Long-Term Prognostic Power of Three-Dimensional Speckle-Tracking Echocardiography-Derived Peak Left Atrial Reservoir Global Longitudinal Strain in Healthy Adults—An Analysis from the MAGYAR-Healthy Study
Abstract
1. Introduction
2. Methods
2.1. Subject Population
2.2. Follow-Up
2.3. Two-Dimensional Doppler Echocardiography
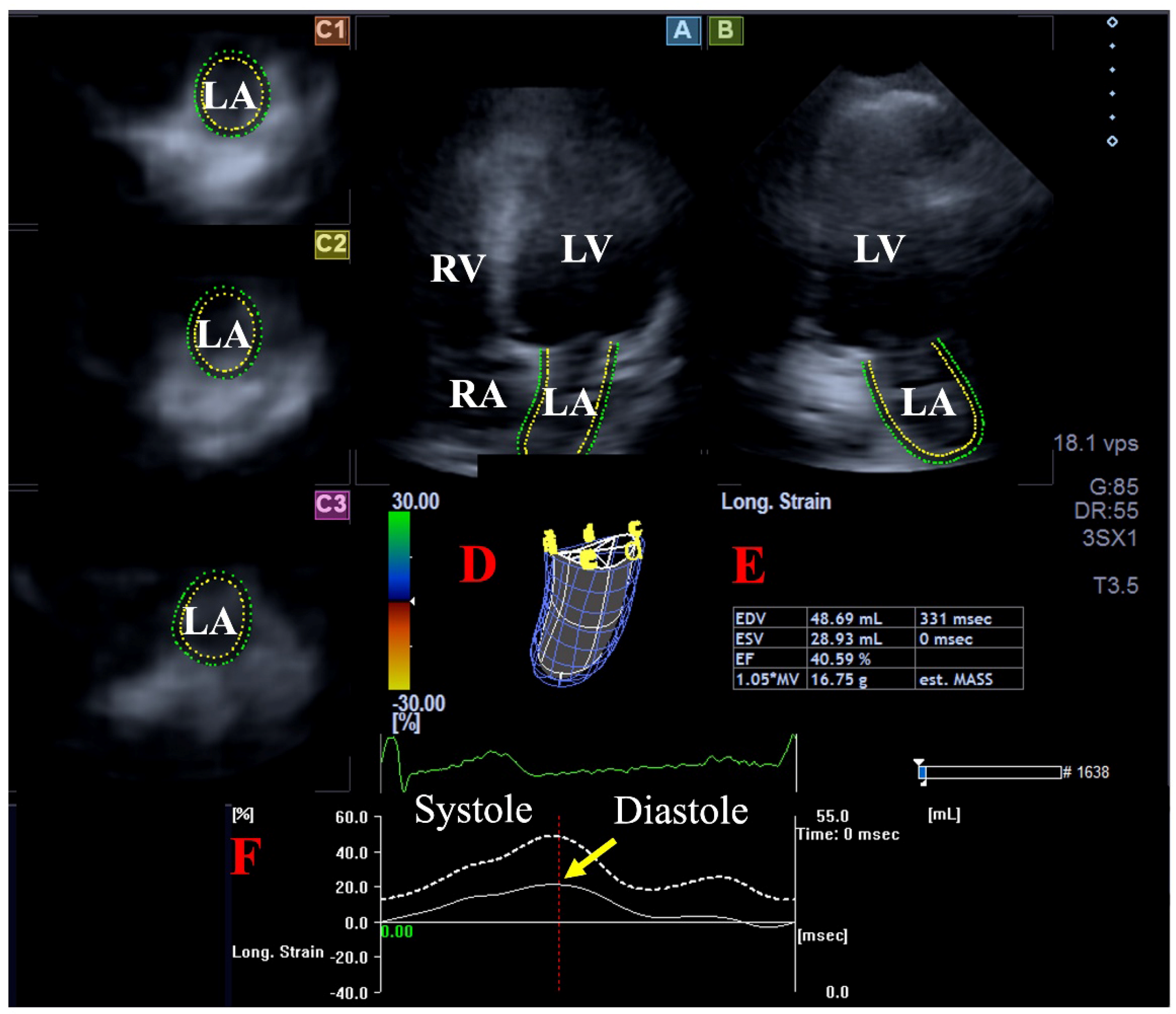
2.4. Three-Dimensional Speckle-Tracking Echocardiography
