The Acute Effects of Moderate-Intensity Aerobic Exercise on Core Executive Functions in Healthy Older Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
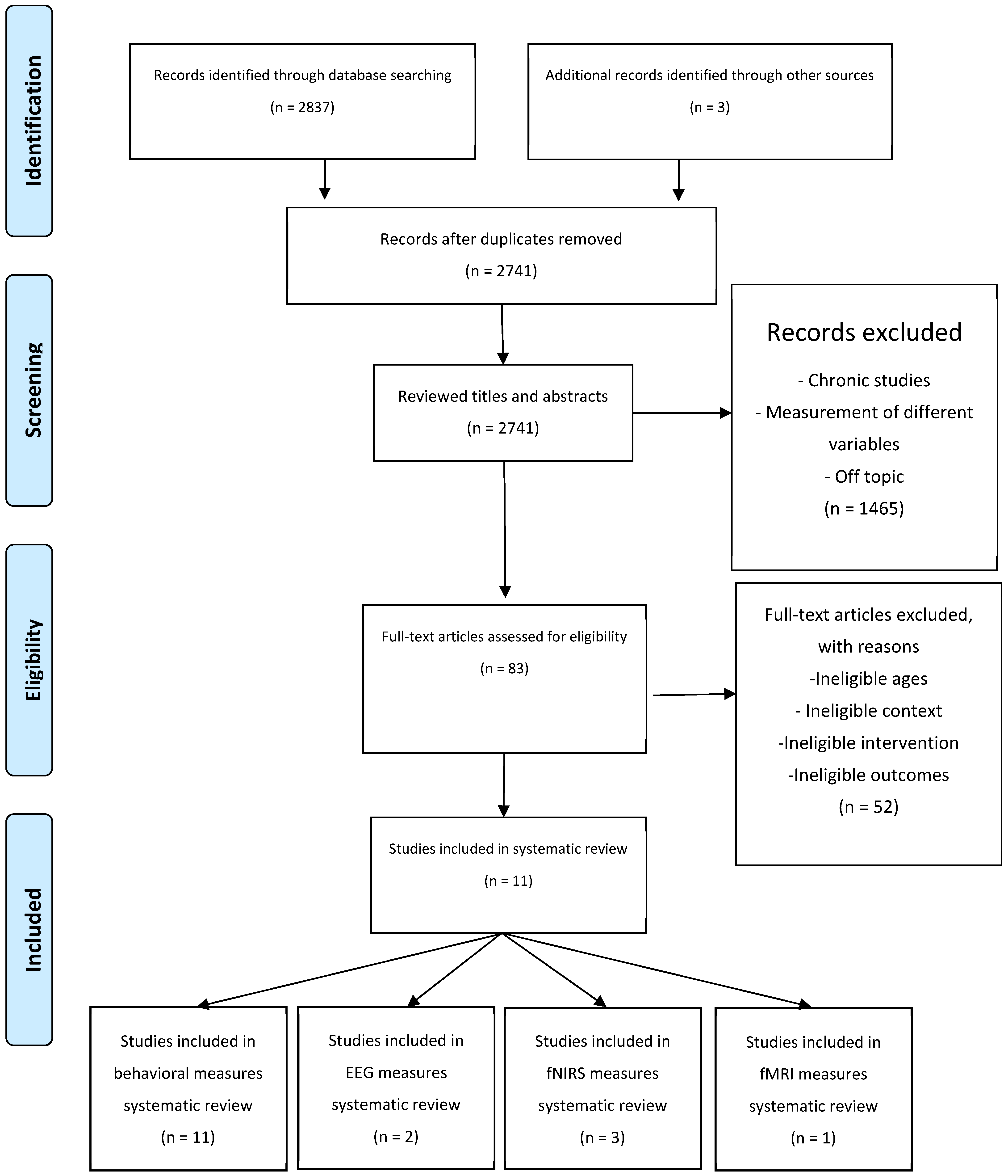
2.1. Search Strategy and Study Selection
2.2. Figures, Tables, and Schemes
2.2.1. The Type of Study
2.2.2. Type of Participants
2.2.3. Type of Interventions
2.2.4. Outcome Measures
2.2.5. Exclusion Criteria
2.2.6. Data Extraction
3. Results
3.1. Study Selection and Characteristics
3.2. Quality and Completeness of Reporting
3.3. Executive Functions Outcome Variables
3.3.1. Inhibition (Interference)
3.3.2. Physiological Outcome Variables for Inhibition
3.3.3. Cognitive Flexibility (Shifting)
3.3.4. Working Memory
3.3.5. Physiological Outcome Variables for Working Memory
4. Discussion
4.1. Acute Effects of MIAE on Inhibition
Physiological Outcome Variables for Inhibition
4.2. The Acute Effects of MIAE on Cognitive Flexibility
4.3. The Acute Effects of MIAE on Working Memory
Physiological Outcome Variables for Working Memory
4.4. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Diamond, A.; Ling, D.S. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev. Cogn. Neurosci. 2016, 18, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.L.; Amso, D.; McLaughlin, K.A. The role of the visual association cortex in scaffolding prefrontal cortex development: A novel mechanism linking socioeconomic status and executive function. Dev. Cogn. Neurosci. 2019, 39, 100699. [Google Scholar] [CrossRef] [PubMed]
- Shaked, D.; Katzel, L.I.; Seliger, S.L.; Gullapalli, R.P.; Davatzikos, C.; Erus, G.; Evans, M.K.; Zonderman, A.B.; Waldstein, S.R. Dorsolateral prefrontal cortex volume as a mediator between socioeconomic status and executive function. Neuropsychology 2018, 32, 985. [Google Scholar] [CrossRef] [PubMed]
- Fiske, A.; Holmboe, K. Neural substrates of early executive function development. Dev. Rev. 2019, 52, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010, 50, 1702–1710. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, T.; Mei, L.; Li, A.; Zhang, C. Influence of acute combined physical and cognitive exercise on cognitive function: An NIRS study. PeerJ 2019, 7, e7418. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Hyodo, K.; Suwabe, K.; Ochi, G.; Sakairi, Y.; Kato, M.; Dan, I.; Soya, H. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage 2014, 98, 336–345. [Google Scholar] [CrossRef]
- Nelson, J.K.; Reuter-Lorenz, P.A.; Persson, J.; Sylvester, C.-Y.C.; Jonides, J. Mapping interference resolution across task domains: A shared control process in left inferior frontal gyrus. Brain Res. 2009, 1256, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Swick, D.; Ashley, V.; Turken, U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008, 9, 102. [Google Scholar] [CrossRef]
- Stone, M.H.; Fleck, S.J.; Triplett, N.T.; Kraemer, W.J. Health-and performance-related potential of resistance training. Sports Med. 1991, 11, 210–231. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Kim, C. Dissociable neural correlates of spatial attention and response inhibition in spatially driven interference. Neurosci. Lett. 2020, 731, 135111. [Google Scholar] [CrossRef] [PubMed]
- Daucourt, M.C.; Schatschneider, C.; Connor, C.M.; Al Otaiba, S.; Hart, S.A. Inhibition, updating working memory, and shifting predict reading disability symptoms in a hybrid model: Project KIDS. Front. Psychol. 2018, 9, 238. [Google Scholar] [CrossRef]
- Di, X.; Rypma, B.; Biswal, B.B. Correspondence of executive function related functional and anatomical alterations in aging brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 41–50. [Google Scholar] [CrossRef]
