On the Origin of Information Dynamics in Early Life
Abstract
1. Introduction
2. Building a Model of Information in Early Life
3. Fulfilling Darwin’s Postulates Under Primordial Conditions
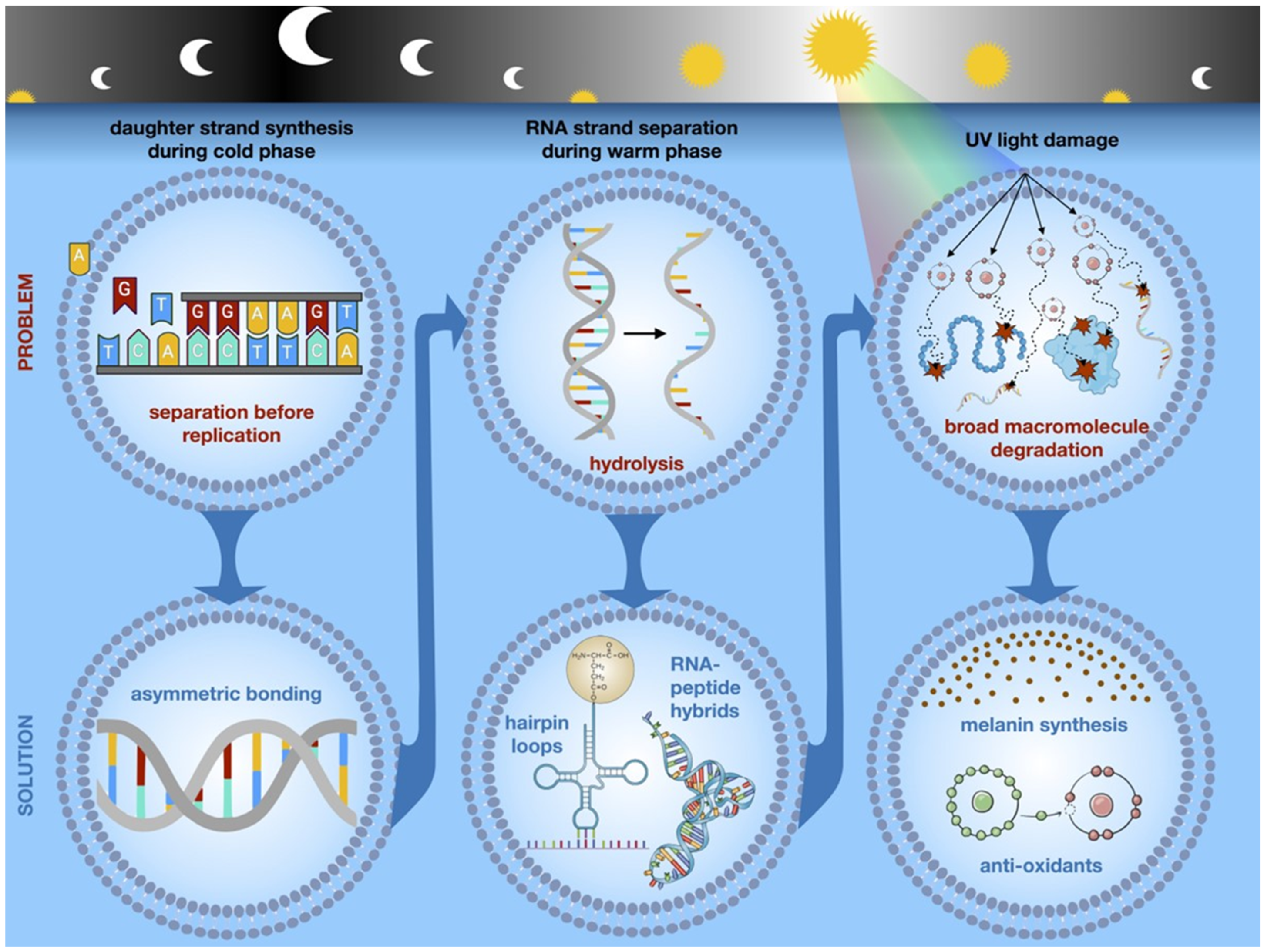
4. Diurnal Variations in Evolutionary Selection Pressures
5. Evolutionary Heritage of Prebiotic Information Acquisition
5.1. Evolution of Circadian Clocks
5.2. The Critical Role of Simultaneous Hazards and Interacting Adaptations
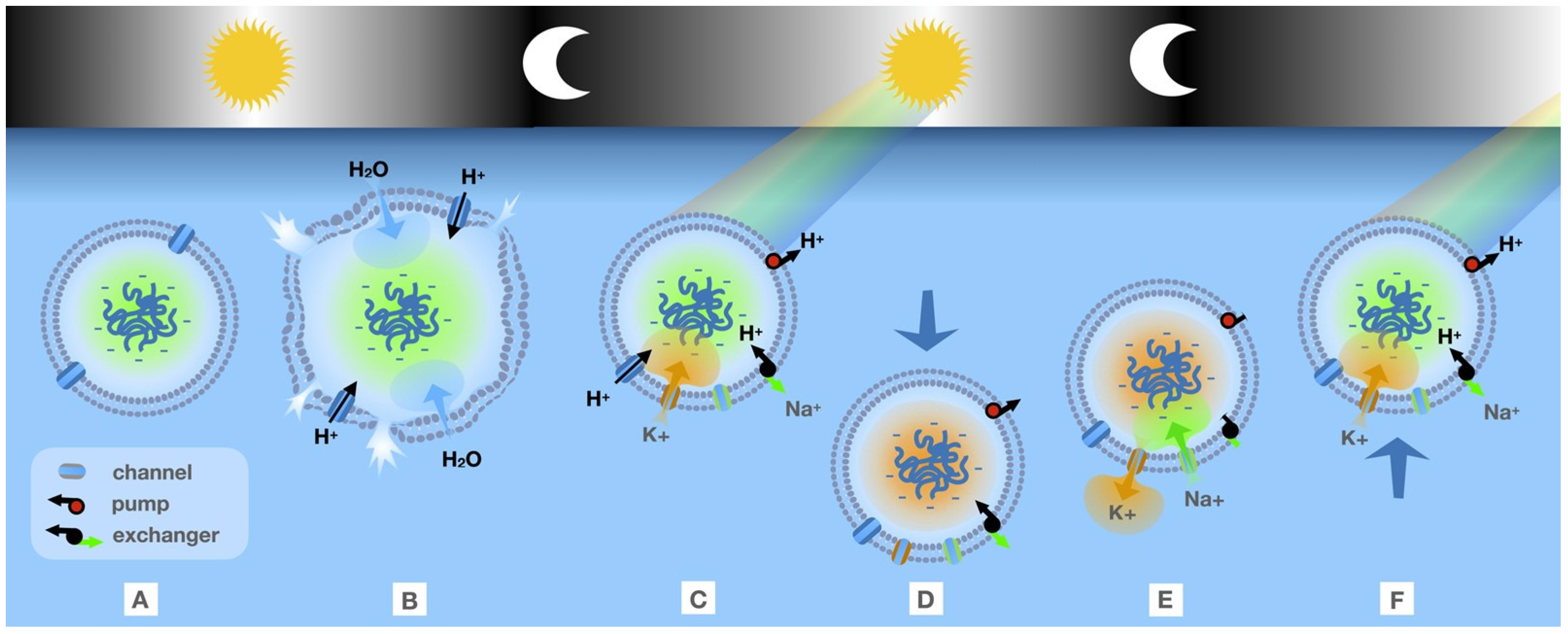
5.3. Evolutionary Selection for the Transmembrane Ion Gradient
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gatenby, R.A.; Frieden, B.R. Information theory in living systems, methods, applications, and challenges. Bull. Math. Biol. 2007, 69, 635–657. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Frieden, B.R. The critical roles of information and nonequilibrium thermodynamics in evolution of living systems. Bull. Math. Biol. 2013, 75, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.A.; Massol, F.; Szymkowiak, J. Resurrecting Shannon’s surprise: Landscape heterogeneity complements information use and population growth. Oikos 2022, 2022, e09305. [Google Scholar] [CrossRef]
- Fiore, M. (Ed.) Prebiotic Chemistry and Life’s Origin; Royal Society of Chemistry: London, UK, 2022; Available online: www.rsc.org (accessed on 16 January 2025).
