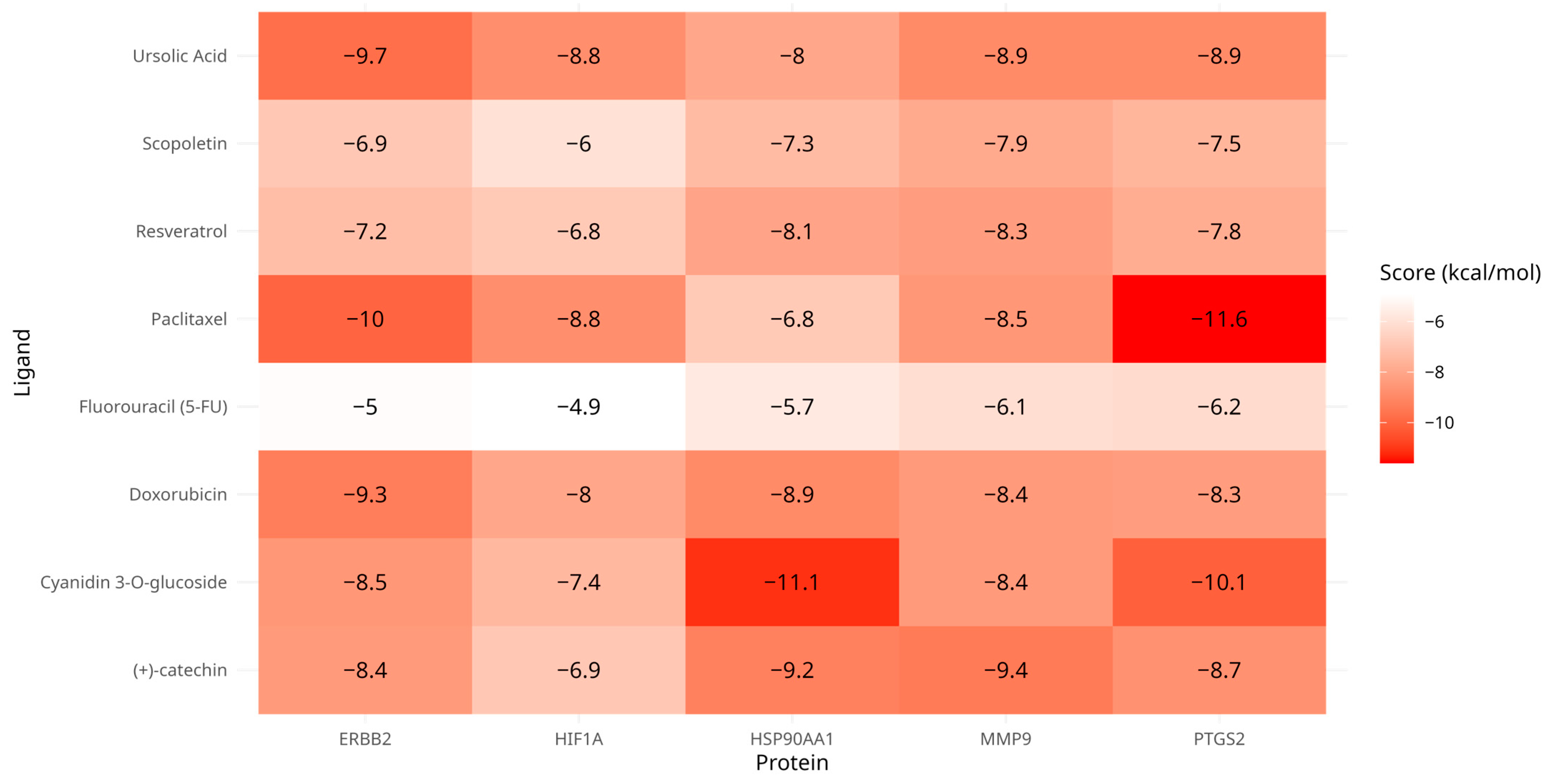
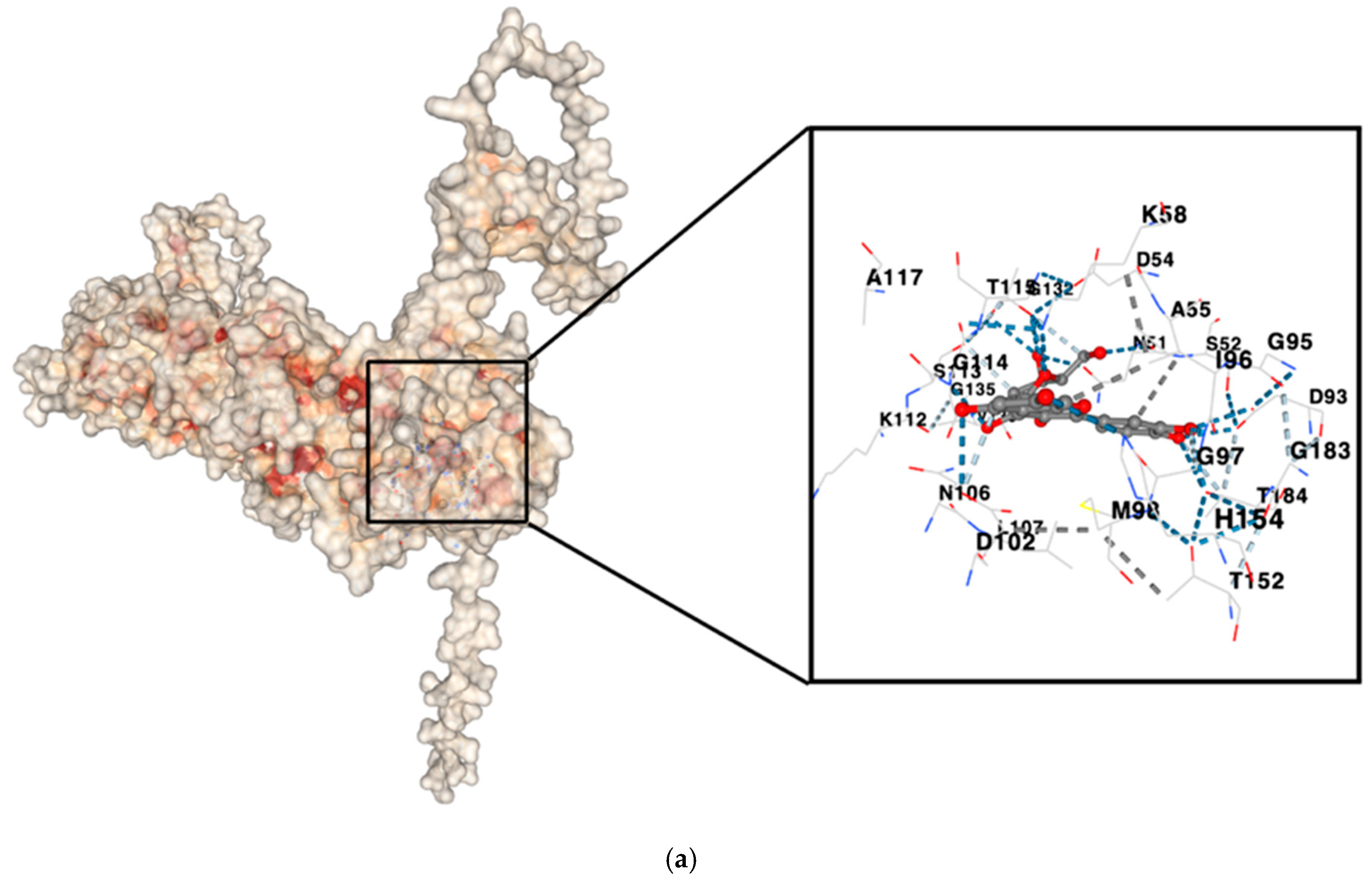
2.6. Molecular Docking
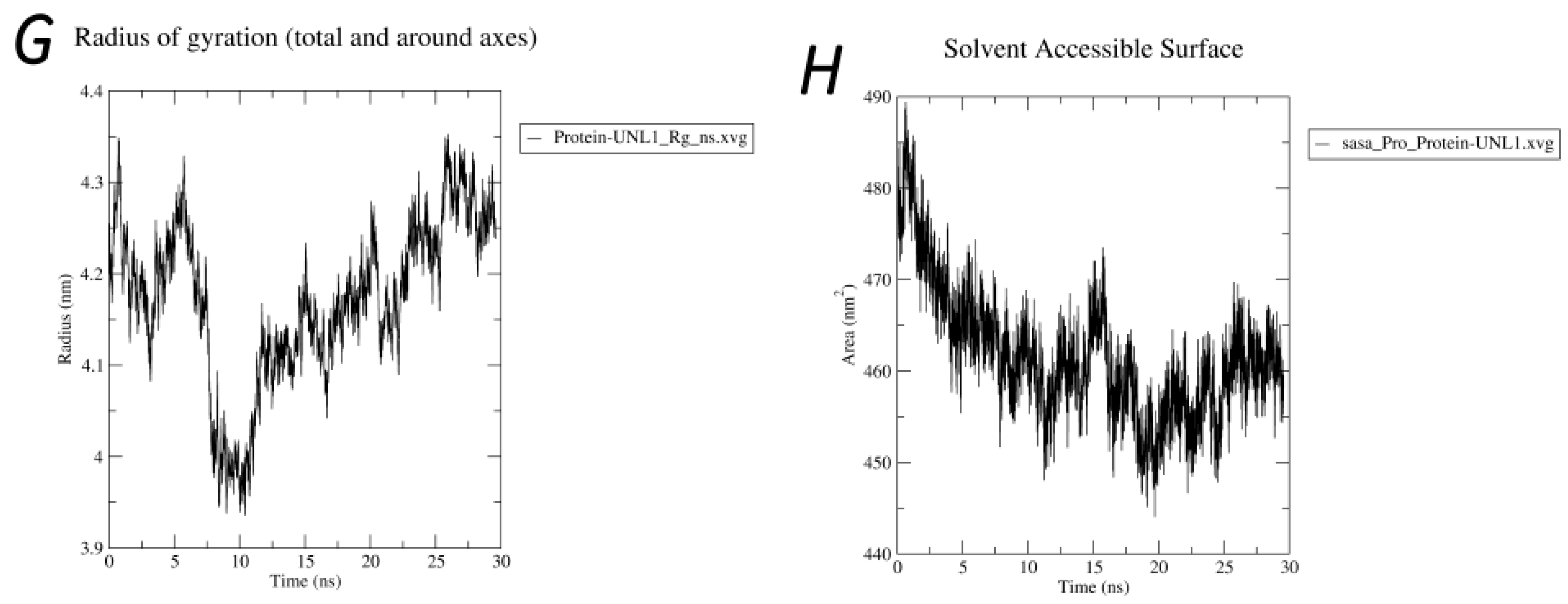
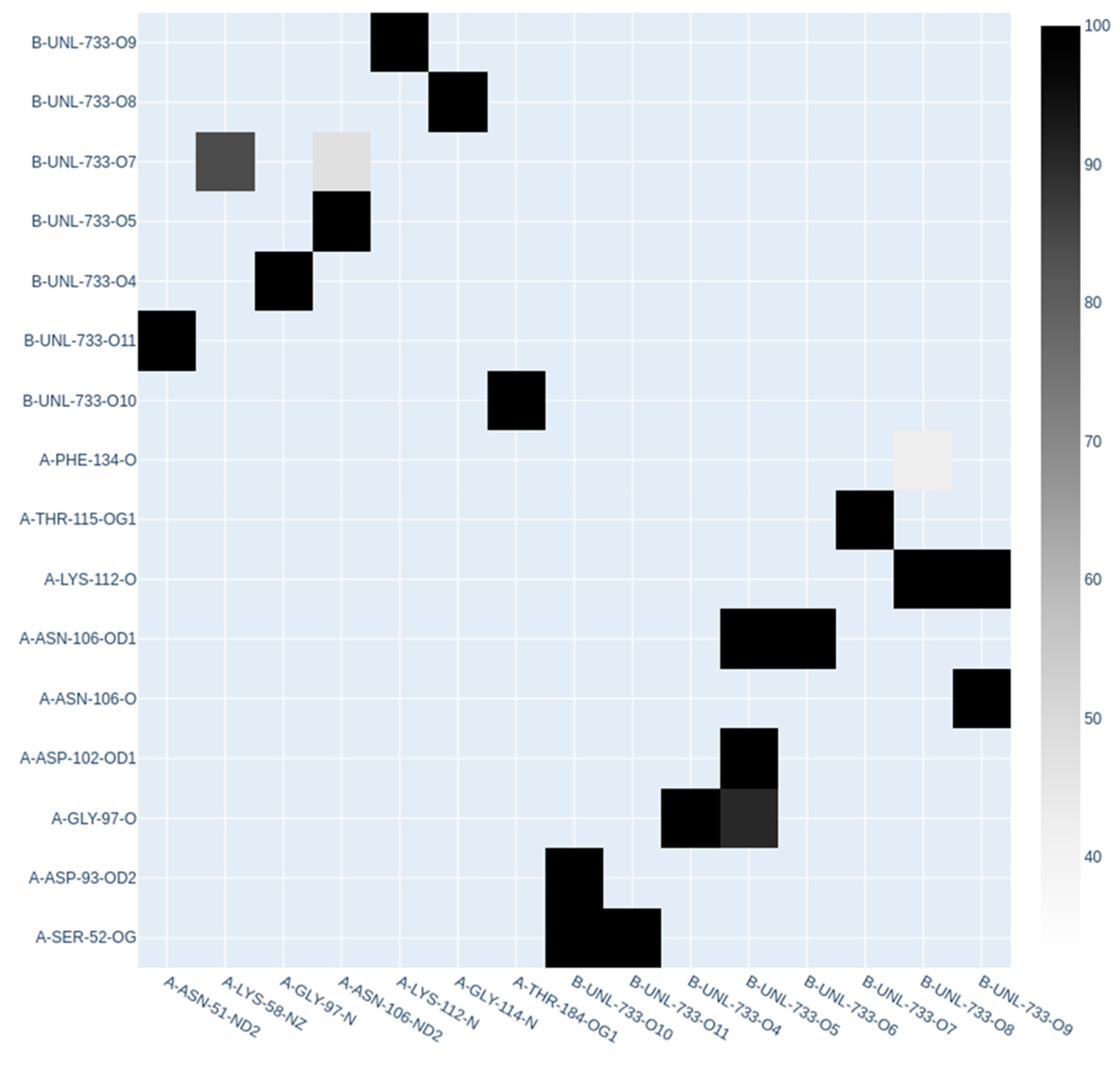
2.7. Molecular Dynamics Simulations
3. Results
3.1. Bioactive Metabolites Present in Vaccinium Species
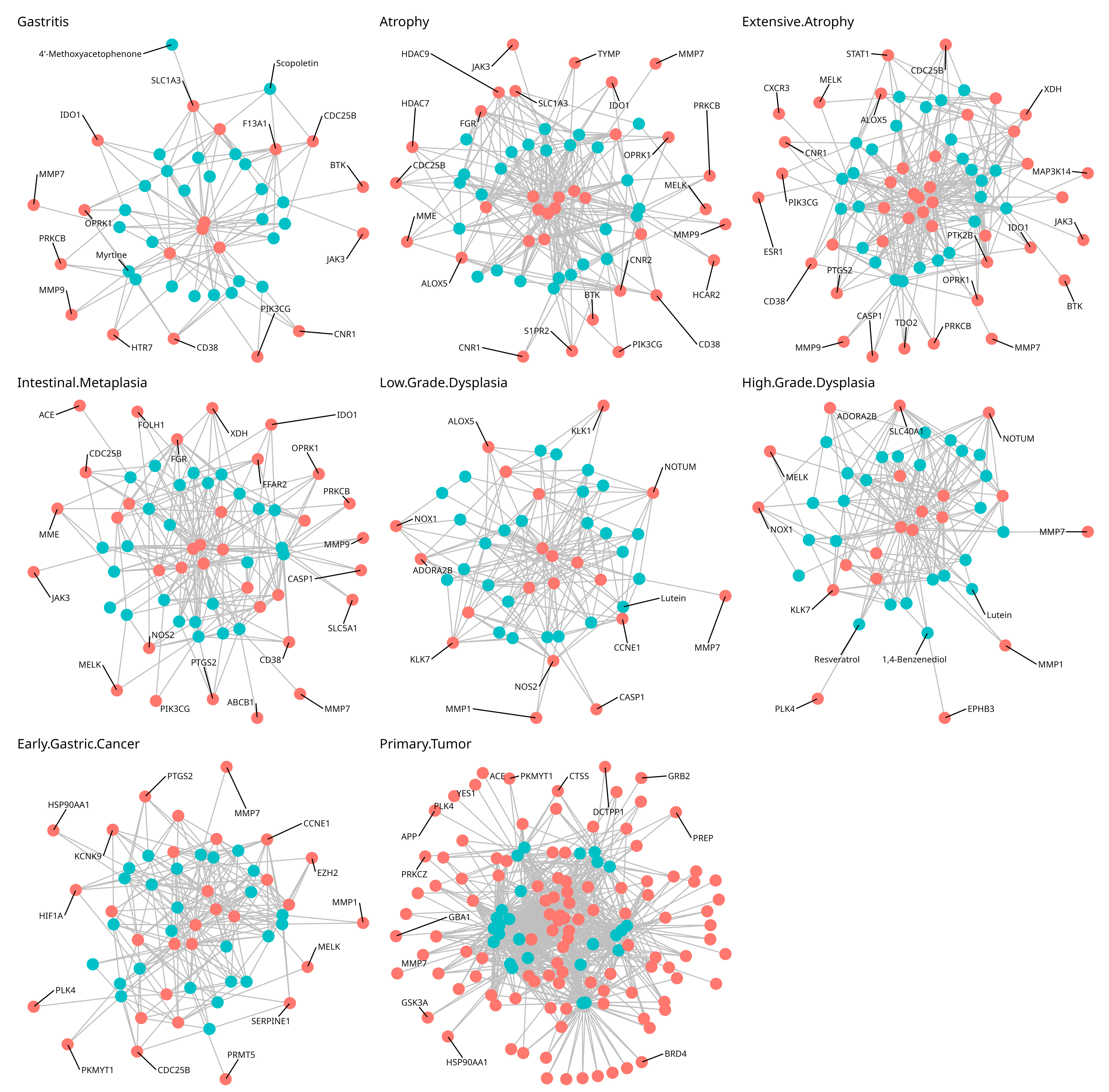
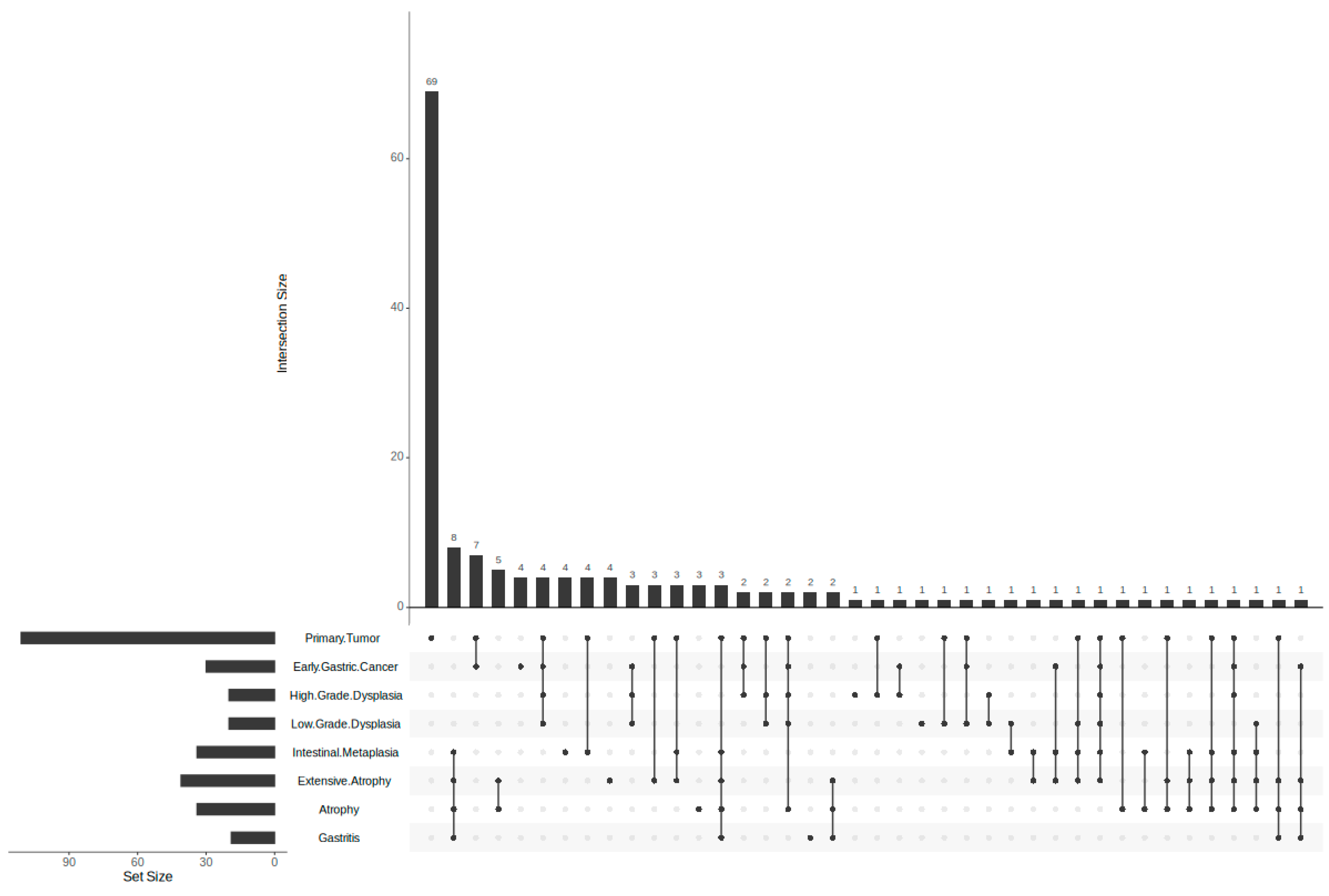
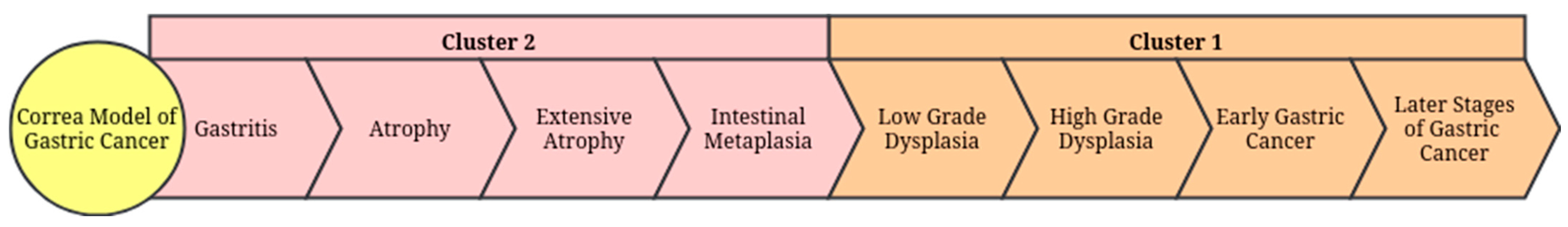
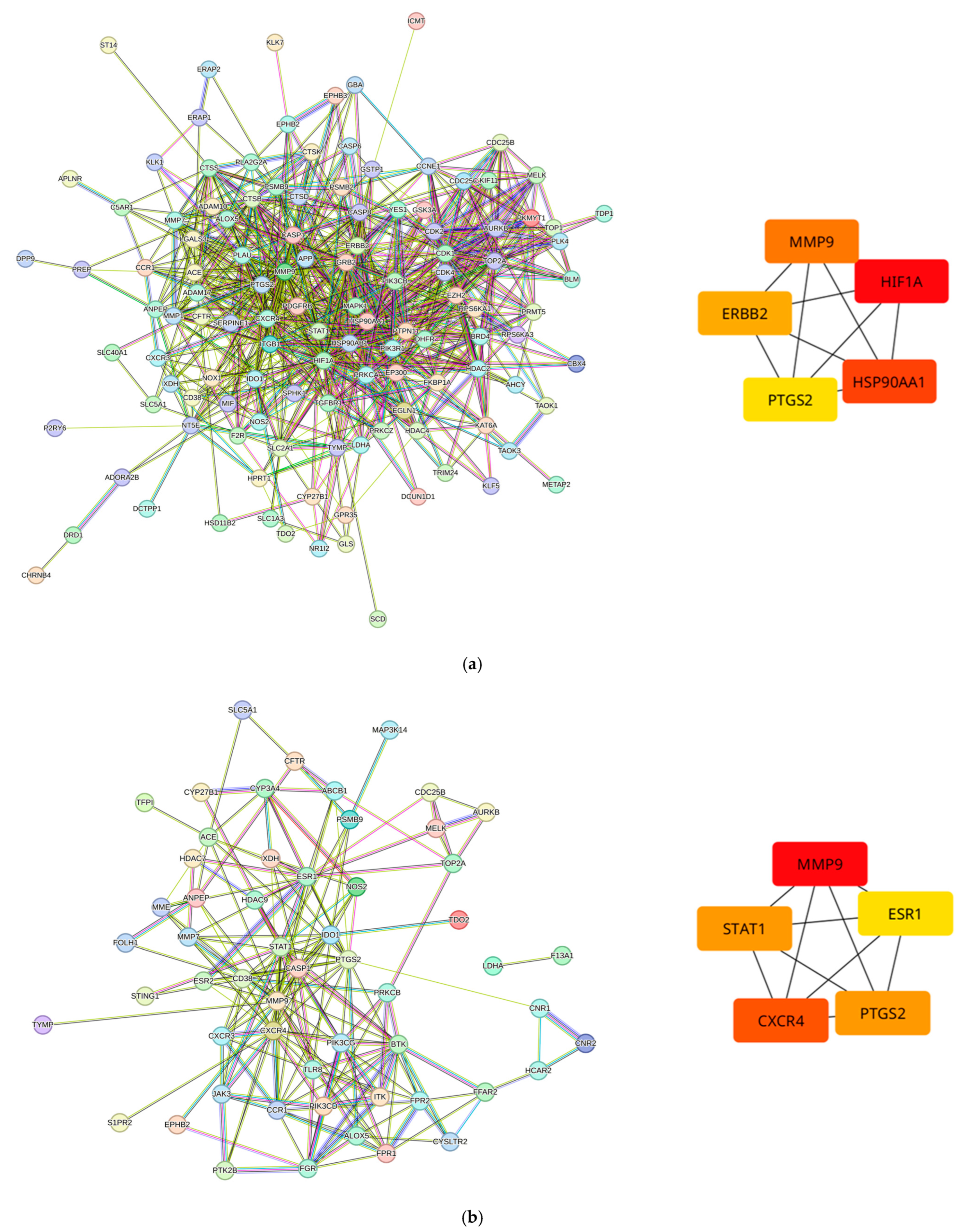
3.2. Predicted Target Proteins of Vaccinium Metabolites and Gastric Cancer
3.3. GO, KEGG, and Reactome Enrichment Analysis
3.4. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migicovsky, Z.; Amyotte, B.; Ulrich, J.C.; Smith, T.; Turner, N.J.; Pico, J.; Ciotir, C.; Sharifi, M.; Meldrum, G.; Stormes, B.; et al. Berries as a Case Study for Crop Wild Relative Conservation, Use, and Public Engagement in Canada. Plants People Planet 2022, 4, 558–578. [Google Scholar] [CrossRef]
- Wunderle, J.M. The Role of Animal Seed Dispersal in Accelerating Native Forest Regeneration on Degraded Tropical Lands. For. Ecol. Manag. 1997, 99, 223–235. [Google Scholar] [CrossRef]
- Agradi, S.; Draghi, S.; Cotozzolo, E.; Barbato, O.; Castrica, M.; Quattrone, A.; Sulce, M.; Vigo, D.; Menchetti, L.; Ceccarini, M.R.; et al. Goji Berries Supplementation in the Diet of Rabbits and Other Livestock Animals: A Mini-Review of the Current Knowledge. Front. Vet. Sci. 2022, 8, 823589. [Google Scholar] [CrossRef]
- Hertel, A.G.; Steyaert, S.M.J.G.; Zedrosser, A.; Mysterud, A.; Lodberg-Holm, H.K.; Gelink, H.W.; Kindberg, J.; Swenson, J.E. Bears and Berries: Species-Specific Selective Foraging on a Patchily Distributed Food Resource in a Human-Altered Landscape. Behav. Ecol. Sociobiol. 2016, 70, 831–842. [Google Scholar] [CrossRef]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef]
- Albert, N.; Iorizzo, M.; Mengist, M.F.; Montanari, S.; Zalapa, J.; Maule, A.; Edger, P.P.; Yocca, A.E.; Platts, A.E.; Pucker, B.; et al. Vaccinium as a Comparative System for Understanding of Complex Flavonoid Accumulation Profiles and Regulation in Fruit. Plant Physiol. 2023, 192, 1696–1710. [Google Scholar] [CrossRef]
- Edger, P.P.; Iorizzo, M.; Bassil, N.V.; Benevenuto, J.; Ferrão, L.F.V.; Giongo, L.; Hummer, K.; Lawas, L.M.F.; Leisner, C.P.; Li, C.; et al. There and Back Again; Historical Perspective and Future Directions for Vaccinium Breeding and Research Studies. Hortic. Res. 2022, 9, uhac083. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.V.; Obotnin, S.I.; Kosolapova, N.V.; Luginina, E.A.; Egoshina, T.L. Wild Berries in Tetraonidae Nutrition. IOP Conf. Ser. Earth Environ. Sci. 2022, 1010, 012119. [Google Scholar] [CrossRef]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Alsharairi, N.A. A Review with a Focus on Vaccinium-Berries-Derived Bioactive Compounds for the Treatment of Reproductive Cancers. Plants 2024, 13, 1047. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Kopystecka, A.; Kozioł, I.; Radomska, D.; Bielawski, K.; Bielawska, A.; Wujec, M. Vaccinium uliginosum and Vaccinium myrtillus—Two Species—One Used as a Functional Food. Nutrients 2023, 15, 4119. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on Global Epidemiology, Risk and Prognostic Factors of Gastric Cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Lin, J.X.; Lin, G.T.; Huang, C.M.; Zheng, C.H.