- Turner, G.R.; Spreng, R.N. Executive functions and neurocognitive aging: Dissociable patterns of brain activity. Neurobiol. Aging 2012, 33, 826.e1–826.e13. [Google Scholar] [CrossRef]
- Cho, I.; Cohen, A.S. Explaining age-related decline in theory of mind: Evidence for intact competence but compromised executive function. PLoS ONE 2019, 14, e0222890. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.; Peltsch, A.; Alahyane, N.; Brien, D.C.; Coe, B.C.; Garcia, A.; Munoz, D.P. Age related prefrontal compensatory mechanisms for inhibitory control in the antisaccade task. Neuroimage 2018, 165, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, M.; Bonyadi, M.R.; Reutens, D.C. Age-related differences in structural and functional prefrontal networks during a logical reasoning task. Brain Imaging Behav. 2020, 15, 1085–1102. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr. Health Sci. 2016, 16, 1045–1055. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Cholerton, B.A.; Plymate, S.R.; Fishel, M.A.; Watson, G. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Sports Med. 2015, 49, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Ye, M.; Wang, L.; Zheng, G. Effects of Physical Exercise on Executive Function in Cognitively Healthy Older Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials: Physical exercise for executive function. Int. J. Nurs. Stud. 2020, 114, 103810. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, J.J.; Chye, Y.; Thompson, S.; Rogasch, N.C.; Suo, C.; Coxon, J.; Yucel, M. The effects of regular aerobic exercise on hippocampal structure and function. bioRxiv 2020, 8, 250688. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Douris, P.C.; Handrakis, J.P.; Apergis, D.; Mangus, R.B.; Patel, R.; Limtao, J.; Platonova, S.; Gregorio, A.; Luty, E. The effects of aerobic exercise and gaming on cognitive performance. J. Hum. Kinet. 2018, 61, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Gejl, A.K.; Bugge, A.; Ernst, M.T.; Tarp, J.; Hillman, C.H.; Have, M.; Froberg, K.; Andersen, L.B. The acute effects of short bouts of exercise on inhibitory control in adolescents. Ment. Health Phys. Act. 2018, 15, 34–39. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Chu, C.-H.; Wang, C.-C.; Wang, Y.-C.; Song, T.-F.; Tsai, C.-L.; Etnier, J.L. Dose–response relation between exercise duration and cognition. Med. Sci. Sports Exerc. 2015, 47, 159–165. [Google Scholar] [CrossRef]
- Chen, A.-G.; Yan, J.; Chen, F.-T.; Kuan, G.; Wei, G.-X.; Hung, T.-M.; Chang, Y.-K. Effects of acute exercise duration on the inhibition aspect of executive function in late middle-aged adults. Front. Aging Neurosci. 2019, 11, 227. [Google Scholar]
- Aly, M.; Kojima, H. Acute moderate-intensity exercise generally enhances neural resources related to perceptual and cognitive processes: A randomized controlled ERP study. Ment. Health Phys. Act. 2020, 19, 100363. [Google Scholar] [CrossRef]
- Mehren, A.; Diaz Luque, C.; Brandes, M.; Lam, A.P.; Thiel, C.M.; Philipsen, A.; Özyurt, J. Intensity-dependent effects of acute exercise on executive function. Neural Plast. 2019, 2019, 8608317. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Kramer, A.F.; Song, T.-F.; Wu, C.-H.; Hung, T.-M.; Chang, Y.-K. Acute exercise and neurocognitive development in preadolescents and young adults: An ERP study. Neural Plast. 2017, 2017, 2631909. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Chi, L.; Etnier, J.L.; Wang, C.-C.; Chu, C.-H.; Zhou, C. Effect of acute aerobic exercise on cognitive performance: Role of cardiovascular fitness. Psychol. Sport Exerc. 2014, 15, 464–470. [Google Scholar] [CrossRef]