2.5. Statistical Analysis
3. Results
3.1. Follow-Up
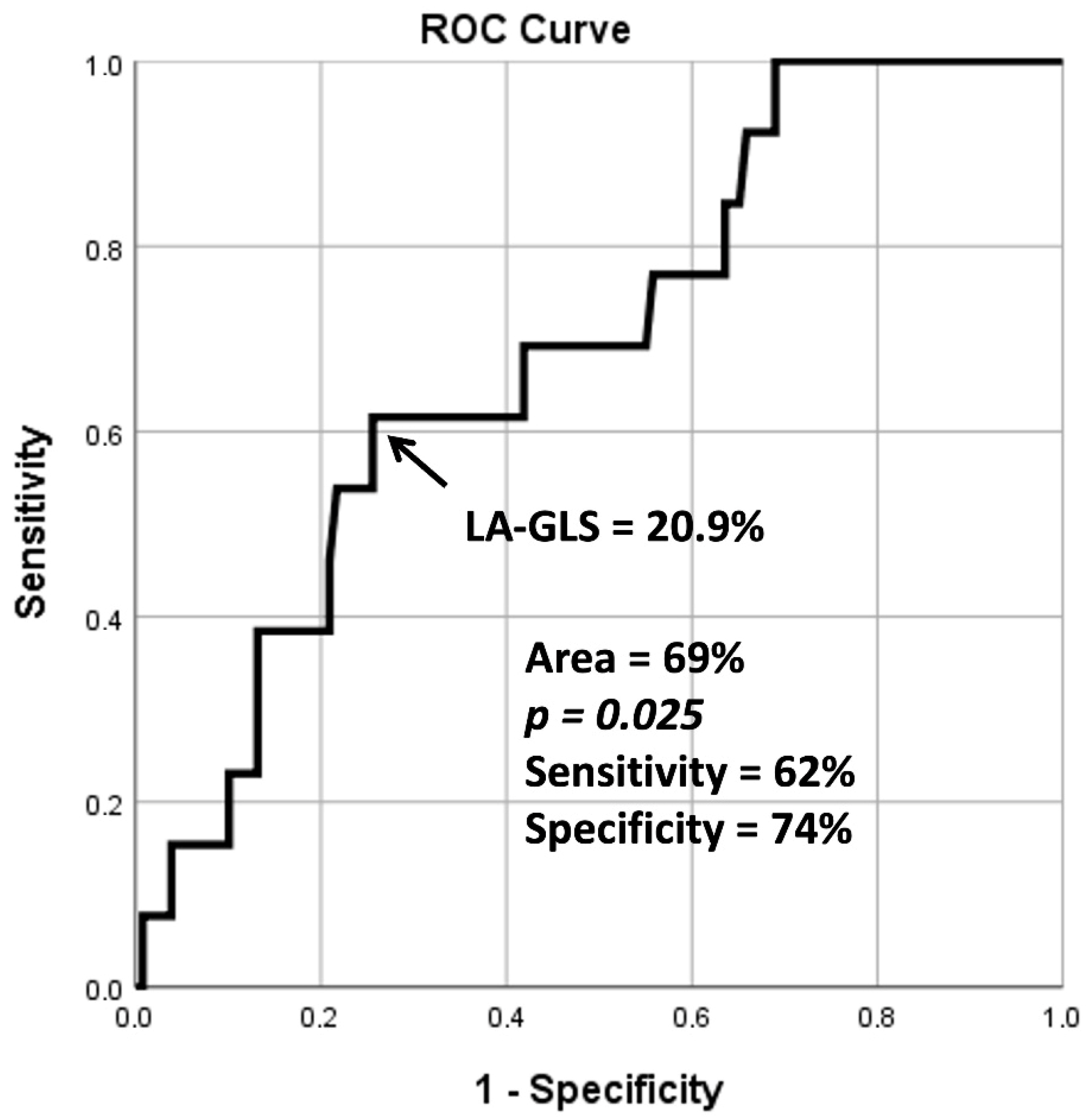
3.2. Global Left Atrial Peak Reservoir Longitudinal Strain
3.3. Comparison of the Clinical Data of the Subgroups
3.4. Comparison of 2D Echocardiographic Data Between the Subgroups
3.5. Comparison of 3DSTE-Derived LA Parameters Between the Subgroups
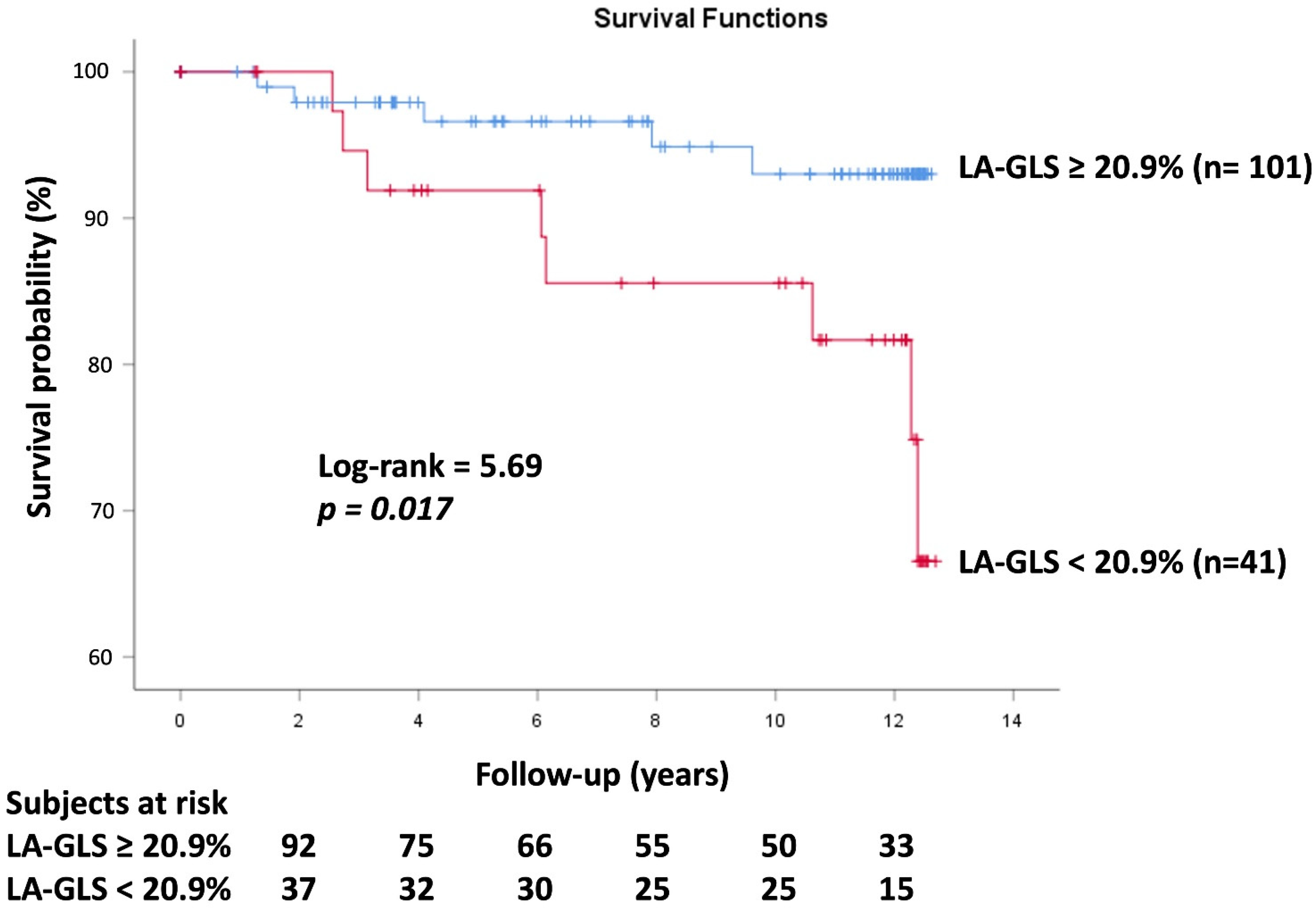
3.6. Events
3.7. Cox Regression Survival Analysis
4. Discussion
Limitations
- -
- At the stage of inclusion all subjects were considered to be healthy, and during the long-term follow-up, therefore, only a low number of events could be realized, limiting the study’s findings. The number of (combined) events limits the most elementary adjustments, such as those that should be included for age and sex in a Cox regression model.