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Li, P. Global Incidence and Mortality Trends of Gastric Cancer and Predicted Mortality of Gastric Cancer by 2035. BMC Public Health 2024, 24, 1763. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Sundar, H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric Cancer: Classification, Histology and Application of Molecular Pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Yin, S.Y.; Wei, W.C.; Jian, F.Y.; Yang, N.S. Therapeutic Applications of Herbal Medicines for Cancer Patients. Evid. Based Complement. Altern. Med. 2013, 2013, 302426. [Google Scholar] [CrossRef]
- Chandra, S.; Gahlot, M.; Choudhary, A.N.; Palai, S.; de Almeida, R.S.; de Vasconcelos, J.E.L.; dos Santos, F.A.V.; de Farias, P.A.M.; Coutinho, H.D.M. Scientific Evidences of Anticancer Potential of Medicinal Plants. Food Chem. Adv. 2023, 2, 100239. [Google Scholar] [CrossRef]
- Graham, J.; Quinn, M.; Fabricant, D.; Farnsworth, N. Plants Used against Cancer—An Extension of the Work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Zou, H.; Li, Y.; Liu, X.; Wu, Z.; Li, J.; Ma, Z. Roles of Plant—Derived Bioactive Compounds and Related microRNAs in Cancer Therapy. Phytother. Res. 2020, 35, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Khan, H. Medicinal Plants in Light of History: Recognized Therapeutic Modality. J. Evid. Based Complement. Altern. Med. 2014, 19, 216–219. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.P.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer Prevention and Therapy through the Modulation of Transcription Factors by Bioactive Natural Compounds. Semin. Cancer Biol. 2016, 40, 35–47. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahashi, H.; Altaf-Ul-Amin, M.; Darusman, L.; et al. KNApSAcK Family Databases: Integrated Metabolite-Plant Species Databases for Multifaceted Plant Research. Plant Cell Physiol. 2012, 53, e1. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.C.; Oler, E. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on Drug Classification and Target Prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Wilks, C.; Zheng, S.C.; Chen, F.Y.; Charles, R.; Solomon, B.; Ling, J.P.; Imada, E.L.; Zhang, D.; Joseph, L.; Leek, J.T.; et al. Recount3: Summaries and Queries for Large-Scale RNA-Seq Expression and Splicing. Genome Biol. 2021, 22, 323. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sherman, B.; Lempicki, R. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Jumper, J. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. CB-Dock2: Improved Protein-Ligand Blind Docking by Integrating Cavity Detection, Docking, and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W408–W415. [Google Scholar] [CrossRef]
- Lee, J.B.; Zgair, A.; Taha, D.A.; Zang, X.; Kagan, L.; Kim, T.H.; Kim, M.G.; Yun, H.; Fischer, P.M.; Gershkovich, P. Quantitative Analysis of Lab-to-Lab Variability in Caco-2 Permeability Assays. Eur. J. Pharm. Biopharm. 2017, 114, 38–42. [Google Scholar] [CrossRef]