- Hussey, E.K.; Fontes, E.B.; Ward, N.; Westfall, D.R.; Kao, S.-C.; Kramer, A.F.; Hillman, C.H. Combined and Isolated Effects of Acute Exercise and Brain Stimulation on Executive Function in Healthy Young Adults. J. Clin. Med. 2020, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Alderman, B.L.; Chu, C.H.; Wang, C.C.; Song, T.F.; Chen, F.T. Acute exercise has a general facilitative effect on cognitive function: A combined ERP temporal dynamic and BDNF study. Psychophysiology 2017, 54, 289–300. [Google Scholar] [CrossRef]
- Chaire, A.; Becke, A.; Düzel, E. Effects of physical exercise on working memory and attention-related neural oscillations. Front. Neurosci. 2020, 14, 239. [Google Scholar] [CrossRef]
- Kao, S.-C.; Drollette, E.S.; Ritondale, J.P.; Khan, N.; Hillman, C.H. The acute effects of high-intensity interval training and moderate-intensity continuous exercise on declarative memory and inhibitory control. Psychol. Sport Exerc. 2018, 38, 90–99. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pan, C.Y.; Chen, F.C.; Wang, C.H.; Chou, F.Y. Effects of acute aerobic exercise on a task-switching protocol and brain-derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Exp. Physiol. 2016, 101, 836–850. [Google Scholar] [CrossRef]
- Razon, S.; Lebeau, J.-C.; Basevitch, I.; Foster, B.; Akpan, A.; Mason, J.; Boiangin, N.; Tenenbaum, G. Effects of acute exercise on executive functioning: Testing the moderators. Int. J. Sport Exerc. Psychol. 2019, 17, 303–320. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- McSween, M.-P.; Coombes, J.S.; MacKay, C.P.; Rodriguez, A.D.; Erickson, K.I.; Copland, D.A.; McMahon, K.L. The immediate effects of acute aerobic exercise on cognition in healthy older adults: A systematic review. Sports Med. 2019, 49, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, I.; Davatzikos, C.; An, Y.; Wu, X.; Shen, D.; Kraut, M.; Resnick, S. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 2009, 72, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.A.; Shaw, M.E.; Cherbuin, N. A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. Neuroimage 2015, 112, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Chu, C.H.; Wang, C.C.; Song, T.F.; Wei, G.X. Effect of acute exercise and cardiovascular fitness on cognitive function: An event-related cortical desynchronization study. Psychophysiology 2015, 52, 342–351. [Google Scholar] [CrossRef]
- Chen, F.-T.; Etnier, J.L.; Wu, C.-H.; Cho, Y.-M.; Hung, T.-M.; Chang, Y.-K. Dose-response relationship between exercise duration and executive function in older adults. J. Clin. Med. 2018, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Chen, A.-G.; Hung, T.-M.; Wang, C.-C.; Chang, Y.-K. Exercise and fitness modulate cognitive function in older adults. Psychol. Aging 2015, 30, 842. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Chang, Y.-C.; Pan, C.-Y.; Wang, T.-C.; Ukropec, J.; Ukropcová, B. Acute effects of different exercise intensities on executive function and oculomotor performance in middle-aged and older adults: Moderate-intensity continuous exercise vs. High-Intensity Interval Exercise. Front. Aging Neurosci. 2021, 13, 743479. [Google Scholar] [CrossRef] [PubMed]
- Stute, K.; Hudl, N.; Stojan, R.; Voelcker-Rehage, C. Shedding light on the effects of moderate acute exercise on working memory performance in healthy older adults: An fNIRS study. Brain Sci. 2020, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Dan, I.; Suwabe, K.; Kyutoku, Y.; Yamada, Y.; Akahori, M.; Byun, K.; Kato, M.; Soya, H. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol. Aging 2012, 33, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Callow, D.D.; Smith, J.C. Brain activation during executive control after acute exercise in older adults. Int. J. Psychophysiol. 2019, 146, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Barella, L.A.; Etnier, J.L.; Chang, Y.-K. The immediate and delayed effects of an acute bout of exercise on cognitive performance of healthy older adults. J. Aging Phys. Act. 2010, 18, 87–98. [Google Scholar] [CrossRef]