- -
- -
- Not only the feasibility of 3DSTE-derived LA-GLS measurement (72%), but the success rate of follow-up (64%) could have significantly influenced the results. These facts have to be considered when interpreting findings.
- -
- This study aimed to examine the prognostic impact of peak LA-GLS by 3DSTE. Although several other parameters would have been measured at the same time, their prognostic powers were not considered during the assessment.
- -
- -
- -
- The present study did not measure right ventricular systolic pressure, whose relationship with LA-GLS would have strengthened the results [34].
- -
- During ROC analysis, the area under the curve being between 0.70 and 0.80 can be considered as acceptable. However, in the present study, it proved to be 0.69, which is very close to these values.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blume, G.G.; Mcleod, C.J.; Barnes, M.E.; Seward, J.B.; Pellikka, P.A.; Bastiansen, P.M.; Tsang, T.S.M. Left atrial function: Physiology, assessment, and clinical implications. Eur. J. Echocardiogr. 2011, 12, 421–430. [Google Scholar] [CrossRef]
- Todaro, M.C.; Choudhuri, I.; Belohlavek, M.; Jahangir, A.; Carerj, S.; Oreto, L.; Khandheria, B.K. New echocardiographic techniques for evaluation of left atrial mechanics. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Nour, A.; Muraru, D. Left atrium as a dynamic three-dimensional entity: Implications for echocardiographic assessment. Rev. Esp. Cardiol. 2013, 66, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.J.; Teixeira, R.; Golçalves, L.; Gersch, B.J. Left atrial mechanics: Echocardiographic assessment and clinical implications. J. Am. Soc. Echocardiogr. 2014, 27, 463–478. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Mignot, A.; Donal, E.; Zaroui, A.; Reant, P.; Salem, A.; Hamon, C.; Monzy, S.; Roudaut, R.; Habib, G.; Lafitte, S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: A multicenter study. J. Am. Soc. Echocardiogr. 2010, 23, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Lengyel, C.; Forster, T. Normal reference values of three-dimensional speckle-tracking echocardiography-derived left atrial strain parameters (results from the MAGYAR-Healthy Study). Int. J. Cardiovasc. Imaging 2019, 35, 991–998. [Google Scholar] [CrossRef]
- Huntjens, P.R.; Zhang, K.W.; Soyama, Y.; Karmpalioti, M.; Lenihan, D.J.; Gorcsan, J., 3rd. Prognostic Utility of Echocardiographic Atrial and Ventricular Strain Imaging in Patients with Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2021, 14, 1508–1519. [Google Scholar] [CrossRef]