- Wang, M.; Peng, M.; Shi, H.; Dong, C.-D.; Cui, L.; Chang, H.; Kan, Z.; Zhen, K.; Si, G.; Li, H. Ping Feng Qingfei Mixture Treats 510 Airway Hyperresponsiveness: A Network Pharmacology and Molecular Docking Study. Ann. Palliat. Med. 2021, 10, 4747–4759. [Google Scholar] [CrossRef]
- Carrasco, G.; Corvalan, A.H. Helicobacter pylori—Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol. Res. Pract. 2013, 2013, 393015. [Google Scholar] [CrossRef]
- Huang, R.; Choi, A.; Truong, C.; Yeh, M.; Hwang, J. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver 2019, 13, 596–603. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, Y.; Yang, H.; Tsai, Y.-C.; Chang, W.; Wu, C.-T.; Kuo, H.; Yu, Y.; Yang, E.-H.; Cheng, W.-J.; et al. Evolution of the Correa’s Cascade Steps: A Long-Term Endoscopic Surveillance among Non-Ulcer Dyspepsia and Gastric Ulcer after H. pylori Eradication. J. Formos. Med. Assoc. 2023, 122, 400–410. [Google Scholar] [CrossRef]
- Caldas, A.M.D.C.; Nunes, W.A.; Taboada, R.G.; Cesca, M.G.; Germano, J.N.; Riechelmann, R.S.P. 353P Loss of CDX2 and High COX2 (PTGS2) Expression in Stage IV Colorectal Cancer. Ann. Oncol. 2022, 33, S697. [Google Scholar] [CrossRef]
- Kim, K.; Lee, H.W.; Lee, E.H.; Park, M.I.; Lee, J.S.; Kim, M.S.; Kim, K.; Roh, M.S.; Pak, M.G.; Oh, J.E.; et al. Differential Expression of HSP90 Isoforms and Their Correlations with Clinicopathologic Factors in Patients with Colorectal Cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 978–986. [Google Scholar]
- Mehta, V.K.; Damerla, R.R.; Parida, P.; Rao, M.; Shenoy Belle, V.; Dikhit, P.S.; Palod, A.; Gireesh, R.; Kumar, N.A.N. Expression of PTGS2 Along with Genes Regulating VEGF Signaling Pathway and Association with High-Risk Factors in Locally Advanced Oral Squamous Cell Carcinoma. Cancer Med. 2024, 13, e6986. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.H.; Im, S.A.; Kim, M.A.; Han, J.K. Human Epidermal Growth Factor Receptor 2 Expression in Unresectable Gastric Cancers: Relationship with CT Characteristics. Korean J. Radiol. 2017, 18, 809–820. [Google Scholar] [CrossRef]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down Regulation of HIF-1α in Cancer Progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Pang, K.; Wang, W.; Qin, J.; Shi, Z.; Hao, L.; Ma, Y.; Xu, H.; Wu, Z.; Pan, D.; Chen, Z.; et al. Role of Protein Phosphorylation in Cell Signaling, Disease, and the Intervention Therapy. MedComm 2022, 3, e175. [Google Scholar] [CrossRef]
- Coopman, P. Protein Phosphorylation in Cancer: Unraveling the Signaling Pathways. Biomolecules 2022, 12, 1036. [Google Scholar] [CrossRef]
- Lintz, M.; Muñoz, A.; Reinhart-King, C.A. The Mechanics of Single Cell and Collective Migration of Tumor Cells. J. Biomech. Eng. 2017, 139, 0210051–0210059. [Google Scholar] [CrossRef]
- Kamińska, K.; Szczylik, C.; Bielecka, Z.F.; Bartnik, E.; Porta, C.; Lian, F.; Czarnecka, A.M. The Role of the Cell—Cell Interactions in Cancer Progression. J. Cell. Mol. Med. 2015, 19, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.; Nasti, T.; Li, Z.; Malla, S.; Buchwald, Z.; Khan, M. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Sahoo, R.K.; Patra, S.K.; Biswal, S.; Biswal, B.K. Molecular Insights to Therapeutic in Cancer: Role of Exosomes in Tumor Microenvironment, Metastatic Progression and Drug Resistance. Drug Discov. Today 2024, 29, 104061. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Song, A.; Julia, Y.; Yap, K.C.-Y.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Frountzas, M.; Karanikki, E.; Toutouza, O.; Sotirakis, D.; Schizas, D.; Theofilis, P.; Tousoulis, D.; Toutouzas, K.G. Exploring the Impact of Cyanidin-3-Glucoside on Inflammatory Bowel Diseases: Investigating New Mechanisms for Emerging Interventions. Int. J. Mol. Sci. 2023, 24, 9399. [Google Scholar] [CrossRef]