- Hogan, C.L.; Mata, J.; Carstensen, L.L. Exercise holds immediate benefits for affect and cognition in younger and older adults. Psychol. Aging 2013, 28, 587. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-S.; Huang, C.-J.; Wu, C.-T.; Chang, Y.-K.; Hung, T.-M. Acute exercise facilitates the N450 inhibition marker and P3 attention marker during stroop test in young and older adults. J. Clin. Med. 2018, 7, 391. [Google Scholar] [CrossRef]
- Tomporowski, P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003, 112, 297–324. [Google Scholar] [CrossRef]
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The effects of mental fatigue on physical performance: A systematic review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, R.L.; Sweet, J.J.; Attix, D.K.; Krull, K.R.; Henry, G.K.; Hart, R.P. Official position of the American Academy of Clinical Neuropsychology on serial neuropsychological assessments: The utility and challenges of repeat test administrations in clinical and forensic contexts. Clin. Neuropsychol. 2010, 24, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zou, L.; Herold, F.; Yu, Q.; Jiao, C.; Zhang, Y.; Chi, X.; Müller, N.G.; Perrey, S.; Li, L. Does Cardiorespiratory Fitness Influence the Effect of Acute Aerobic Exercise on Executive Function? Front. Hum. Neurosci. 2020, 14, 569010. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Snook, E.M.; Jerome, G.J. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 2003, 48, 307–314. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ekkekakis, P.; Hall, E.E.; Petruzzello, S.J. Practical markers of the transition from aerobic to anaerobic metabolism during exercise: Rationale and a case for affect-based exercise prescription. Prev. Med. 2004, 38, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef] [PubMed]
- MacLean, M.H.; Arnell, K.M. Greater attentional blink magnitude is associated with higher levels of anticipatory attention as measured by alpha event-related desynchronization (ERD). Brain Res. 2011, 1387, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Hu, L.; Chen, A. The neural oscillations of conflict adaptation in the human frontal region. Biol. Psychol. 2013, 93, 364–372. [Google Scholar] [CrossRef]
- Hogan, M.J.; O’Hora, D.; Kiefer, M.; Kubesch, S.; Kilmartin, L.; Collins, P.; Dimitrova, J. The effects of cardiorespiratory fitness and acute aerobic exercise on executive functioning and EEG entropy in adolescents. Front. Hum. Neurosci. 2015, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gronwald, T.; Hottenrott, K. The athlete’s brain: Cross-sectional evidence for neural efficiency during cycling exercise. Neural Plast. 2016, 2016, 4583674. [Google Scholar] [CrossRef]
- Del Percio, C.; Infarinato, F.; Iacoboni, M.; Marzano, N.; Soricelli, A.; Aschieri, P.; Eusebi, F.; Babiloni, C. Movement-related desynchronization of alpha rhythms is lower in athletes than non-athletes: A high-resolution EEG study. Clin. Neurophysiol. 2010, 121, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Maffei, L.; Picano, E.; Andreassi, M.; Angelucci, A.; Baldacci, F.; Baroncelli, L.; Begenisic, T.; Bellinvia, P.; Berardi, N.; Biagi, L. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: The Train the Brain study. Sci. Rep. 2017, 7, 39471. [Google Scholar]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Larson, M.J.; Clayson, P.E.; Clawson, A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. Int. J. Psychophysiol. 