- Nogal, N.; Sanz-Sánchez, M.; Vela-Gallego, S.; Ruiz-Mirazo, K.; de la Escosura, A. The protometabolic nature of prebiotic chemistry. Chem. Soc. Rev. 2023, 52, 7359–7388. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fu, S.; Ying, J.; Zhao, Y. Prebiotic chemistry: A review of nucleoside phosphorylation and polymerization. Open Biol. 2023, 13, 220234. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.D. The origin of life—Out of the blue. Angew. Chem. Int. Edit. 2016, 55, 104–121. [Google Scholar] [CrossRef]
- Mayr, E. Cause and effect in biology. Science 1961, 134, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Tinbergen, N. On aims and methods of ethology. Z. Tierpsychol. 1963, 20, 410–433. [Google Scholar] [CrossRef]
- Garland, T.; Downs, C.J., Jr.; Ives, A.R. Trade-offs (and constraints) in organismal biology. Physiol. Biochem. Zool. 2022, 95, 82–112. [Google Scholar] [CrossRef]
- Egbert, M.; Hanczyc, M.M.; Harvey, I.; Virgo, N.; Parke, E.C.; Froese, T.; Sayama, H.; Penn, A.S.; Bartlett, S. Behaviour and the origin of organisms. Origins Life Evol. B 2023, 53, 87–112. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gatenby, R.A. Evolutionary advantage of anti-parallel strand orientation of duplex DNA. Sci. Rep. 2020, 10, 9883. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gatenby, R.A. Evolutionary advantage of directional symmetry breaking in self-replicating polymers. J. Theor. Biol. 2018, 446, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Brown, J.; Gatenby, R. Prebiotic competition and evolution in self-replicating polynucleotides can explain the properties of DNA/RNA in modern living systems. BMC Evol. Biol. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Burton, Z.F. The 3 31 Nucleotide minihelix tRNA evolution theorem and the origin of life. Life 2023, 13, 2224. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Burton, Z.F. Evolution of Life on Earth: tRNA, Aminoacyl-tRNA Synthetases and the Genetic Code. Life 2020, 10, 21. [Google Scholar] [CrossRef]
- D’Alba, L.; Shawkey, M.D. Melanosomes: Biogenesis, properties, and evolution of an ancient organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.P.; Manning, P.L.; Wogelius, R.A. Pigments through time. Pigment. Cell Melanoma Res. 2014, 27, 684–685. [Google Scholar] [CrossRef] [PubMed]
- Brierley, A.S. Diel vertical migration. Curr. Biol. 2014, 24, R1074–R1076. [Google Scholar] [CrossRef]
- Harrison, T.M. Hadean Earth; Springer Nature: Cham, Switzerland, 2020; 291p. [Google Scholar] [CrossRef]
- Deamer, D.W. Assembling Life: How Can Life Begin on Earth and Other Habitable Planets? Oxford University Press: New York, NY, USA, 2019; 166p. [Google Scholar]
- Lepot, K. Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Sci. Rev. 2020, 209, 103296. [Google Scholar] [CrossRef]
- Bottke, W.F.; Norman, M.D. The late heavy bombardment. Ann. Rev. Earth Planet. Sci. 2017, 45, 619–647. [Google Scholar] [CrossRef]
- Obridko, V.N.; Ragulskaya, M.V.; Khramova, E.G. Young Sun, galactic processes, and origin of life. J. Atmos. Solar-Terrest. Physics. 2020, 208, 105395. [Google Scholar] [CrossRef]