- Yang, M.; Abdullah, N.; Ahmad, N.; Hussain, M.; Lu, X.; Xu, J.; Zhong, H.; Guan, R. A Review of Recent Advances on Cyanidin-3-Glucoside: The Biotransformation, Absorption, Bioactivity and Applications of Nano-Encapsulation. Food Funct. 2023, 14, 6320–6345. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, M.; Li, X.; Wang, D.; Yu, L.; Ma, F.; Jiang, J.; Zhang, L.; Li, P. Contributions of Common Foods to Resveratrol Intake in the Chinese Diet. Foods 2024, 13, 1267. [Google Scholar] [CrossRef]
- Liu, G.; Qin, P.; Cheng, X.; Wu, L.; Wang, R.; Gao, W. Ursolic acid: Biological functions and application in animal husbandry. Front. Vet. Sci. 2023, 10, 1251248. [Google Scholar] [CrossRef]
- Pandy, V.; Narasingam, M.; Kunasegaran, T.; Murugan, D.D.; Mohamed, Z. Effect of Noni (Morinda citrifolia Linn.) Fruit and Its Bioactive Principles Scopoletin and Rutin on Rat Vas Deferens Contractility: An Ex Vivo Study. Sci. World J. 2014, 2014, 909586. [Google Scholar] [CrossRef]
- Wu, C.-F.; Wu, C.-Y.; Lin, C.-F.; Liu, Y.-W.; Lin, T.-C.; Liao, H.-J.; Chang, G.-R. The Anticancer Effects of Cyanidin 3-O-Glucoside Combined with 5-Fluorouracil on Lung Large-Cell Carcinoma in Nude Mice. Biomedicines 2022, 151, 113128. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, N.; Zhang, T.; Li, Y.-N.; Xue, H.; Cao, J.; Hou, W.-S.; Liu, J.; Wang, Y.; Jin, C. Cyanidin-3-O-Glucoside Induces the Apoptosis of Human Gastric Cancer MKN-45 Cells through ROS-Mediated Signaling Pathways. Molecules 2023, 28, 652. [Google Scholar] [CrossRef] [PubMed]
- Mirmalek, S.A.; Faraji, S.; Ranjbaran, S.; Aryan, H.; Arani, H.Z.; Jangholi, E.; Marzouni, H.Z.; Salimi-Tabatabaee, S.A. Cyanidin 3-Glycoside Induced Apoptosis in MCF-7 Breast Cancer Cell Line. Arch. Med. Sci. 2020, 19, 1092–1098. [Google Scholar] [CrossRef]
- Tian, J.; Si, X.; Wang, Y.; Gong, E.; Xie, X.; Zhang, Y.; Shu, C.; Li, B. Cyanidin-3-O-Glucoside Protects Human Gastric Epithelial Cells against Helicobacter pylori Lipopolysaccharide-Induced Disorders by Modulating TLR-Mediated NF-κB Pathway. J. Funct. Foods 2020, 68, 103899. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Rivera-Piza, A.; Kim, Y.-J.; Lee, J.H.; Lee, S.-J. Mechanism of Action of Cyanidin 3-O-Glucoside in Gluconeogenesis and Oxidative Stress-Induced Cancer Cell Senescence. Antioxidants 2022, 11, 749. [Google Scholar] [CrossRef]
- Tan, C.; Liu, L.; Liu, X.; Qi, L.; Wang, W.; Zhao, G.; Wang, L.; Dai, Y. Activation of PTGS2/NF-κB Signaling Pathway Enhances Radiation Resistance of Glioma. Cancer Med. 2019, 8, 1175–1185. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Li, Y.; Yang, D.; Li, X.; Shen, C.; Liu, Y.; Ke, X.; Guo, S.; Guo, Z. HSP90AA1-Mediated Autophagy Promotes Drug Resistance in Osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 201. [Google Scholar] [CrossRef]