- Bakija, F.Z.; Bagyura, Z.; Fábián, A.; Ferencz, A.; Kiss, L.; Szenczi, O.; Vadas, R.; Dósa, E.; Nguyen, D.T.; Csobay-Novák, C.; et al. Long-term prognostic value of left atrial longitudinal strain in an elderly community-based cohort. Geroscience 2023, 45, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Olajos, D.L.; Achim, A.; Ruzsa, Z.; Ambrus, N.; Lengyel, C. Long-Term Prognostic Significance of Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Twist in Healthy Adults-Results from the MAGYAR-Healthy Study. Rev. Cardiovasc. Med. 2024, 25, 324. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Olajos, D.L.; Achim, A.; Ruzsa, Z.; Ambrus, N.; Lengyel, C. Long-Term Prognostic Impact of Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Global Longitudinal Strain in Healthy Adults-Insights from the MAGYAR-Healthy Study. J. Cardiovasc. Dev. Dis. 2024, 11, 237. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Comparison between direct volumetric and speckle tracking methodologies for left ventricular and left atrial chamber quantification by three-dimensional echocardiography. Am. J. Cardiol. 2011, 108, 1038–1044. [Google Scholar] [CrossRef]
- Nagaya, M.; Kawasaki, M.; Tanaka, R.; Onishi, N.; Sato, N.; Ono, K.; Watanabe, T.; Minatoguchi, S.; Miwa, H.; Goto, Y.; et al. Quantitative validation of left atrial structure and function by two-dimensional and three-dimensional speckle tracking echocardiography: A comparative study with three-dimensional computed tomography. J. Cardiol. 2013, 62, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, A.; Yuda, S.; Oi, Y.; Kawamukai, M.; Nishida, J.; Kouzu, H.; Muranaka, A.; Kokubu, N.; Shimoshige, S.; Hashimoto, A.; et al. Assessment of left atrial deformation and synchrony by three-dimensional speckle-tracking echocardiography: Comparative studies in healthy subjects and patients with atrial fibrillation. J. Am. Soc. Echocardiogr. 2013, 26, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Domsik, P.; Kalapos, A.; Lengyel, C.; Orosz, A.; Forster, T. Comparison of three-dimensional speckle tracking echocardiography and two-dimensional echocardiography for evaluation of left atrial size and function in healthy volunteers (results from the MAGYAR-Healthy study). Echocardiography 2014, 31, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Dell’Angela, L.; Nicolosi, G.L. From ejection fraction, to myocardial strain, and myocardial work in echocardiography: Clinical impact and controversies. Echocardiography 2024, 41, e15758. [Google Scholar] [CrossRef]
- Liao, H.; Yang, S.; Yu, S.; Hu, X.; Meng, X.W.; Wu, K. Prognostic Value of Left Ventricular Global Longitudinal Strain for Major Adverse Cardiovascular Events in Patients with Aortic Valve Disease: A Meta-Analysis. Cardiology 2024, 149, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, X.; Xie, Q. Prognostic value of left ventricular global longitudinal strain on speckle echocardiography for predicting chemotherapy-induced cardiotoxicity in breast cancer patients: A systematic review and meta-analysis. Echocardiography 2023, 40, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.C.W.; Lee, N.H.C.; Poon, J.W.L.; Chin, C.W.L.; He, B.; Luo, L.; Chen, C.; Wan, E.Y.F.; Pennell, D.J.; Mohiaddin, R.; et al. Prognostic value of cardiac magnetic resonance derived global longitudinal strain analysis in patients with ischaemic and non-ischaemic dilated cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2022, 38, 2707–2721. [Google Scholar] [CrossRef] [PubMed]