- Cockell, C.S.; Horneck, G. The history of the UV radiation climate of the earth—Theoretical and space-based observations. Photochem. Photobiol. 2007, 73, 447–451. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cy-anosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Deamer, D. Dry/wet cycling and the thermodynamics and kinetics of prebiotic polymer synthesis. Life 2016, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Dworkin, J.P.; Sandford, S.A.; Bernstein, M.P.; Allamandola, L.J. The first cell membranes. Astrobiology 2002, 2, 371–381. [Google Scholar] [CrossRef]
- Joyce, G.; Szostak, J.W. Protocells and RNA selfreplication. Cold Spring Harb. Perspect. Biol. 2018, 10, a034801. [Google Scholar] [CrossRef]
- Toner, J.D.C.D. Alkaline lake settings for concentrated prebiotic cyanide and the origin of life. Geochim. Cosmochim. Acta 2019, 260, 124–132. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Pfennig, D.W. Testing Darwin’s postulates. Trends Eco. Evol. 2001, 16, 165. [Google Scholar] [CrossRef]
- Baum, D.A.; Peng, Z.; Dolson, E.; Smith, E.; Plum, A.M.; Gagrani, P. The ecology-evolution continuum and the origin of life. J. R. Soc. Interface 2023, 20, 20230346. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C. Internal tides and ocean mixing. Science 2003, 301, 1858–1859. [Google Scholar] [CrossRef]
- Mat, A.M.; Sarrazin, J.; Markov, G.V.; Apremont, V.; Dubreuil, C.; Eché, C.; Fabioux, C.; Klopp, C.; Sarradin, P.-M.; Tanguy, A.; et al. Biological rhythms in the deep-sea hydrothermal mussel Bathymodiolus azoricus. Nat. Commun. 2020, 11, 3454. [Google Scholar] [CrossRef] [PubMed]
- Bonfio, C.; Valer, L.; Scintilla, S.; Shah, S.; Evans, D.J.; Jin, L.; Szostak, J.W.; Sasselov, D.D.; Sutherland, J.D.; Mansy, S.S. UV-light-driven prebiotic synthesis of iron-sulfur clusters. Nat. Chem. 2017, 94, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Green, N.J.; Xu, J.; Sutherland, J.D. Illuminating life’s origins: UV photochemistry in abiotic synthesis of biomolecules. J. Amer. Chem. Soc. 2021, 143, 7219–7236. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, D.E.C.B.; Powner, M.W. Nucleotide photochemistry on the early earth. In Conflicting Models for the Origin of Life; Stoyan, K., Smoukov, J., Gordon, R., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 369–405. [Google Scholar] [CrossRef]
- Nunes Palmeira, R.; Colnaghi, M.; Harrison, S.A.; Pomiankowski, A.; Lane, N. The limits of metabolic heredity in protocells. Proc. R. Soc. B 2022, 289, 28920221469. [Google Scholar] [CrossRef]
- Ranjan, S.; Kufner, C.L.; Lozano, G.G.; Todd, Z.R.; Haseki, A.; Sasselov, D.D. UV transmission in natural waters on prebiotic earth. Astrobiology 2022, 22, 242–262. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Junge, W. Primordial UV-protectors as ancestors of the photosynthetic pigment-proteins. In The Phototrophic Prokaryotes; Peschek, G.A., Löffelhardt, W., Schmetterer, G., Eds.; Springer: Boston, MA, USA, 1999; pp. 806–812. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Junge, W. On the origin of photosynthesis as inferred from sequence analysis. Photosyn. Res. 1997, 51, 27–42. [Google Scholar] [CrossRef]