- Pang, L.Y.; Hurst, E.A.; Argyle, D.J. Cyclooxygenase-2: A Role in Cancer Stem Cell Survival and Repopulation of Cancer Cells during Therapy. Stem Cells Int. 2016, 2016, 2048731. [Google Scholar] [CrossRef]
- Khorshidi, F.; Haghighi, M.M.; Nazemalhosseini Mojarad, E.; Azimzadeh, P.; Damavand, B.; Vahedi, M.; Almasi, S.; Aghdaei, H.A.; Zali, M.R. The Prostaglandin Synthase 2/Cyclooxygenase 2 (PTGS2/COX2) Rs5277 Polymorphism Does Not Influence Risk of Colorectal Cancer in an Iranian Population. Asian Pac. J. Cancer Prev. 2014, 15, 3507–3511. [Google Scholar] [CrossRef]
- Mehta, V.K.; Damerla, R.R.; Dikhit, P.S.; Kumar, N.A.N. Prostaglandin-Endoperoxide Synthase 2 (PTGS2) Gene Expression and Its Association with Genes Regulating the VEGF Signaling Pathway in Head and Neck Squamous Cell Carcinoma. J. Oral Biol. Craniofacial Res. 2023, 13, 567–574. [Google Scholar] [CrossRef]
- Cheng, J. Role of Cyclooxygenase-2 in Gastric Cancer Development and Progression. World J. Gastroenterol. 2013, 19, 7361. [Google Scholar] [CrossRef]
- Saindane, M.; Rallabandi, H.R.; Park, K.S.; Heil, A.; Nam, S.E.; Yoo, Y.B.; Yang, J.-H.; Yun, I.J. Prognostic Significance of Prostaglandin-Endoperoxide Synthase-2 Expressions in Human Breast Carcinoma: A Multiomic Approach. Cancer Inform. 2020, 19, 117693512096969. [Google Scholar] [CrossRef] [PubMed]
- Kosumi, K.; Hamada, T.; Zhang, S.; Liu, L.; da Silva, A.; Koh, H.; Twombly, T.S.; Mima, K.; Morikawa, T.; Song, M.; et al. Prognostic Association of PTGS2 (COX-2) over-Expression According to BRAF Mutation Status in Colorectal Cancer: Results from Two Prospective Cohorts and CALGB 89803 (Alliance) Trial. Eur. J. Cancer 2019, 111, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Shu, H.K. EGFR Activation Results in Enhanced Cyclooxygenase-2 Expression through p38 Mitogen-Activated Protein Kinase-Dependent Activation of the Sp1/Sp3 Transcription Factors in Human Gliomas. Cancers 2007, 67, 6121–6129. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Sikora, A.T.; Fu, S. Dual EGFR and COX-2 Inhibition as a Novel Approach to Targeting Head and Neck Squamous Cell Carcinoma. Curr. Cancer Drug Targets 2009, 9, 931–937. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined Chemotherapy with Cyclooxygenase-2 (COX-2) Inhibitors in Treating Human Cancers: Recent Advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef]
- Urban, M. COX-2 Specific Inhibitors Offer Improved Advantages over Traditional NSAIDs. Orthopedics 2000, 23, S761–S764. [Google Scholar] [CrossRef]
- Mansour, H.M.; Mohamed, A.F.; Khattab, M.; El-Khatib, A.S. Heat Shock Protein 90 in Parkinson’s Disease: Profile of a Serial Killer. Neuroscience 2024, 537, 32–46. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Liu, Y.; Zhao, K.; Wei, S.; Sugarman, E.T.; Liu, L.; Zhang, G. Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance. Cells 2022, 11, 2778. [Google Scholar] [CrossRef]
- Li, J.; Buchner, J. Structure, Function and Regulation of the Hsp90 Machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [CrossRef]