2014, 93, 283–297. [Google Scholar] [CrossRef]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging 2002, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.; Bourbeau, K.; Bellovary, B.; Zuhl, M.N. Exercise intensity influences prefrontal cortex oxygenation during cognitive testing. Behav. Sci. 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Halaki, M.; Chow, C.M.; O’Dwyer, N. The effects of multi-stage exercise with and without concurrent cognitive performance on cardiorespiratory and cerebral haemodynamic responses. Eur. J. Appl. Physiol. 2018, 118, 2121–2132. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Pan, C.-Y.; Chen, F.-T.; Tsai, C.-L.; Huang, C.-C. Effect of resistance-exercise training on cognitive function in healthy older adults: A review. J. Aging Phys. Act. 2012, 20, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K.; Satariano, W.A.; Tager, I.B. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J. Am. Geriatr. Soc. 2003, 51, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Scisco, J.L.; Leynes, P.A.; Kang, J. Cardiovascular fitness and executive control during task-switching: An ERP study. Int. J. Psychophysiol. 2008, 69, 52–60. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Albany, NY, USA, 2018. [Google Scholar]
- Tsujii, T.; Komatsu, K.; Sakatani, K. Acute effects of physical exercise on prefrontal cortex activity in older adults: A functional near-infrared spectroscopy study. In Oxygen Transport to Tissue XXXIV; Springer: Berlin/Heidelberg, Germany, 2013; pp. 293–298. [Google Scholar]
- Kramer, A.F.; Erickson, K.I.; Colcombe, S.J. Exercise, cognition, and the aging brain. J. Appl. Physiol. 2006, 101, 1237–1242. [Google Scholar] [CrossRef]
- Cansino, S.; Torres-Trejo, F.; Estrada-Manilla, C.; Pérez-Loyda, M.; Ramírez-Barajas, L.; Hernández-Ladrón-deGuevara, M.; Nava-Chaparro, A.; Ruiz-Velasco, S. Predictors of Working Memory Maintenance and Decline in Older Adults. Arch. Gerontol. Geriatr. 2020, 89, 104074. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.-C.; Mohammed, A.K.H. BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: Associations with working memory function. J. Alzheimer’s Dis. 2017, 55, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Shishido, T.; Nagatomi, R. Executive function during and after acute moderate aerobic exercise in adolescents. Psychol. Sport Exerc. 2015, 16, 7–17. [Google Scholar] [CrossRef]
- Li, L.; Men, W.-W.; Chang, Y.-K.; Fan, M.-X.; Ji, L.; Wei, G.-X. Acute aerobic exercise increases cortical activity during working memory: A functional MRI study in female college students. PLoS ONE 2014, 9, e99222. [Google Scholar] [CrossRef] [PubMed]
- Lindheimer, J.B.; O’Connor, P.J.; McCully, K.K.; Dishman, R.K. The effect of light-intensity cycling on mood and working memory in response to a randomized, placebo-controlled design. Psychosom. Med. 2017, 79, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Gothe, N.; Pontifex, M.B.; Hillman, C.; McAuley, E. The acute effects of yoga on executive function. J. Phys. Act. Health 2013, 10, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-G.; Zhu, L.-N.; Yan, J.; Yin, H.-C. Neural basis of working memory enhancement after acute aerobic exercise: fMRI study of preadolescent children. Front. Psychol. 2016, 7, 1804. [Google Scholar] [CrossRef]
- Chen, A.-G.; Yan, J.; Yin, H.-C.; Pan, C.-Y.; Chang, Y.-K. Effects of acute aerobic exercise on multiple aspects of executive function in preadolescent children. Psychol. Sport Exerc. 2014, 15, 627–636. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Hillman, C.H.; Fernhall, B.; Thompson, K.M.; Valentini, T.A. The effect of acute aerobic and resistance exercise on working memory. Med. Sci. Sports Exerc. 2009, 41, 927–934. [Google Scholar] [CrossRef]