- Zhang, K.; Hodge, J.; Chatterjee, A.; Moon, T.S.; Parker, K.M. Duplex structure of double-stranded RNA provides stability against hydrolysis relative to single-stranded RNA. Environ. Sci. Technol. 2021, 55, 8045–8053. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.; Bobonis, J.; Kurzawa, N.; Stein, F.; Helm, D.; Hevler, J.; Typas, A.; Savitski, M.M. Thermal proteome profiling in bacteria: Probing protein state in vivo. Mol. Syst. Biol. 2018, 14, e8242. [Google Scholar] [CrossRef] [PubMed]
- Majidi, D.; Aksöz, N. Stability of tyrosinase enzyme from Funalia trogii. Am. J. Microbiol. Res. 2013, 1, 1–3. [Google Scholar] [CrossRef]
- Baluska, F.; Reber, A.S. CBC-Clock theory of life—Integration of cellular circadian clocks and cellular sentience is essential for cognitive basis of life. Bioessays 2021, 43, e2100121. [Google Scholar] [CrossRef]
- Eelderink-Chen, Z.; Bosman, J.; Sartor, F.; Dodd, A.N.; Kovács, Á.T.; Merrow, M. A circadian clock in a nonphotosynthetic prokaryote. Sci. Adv. 2021, 7, eabe2086. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lazar, M.A. Transcriptional control of circadian rhythms and metabolism: A matter of time and space. Endocr. Rev. 2020, 41, 707–732. [Google Scholar] [CrossRef]
- Müller, F.; Escobar, L.; Xu, F.; Węgrzyn, E.; Nainytė, M.; Amatov, T.; Chan, C.; Pichler, A.; Carell, T. A prebiotically plausible scenario of an RNA-peptide world. Nature 2022, 605, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wayment-Steele, H.K.; Kim, D.S.; Choe, C.A.; Nicol, J.J.; Wellington-Oguri, R.; Watkins, A.M.; Sperberg, R.A.P.; Huang, P.-S.; Participants, E.; Das, R. Theoretical basis for stabilizing messenger RNA through secondary structure design. Nucleic Acids Res. 2021, 49, 10604–10617. [Google Scholar] [CrossRef]
- Netzer, A.; Katzir, I.; Baruch Leshem, A.; Weitman, M.; Lampel, A. Emergent properties of melanin-inspired peptide/RNA condensates. Proc. Natl. Acad. Sci. USA 2023, 120, e2310569120. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. The atmosphere of the primitive Earth and the prebiotic synthesis of amino acids. Orig. Life Evol. Biosph. 1974, 5, 139–151. [Google Scholar] [CrossRef]
- Raposo, G.; Marks, M.S. Melanosomes--dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007, 8, 786–797. [Google Scholar] [CrossRef]
- Hurbain, I.; Geerts, W.J.C.; Boudier, T.; Marco, S.; Verkleij, A.J.; Marks, M.S.; Raposo, G. Electron tomography of early melanosomes: Implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc. Natl. Acad. Sci. USA 2008, 105, 19726–19731. [Google Scholar] [CrossRef] [PubMed]
- Bush, W.D.; Simon, J.D. Quantification of Ca(2+) binding to melanin supports the hypothesis that melanosomes serve a functional role in regulating calcium homeostasis. Pigment. Cell Res. 2007, 20, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Malhotra, A.G.; Pandey, A.; Pandey, K.M. Computational model for pathway reconstruction to unravel the evolutionary significance of melanin synthesis. Bioinformation 2013, 9, 94–100. [Google Scholar] [CrossRef]
- Vinther, J. A guide to the field of palaeo colour: Melanin and other pigments can fossilise: Reconstructing colour patterns from ancient organisms can give new insights to ecology and behaviour. Bioessays 2015, 37, 643–656. [Google Scholar] [CrossRef]