- Liu, B.; Qian, D. Hsp90α and Cell Death in Cancers: A Review. Discov. Oncol. 2024, 15, 151. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Chen, C. Analysis of the Prognostic, Diagnostic and Immunological Role of HSP90α in Malignant Tumors. Front. Oncol. 2022, 12, 963719. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, S.; Zhang, X.; Wu, Y.; Tang, H. High Expression of Heat Shock Protein 90 Is Associated with Tumor Aggressiveness and Poor Prognosis in Patients with Advanced Gastric Cancer. PLoS ONE 2013, 8, e62876. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yu, D. Molecular Mechanisms of ErbB2-Mediated Breast Cancer Chemoresistance; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Lemoine, N.; Jain, S.; Silvestre, F.; Lopes, C.; Hughes, C.; McLelland, E.; Gullick, W.; Filipe, M. Amplification and Overexpression of the EGF Receptor and C-erbB-2 Proto-Oncogenes in Human Stomach Cancer. Br. J. Cancer 1991, 64, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Sauter, G.; Moch, H.; Moore, D.; Carroll, P.; Kerschmann, R.; Chew, K.; Mihatsch, M.J.; Gudat, F.; Waldman, F. Heterogeneity of erbB-2 Gene Amplification in Bladder Cancer. Cancer Res. 1993, 53, 2199–2203. [Google Scholar]
- Tateishi, M.; Ishida, T.; Mitsudomi, T.; Kaneko, S.; Sugimachi, K. Prognostic Value of C-erbB-2 Protein Expression in Human Lung Adenocarcinoma and Squamous Cell Carcinoma. Eur. J. Cancer Clin. Oncol. 1991, 27, 1372–1375. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Stenman, G.; Sandros, J.; Nordkvist, A.; Mark, J.; Sahlin, P. Expression of the ERBB2 Protein in Benign and Malignant Salivary Gland Tumors. Genes Chromosomes Cancer 1991, 3, 128–135. [Google Scholar] [CrossRef]
- Kumar, R. The Role of HER2 in Angiogenesis. Semin. Oncol. 2001, 28, 27–32. [Google Scholar] [CrossRef]
- Cheng, X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef]
- Weng, H.; Tan, Z.J.; Hu, Y.P.; Shu, Y.J.; Bao, R.F.; Jiang, L.; Wu, X.S.; Li, M.L.; Ding, Q.; Wang, X.A.; et al. Ursolic Acid Induces Cell Cycle Arrest and Apoptosis of Gallbladder Carcinoma Cells. Cancer Cell Int. 2014, 14, 96. [Google Scholar] [CrossRef]
- Chakravarti, B.; Maurya, R.; Siddiqui, J.A.; Kumar Bid, H.; Rajendran, S.M.; Yadav, P.P.; Konwar, R. In Vitro Anti-Breast Cancer Activity of Ethanolic Extract of Wrightia tomentosa: Role of Pro-Apoptotic Effects of Oleanolic Acid and Ursolic Acid. J. Ethnopharmacol. 2012, 142, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Sudhagar, S.; Sarathkumar, B.; Lakshmi, B.S. EGFR Inhibition by Pentacyclic Triterpenes Exhibit Cell Cycle and Growth Arrest in Breast Cancer Cells. Life Sci. 2014, 95, 53–62. [Google Scholar] [CrossRef]
- Zhao, C.; Yin, S.; Dong, Y.; Guo, X.; Fan, L.; Ye, M.; Hu, H. Autophagy-Dependent EIF2AK3 Activation Compromises Ursolic Acid-Induced Apoptosis through Upregulation of MCL1 in MCF-7 Human Breast Cancer Cells. Autophagy 2013, 9, 196–207. [Google Scholar] [CrossRef]
- Kim, K.H.; Seo, H.S.; Choi, H.S.; Choi, I.; Shin, Y.C.; Ko, S.-G. Induction of Apoptotic Cell Death by Ursolic Acid through Mitochondrial Death Pathway and Extrinsic Death Receptor Pathway in MDA-MB-231 Cells. Arch. Pharm. Res. 2011, 34, 1363–1372. [Google Scholar] [CrossRef]
- Eide, S.; Feng, Z.-P. Doxorubicin Chemotherapy-Induced “Chemo-Brain”: Meta-Analysis. Eur. J. Pharmacol. 2020, 881, 173078. [Google Scholar] [CrossRef]