- Tangen, J.; Nguyen, T.M.; Melichova, D.; Klaeboe, L.G.; Forsa, M.; Andresen, K.; Wazzan, A.A.; Lie, O.; Haugaa, K.; Skulstad, H.; et al. The Prognostic Value of Left Atrial Function in Patients with Acute Myocardial Infarction. Diagnostics 2024, 14, 2027. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Gertz, R.J.; Schultz, A.; Backhaus, S.J.; Evertz, R.; Kowallick, J.T.; Hasenfuß, G.; Desch, S.; Thiele, H.; Stiermaier, T.; et al. Impact of myocardial deformation on risk prediction in patients following acute myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1199936. [Google Scholar] [CrossRef]
- Lu, S.R.; Zhu, Y.; Zhou, W.; Zhang, J.; Deng, Y.B.; Liu, Y.N. Incremental prognostic utility of left ventricular and left atrial strains in coronary artery disease patients with reduced systolic function. Echocardiography 2024, 41, e15740. [Google Scholar] [CrossRef]
- Han, P.L.; Shen, M.T.; Jiang, Y.; Jiang, Z.K.; Li, K.; Yang, Z.G. Prognostic Value of Left Atrial Reservoir Strain in Left Ventricular Myocardial Noncompaction: A 3.0 T Cardiac Magnetic Resonance Feature Tracking Study. J. Magn. Reson. Imaging 2023, 57, 559–575. [Google Scholar] [CrossRef]
- Huurman, R.; Bowen, D.J.; Mutluer, F.O.; Barreto, B.L.; van Slegtenhorst, M.A.; Verhagen, J.M.A.; Hirsch, A.; van den Bosch, A.E.; Michels, M.; Schinkel, A.F.L. Prognostic significance of left atrial strain in sarcomere gene variant carriers without hypertrophic cardiomyopathy. Echocardiography 2022, 39, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.G.; Sequeiros, M.A.; Gómez, A.G.G.; Díaz, L.M.R.; Ruiz, J.M.M.; Baydés, R.H.; Mur, J.L.M.; Zamorano, J.L.; Fernández-Golfin, C. Prognostic value of diastolic function parameters in significant aortic regurgitation: The role of the left atrial strain. J. Echocardiogr. 2022, 20, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Sanoglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Incremental prognostic role of left atrial reservoir strain in asymptomatic patients with moderate aortic stenosis. Int. J. Cardiovasc. Imaging 2021, 37, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Seo, J.H.; Choi, K.H.; Lee, S.H.; Choi, J.O.; Jeon, E.S.; Yang, J.H. Prognostic implications of left atrial stiffness index in heart failure patients with preserved ejection fraction. JACC Cardiovasc. Imaging 2023, 16, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P.; Śmigielski, W. Risk Factors of Postoperative Hospital-Acquired Pneumonia in Patients Undergoing Cardiac Surgery. Medicina 2023, 59, 1993. [Google Scholar] [CrossRef]
| All Subjects | Peak LA-GLS ≥ 20.9% | Peak LA-GLS < 20.9% | No Event | Event | |
|---|---|---|---|---|---|
| No. of patients | 142 | 101 (71) | 41 (29) | 129 (91) | 13 (9) |
| Male (%) | 70 (49) | 50 (50) | 20 (49) | 63 (49) | 7 (54) |
| Age (years) | 32.1 ± 12.7 | 30.8 ± 11.3 | 35.4 ± 15.1 * | 29.1 ± 9.6 | 52.2 ± 14.8 † |