- Wiriyasermkul, P.; Moriyama, S.; Nagamori, S. Membrane transport proteins in melanosomes: Regulation of ions for pigmentation. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183318. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.L.; Boyle, J.A.; Aradi, A.E.; Christian, K.A.; Di Pietro, S.M. TPC2 controls pigmentation by regulating melanosome pH and size. Proc. Natl. Acad. Sci. USA 2016, 113, 5622–5627. [Google Scholar] [CrossRef] [PubMed]
- Guppy, M.; Kong, S.E.; Niu, X.; Busfield, S.; Klinken, S.P. Method for measuring a comprehensive energy budget in a proliferating cell system over multiple cell cycles. J. Cell Physiol. 1997, 170, 1–7. [Google Scholar] [CrossRef]
- Hansma, H.G. Potassium at the origins of life: Did biology emerge from biotite in micaceous clay? Life 2022, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.T. The gas vacuole: An early organelle of prokaryote motility? Discov. Life 1980, 10, 111–116. [Google Scholar] [CrossRef]
- Naranjo, D. A scenario for the origin of life: Volume regulation by bacteriorhodopsin required extremely voltage sensitive Na-channels and very selective K-channels. Bioessays 2022, 44, e2100210. [Google Scholar] [CrossRef]
- Sephus, C.D.; Fer, E.; Garcia, A.K.; Adam, Z.R.; Schwieterman, E.W.; Kacar, B. Earliest Photic Zone Niches Probed by Ancestral Microbial Rhodopsins. Mol. Biol. Evol. 2022, 39, msac100. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, H.J. Beginnings of Cellular Life: Metabolism Recapitulates Biogenesis; Yale University Press: New Haven, CT, USA, 1992; 195p, ISBN 0-300-05483-1. [Google Scholar]
- Mahendrarajah, T.A.; Moody, E.R.R.; Schrempf, D.; Szánthó, L.L.; Dombrowski, N.; Davín, A.A.; Pisani, D.; Donoghue, P.C.J.; Szöllősi, G.J.; Williams, T.A.; et al. ATP synthase evolution on a cross-braced dated tree of life. Nat. Commun. 2023, 14, 7456. [Google Scholar] [CrossRef]
- Goldman, A.D.; Weber, J.M.; LaRowe, D.E.; Barge, L.M. Electron transport chains as a window into the earliest stages of evolution. Proc. Natl. Acad. Sci. USA 2023, 120, e2210924120. [Google Scholar] [CrossRef]
- Tosteson, D.C.; Hoffman, J.F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J. Gen. Physiol. 1960, 44, 169–194. [Google Scholar] [CrossRef] [PubMed]
- Trapp, O. Origins of life research: A roadmap for the transition from chemistry to biology. Bioessays 2022, 44, e2200157. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication Bell Syst. Tech. J. 1948, 27, 379–623. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatenby, R.A.; Gallaher, J.; Subramanian, H.; Hammarlund, E.U.; Whelan, C.J. On the Origin of Information Dynamics in Early Life. Life 2025, 15, 234. https://doi.org/10.3390/life15020234
Gatenby RA, Gallaher J, Subramanian H, Hammarlund EU, Whelan CJ. On the Origin of Information Dynamics in Early Life. Life. 2025; 15(2):234. https://doi.org/10.3390/life15020234
Chicago/Turabian StyleGatenby, Robert A., Jill Gallaher, Hemachander Subramanian, Emma U. Hammarlund, and Christopher J. Whelan. 2025. "On the Origin of Information Dynamics in Early Life" Life 15, no. 2: 234. https://doi.org/10.3390/life15020234
APA StyleGatenby, R. A., Gallaher, J., Subramanian, H., Hammarlund, E. U., & Whelan, C. J. (2025). On the Origin of Information Dynamics in Early Life. Life, 15(2), 234. https://doi.org/10.3390/life15020234