- Peng, M.; Darko, K.O.; Tao, T.; Huang, Y.; Su, Q.; He, C.; Yin, T.; Liu, Z.; Yang, X. Combination of Metformin with Chemotherapeutic Drugs via Different Molecular Mechanisms. Cancer Treat. Rev. 2017, 54, 24–33. [Google Scholar] [CrossRef]
- Murphy, F.; Middleton, M. Cytostatic and Cytotoxic Drugs. In Side Effects of Drugs Annual; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Reva, B.A.; Finkelstein, A.V.; Skolnick, J. What Is the Probability of a Chance Prediction of a Protein Structure with an RMSD of 6 Å? Fold. Des. 1998, 3, 141–147. [Google Scholar] [CrossRef]
- Aier, I.; Varadwaj, P.K.; Raj, U. Structural Insights into Conformational Stability of Both Wild-Type and Mutant EZH2 Receptor. Sci. Rep. 2016, 6, 34984. [Google Scholar] [CrossRef]
- Schiebel, J.; Gaspari, R.; Wulsdorf, T.; Ngo, K.; Sohn, C.; Schrader, T.E.; Cavalli, A.; Ostermann, A.; Heine, A.; Klebe, G. Intriguing Role of Water in Protein-Ligand Binding Studied by Neutron Crystallography on Trypsin Complexes. Nat. Commun. 2018, 9, 3889. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Craig, E.A. The HSP70 Chaperone Machinery: J Proteins as Drivers of Functional Specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Mimnaugh, E.G.; Costa, B.D.; Myers, C.E.; Neckers, L.M. Inhibition of Heat Shock Protein HSP90-pp60v-src Heteroprotein Complex Formation by Benzoquinone Ansamycins: Essential Role for Stress Proteins in Oncogenic Transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Trepel, J.B. Stressing the Development of Small Molecules Targeting HSP90. Clin. Cancer Res. 2013, 20, 275–277. [Google Scholar] [CrossRef]
- Ren, X.; Li, T.; Zhang, W.; Yang, X. Targeting Heat-Shock Protein 90 in Cancer: An Update on Combination Therapy. Cells 2022, 11, 2556. [Google Scholar] [CrossRef]
- Kristo, A.; Klimis-Zacas, D.; Sikalidis, A. Protective Role of Dietary Berries in Cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-Based Combinatorial Strategies for Cancer Management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef]
- Meilawati, L.; Dewi, R.M.; Tasfiyati, A.N.; Septama, A.W.; Antika, L.D. Scopoletin: Anticancer Potential and Mechanism of Action. Asian Pac. J. Trop. Biomed. 2023, 13, 1–8. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Dong, S.; Ou, C.-S.; Lu, J.-L.; Ye, J.-H.; Liang, Y.-R.; Zheng, X.-Q. Anticarcinogenic Potentials of Tea Catechins. Front. Nutr. 2022, 9, 1060783. [Google Scholar] [CrossRef]



| No. | Compound | Lipinski’s Rule | Caco-2 | 2D Chemical Structure |
|---|---|---|---|---|
| 1 | Resveratrol | Passed | −4.92 | 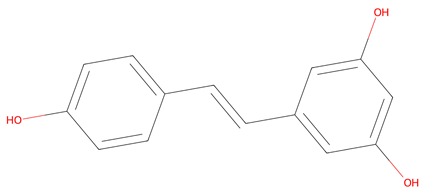 |
| 2 | Ursolic Acid | Passed | −5.54 |  |
| 3 | Scopoletin | Passed | −4.71 |  |
| 4 | (+)-catechin | Passed | −6.17 | 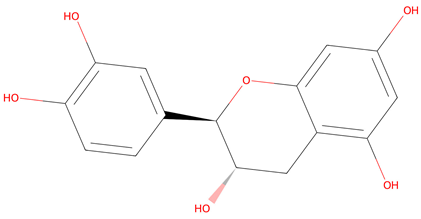 |
| 5 | Cyanidin 3-O-glucoside * | Failed | −6.35 | 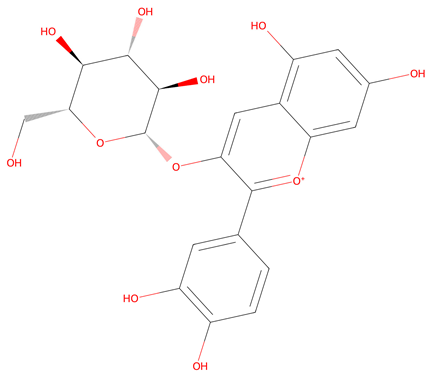 |
| Symbol | Gene Name | Cluster |
|---|---|---|
| MMP9 | Matrix metallopeptidase 9 | 1, 2 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | 1, 2 |
| HIF1A | Hypoxia-inducible factor 1 subunit alpha | 1 |
| ERBB2 | Erb-b2 receptor tyrosine kinase 2 | 1 |
| HSP90AA1 | Heat shock protein 90 alpha family class A member 1 | 1 |
| CXCR4 | C-X-C motif chemokine receptor 4 | 2 |
| ESR1 | Signal transducer and activator of transcription 1 | 2 |