| Two-dimensional echocardiography | |||||
| LA diameter in PLA view (mm) | 36.4 ± 4.1 | 35.6 ± 4.0 | 37.5 ± 4.2 * | 36.4 ± 4.1 | 38.8 ± 4.6 † |
| LA cross-sectional diameter in AP4CH (mm) | 40.4 ± 3.8 | 40.1 ± 3.4 | 41.1 ± 4.6 | 40.2 ± 3.7 | 41.8 ± 4.7 |
| LA longitudinal diameter in AP4CH (mm) | 43.6 ± 5.2 | 43.2 ± 5.1 | 44.5 ± 5.3 | 43.1 ± 5.1 | 47.8 ± 4.3 † |
| LV-EDD (mm) | 48.1 ± 3.5 | 47.6 ± 3.6 | 49.4 ± 2.9 * | 48.1 ± 3.5 | 49.4 ± 2.6 |
| LV-EDV (mL) | 105.2 ± 22.3 | 102.8 ± 20.6 | 110.9 ± 25.0 * | 105.2 ± 22.3 | 112.0 ± 14.6 |
| LV-ESD (mm) | 31.8 ± 3.1 | 31.7 ± 3.4 | 32.2 ± 2.4 | 31.8 ± 3.1 | 31.7 ± 1.7 |
| LV-ESV (mL) | 35.8 ± 8.6 | 34.5 ± 9.0 | 38.7 ± 6.7 * | 35.8 ± 8.6 | 38.1 ± 5.4 |
| IVS (mm) | 9.0 ± 1.6 | 8.9 ± 1.6 | 9.2 ± 1.4 | 9.0 ± 1.6 | 10.1 ± 1.6 † |
| LV-PW (mm) | 9.1 ± 1.6 | 9.0 ± 1.6 | 9.1 ± 1.6 | 9.1 ± 1.6 | 10.0 ± 1.3 † |
| LV-EF (%) | 66.3 ± 5.0 | 66.6 ± 5.5 | 65.4 ± 3.4 | 66.3 ± 5.0 | 65.8 ± 4.2 |
| Three-dimensional speckle-tracking echocardiography | |||||
| LV-GLS | −16.2 ± 2.3 | −16.4 ± 2.1 | −15.8 ± 2.6 | −16.3 ± 2.1 | −15.8 ± 3.1 |
| LA-Vmax (mL) | 40.3 ± 12.5 | 40.7 ± 11.8 | 39.3 ± 14.0 | 39.2 ± 11.3 | 50.9 ± 17.7 † |
| Peak LA-GLS (%) | 26.3 ± 9.2 | 30.3 ± 7.6 | 16.4 ± 3.6 * | 26.3 ± 9.2 | 20.9 ± 6.8 † |
| Subjects with peak LA-GLS < 20.9% | 41 (29) | 0 (0) | 41 (100) * | 34 (26) | 7 (55) † |
| Events | |||||
| Subjects with events (%) | 13 (9) | 5 (5) | 8 (20) * | 0 (0) | 13 (100) † |
| Subjects who died (%) | 2 (1) | 1 (1) | 1 (2) | 0 (0) | 2 (15) † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Olajos, D.L.; Achim, A.; Ruzsa, Z.; Ambrus, N.; Lengyel, C. Long-Term Prognostic Power of Three-Dimensional Speckle-Tracking Echocardiography-Derived Peak Left Atrial Reservoir Global Longitudinal Strain in Healthy Adults—An Analysis from the MAGYAR-Healthy Study. Life 2025, 15, 232. https://doi.org/10.3390/life15020232
Nemes A, Olajos DL, Achim A, Ruzsa Z, Ambrus N, Lengyel C. Long-Term Prognostic Power of Three-Dimensional Speckle-Tracking Echocardiography-Derived Peak Left Atrial Reservoir Global Longitudinal Strain in Healthy Adults—An Analysis from the MAGYAR-Healthy Study. Life. 2025; 15(2):232. https://doi.org/10.3390/life15020232
Chicago/Turabian StyleNemes, Attila, Dorottya Lilla Olajos, Alexandru Achim, Zoltán Ruzsa, Nóra Ambrus, and Csaba Lengyel. 2025. "Long-Term Prognostic Power of Three-Dimensional Speckle-Tracking Echocardiography-Derived Peak Left Atrial Reservoir Global Longitudinal Strain in Healthy Adults—An Analysis from the MAGYAR-Healthy Study" Life 15, no. 2: 232. https://doi.org/10.3390/life15020232
APA StyleNemes, A., Olajos, D. L., Achim, A., Ruzsa, Z., Ambrus, N., & Lengyel, C. (2025). Long-Term Prognostic Power of Three-Dimensional Speckle-Tracking Echocardiography-Derived Peak Left Atrial Reservoir Global Longitudinal Strain in Healthy Adults—An Analysis from the MAGYAR-Healthy Study. Life, 15(2), 232. https://doi.org/10.3390/life15020232