| STAT1 | Signal transducer and activator of transcription 1 | 2 |
| Ligand | Receptor | Binding Energy (kcal/mol) | Binding Sites |
|---|---|---|---|
| Cyanidin 3-O-glucoside | HSP90AA1 | −11.1 | Chain A: LEU48 ILE49 ASN51 SER52 ASP54 ALA55 LYS58 ILE91 ASP93 GLY95 ILE96 GLY97 MET98 ASP102 ASN106 LEU107 THR109 ALA111 LYS112 SER113 GLY114 THR115 LYS116 ALA117 PHE118 GLY132 GLN133 PHE134 GLY135 VAL136 GLY137 PHE138 TYR139 VAL150 THR152 HIS154 GLY183 THR184 VAL186 |
| Cyanidin 3-O-glucoside | PTGS2 | −10.1 | Chain A: CYS21 CYS22 HIS24 PRO25 CYS26 GLN27 ASN28 ARG29 GLY30 VAL31 CYS32 MET33 ASP43 CYS44 THR45 ARG46 THR47 GLY48 PHE49 SER107 HIS108 LEU109 ILE110 ASP111 SER112 PRO113 THR115 TYR116 GLY121 TYR122 LYS123 ARG136 ALA137 LEU138 PRO139 PRO140 PRO142 GLN356 GLN358 GLN447 GLU451 LYS454 ARG455 MET457 |
| Ursolic Acid | ERBB2 | −9.7 | Chain A: GLN81 ARG103 GLY104 THR105 LEU149 LYS150 GLY151 HIS174 ASN176 ASN177 GLN178 CYS255 LEU256 HIS257 PHE258 LEU266 GLY292 ALA293 PRO702 SER703 ARG840 ILE872 ASP873 |
| Paclitaxel * | PTGS2 | −11.8 | Chain A: ASN19 CYS21 ARG29 VAL31 CYS32 MET33 SER34 ASP43 THR45 ARG46 THR47 ILE110 ASP111 SER112 PRO113 PRO114 THR115 TYR116 ASP119 TYR120 GLY121 TYR122 LYS123 SER124 TRP125 PHE128 ALA137 PRO139 PRO140 VAL141 PRO142 ASP143 ASP144 GLN447 ARG455 |
| Fluorouracil (5-FU) * | PTGS2 | −6.2 | Chain A: TYR134 PHE184 ALA185 PHE186 ALA188 GLN189 THR192 HIS193 PHE196 LYS197 THR198 HIS200 GLN275 VAL277 ASN368 TYR371 HIS372 TRP373 HIS374 LEU376 LEU377 LYS432 VAL433 GLN435 ALA436 GLN440 LEU597 LYS598 GLU599 ARG600 SER601 THR602 GLU603 LEU604 |
| Doxorubicin * | ERBB2 | −9.3 | Chain A: LEU726 GLY727 SER728 GLY729 ALA730 PHE731 GLY732 VAL734 ALA751 LYS753 LEU755 ARG756 ASN758 ILE767 GLU770 SER783 THR798 GLN799 LEU800 MET801 GLY804 CYS805 LEU807 ASP808 HIS809 ARG811 ARG844 ASP845 ARG849 ASN850 VAL851 LEU852 THR862 ASP863 LEU866 ARG868 TYR877 ALA879 ASP880 GLY881 GLY882 LYS883 VAL884 PRO885 ILE886 LYS887 TRP888 GLU914 GLY919 ALA920 LYS921 PRO922 PHE1004 PRO1241 THR1242 ALA1243 GLU1244 ASN1245 PRO1246 GLU1247 TYR1248 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpio, A.R.; Talubo, N.D.; Tsai, P.-W.; Chen, B.-Y.; Tayo, L.L. Berries as Nature’s Therapeutics: Exploring the Potential of Vaccinium Metabolites in Gastric Cancer Treatment Through Computational Insights. Life 2025, 15, 406. https://doi.org/10.3390/life15030406
Carpio AR, Talubo ND, Tsai P-W, Chen B-Y, Tayo LL. Berries as Nature’s Therapeutics: Exploring the Potential of Vaccinium Metabolites in Gastric Cancer Treatment Through Computational Insights. Life. 2025; 15(3):406. https://doi.org/10.3390/life15030406
Chicago/Turabian StyleCarpio, Angelica Rachel, Nicholas Dale Talubo, Po-Wei Tsai, Bor-Yann Chen, and Lemmuel L. Tayo. 2025. "Berries as Nature’s Therapeutics: Exploring the Potential of Vaccinium Metabolites in Gastric Cancer Treatment Through Computational Insights" Life 15, no. 3: 406. https://doi.org/10.3390/life15030406
APA StyleCarpio, A. R., Talubo, N. D., Tsai, P.-W., Chen, B.-Y., & Tayo, L. L. (2025). Berries as Nature’s Therapeutics: Exploring the Potential of Vaccinium Metabolites in Gastric Cancer Treatment Through Computational Insights. Life, 15(3), 406. https://doi.org/10.3390/life15030406