- Weng, T.B.; Pierce, G.L.; Darling, W.G.; Voss, M.W. Differential effects of acute exercise on distinct aspects of executive function. Med. Sci. Sports Exerc. 2015, 47, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Blasiman, R.N.; Was, C.A. Why is working memory performance unstable? A review of 21 factors. Eur. J. Psychol. 2018, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Dajani, D.R.; Uddin, L.Q. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci. 2015, 38, 571–578. [Google Scholar] [CrossRef] [PubMed]
| Study | C 1 | C 2 | C 3 | C 4 | C 5 | C 6 | C 7 | C 8 | C 9 | C 10 | C 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barella et al. [56] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Chang et al. [49] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Chen et al. [50] | N | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Chu et al. [51] | N | Y | N | N | N | N | N | N | N | Y | Y | 3 |
| Hogan et al. [57] | N | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Hsieh et al. [58] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Hyodo et al. [54] | Y | Y | N | N | N | N | N | N | N | Y | Y | 3 |
| Ji et al. [7] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Stute et al. [53] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Tsai et al. [52] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Won et al. [55] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Author(s) | Study Design | Participants | Exercise Intervention | Cognitive Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age, Years (SD) | n | Type | Duration | Intensity | Task | Domain | Timing of Test | ||
| Barella et al. [56] (2010) | Randomized Controlled Trial | 69.5 ± 8.3 | CG: 20 EG: 20 (32F, 8M) | CG: Sitting at the end of the treadmill EG: Treadmill walking | CG: 25 min. EG: 25 Min (including 5 min WU) | CG: NA EG: 60% HRR | The Stroop test | Inhibition | Immediately after 5, 10, 15, 20, 30, 45, 60, 75, 90, 105 and 120 min after |
| Chang et al. [49] (2015) | Counter-balanced randomized and controlled group post-test | 63.10 ± 2.89 | LFG: 21 HFG: 21 (42M) | CG: Reading a book EG: Cycling | CG: 30 min EG: 30 Min (including 5 min WU and 5 min CD) | CG: NA EG: 50% to 60% HRR | Computerized version of the Stroop test | Inhibition | 15 Min after |
| Chen et al. [50] (2018) | Counter-balanced randomized and controlled group post-test | 57.67 ± 5.06 | Total: 45 (26F, 19M) | CG: Reading a book EG: Cycling | CG: 30 Min. EG1: 20 Min; EG2: 30 Min; EG3: 55 min. (All EC including 5 min WU and 5 min CD) | CG: NA EG: 65% to 70% HRR | The task switching task | Cognitive Flexibility | Immediately after |
| Chu et al. [51] (2015) | Counter-balanced randomized and controlled group post-test | HFG: 63.8 ± 2.3 LFG: 64.9 ± 4.0 | HFG: 22 LFG: 24 (22F, 24M) | CG: Reading a book EG: Cycling | CG: 30 Min EG: 30 Min (including 5 min WU and 5 min CD) | CG: NA EG: 60% HRR | Computerized version of the Stroop test | Inhibition | <5 min after |
| Hogan et al. [57] (2013) | Stratified randomized and controlled | YG: 19–39 years MAG: 40–64 years OG: 65+ | Total: 144 (73FM, 71M) | CG: Subjective picture quality rating EG: Cycling | CG: 15 to 25 min EG: 23 Min (including 5 min WU and 3 min CD) | CG: NA EG: 50% HRR | N-back task | Working Memory | Immediately after |
| Hsieh et al. [58] (2018) | Counter-balanced randomized and controlled group post-test | YG: 24.0 ± 3.1 OG: 70.0 ± 3.3 | YG: 24 (24M) OG: 20 (20M) | CG: Watching videos EG: Treadmill walking | CG: 30 Min EG: 30 Min (including 5 min WU and 5 min CD) | CG: NA EG: 60–70% HRR | Modified Stroop Color-Word Test | Inhibition | 15 Min after |
| Hyodo et al. [54] (2012) | Counterbalanced, randomized, and controlled | 69.3 ± 3.5 | Total: 16 (3F, 13M) | CG: Resting EG: Cycling | CG: 10 Min EG: 10 Min (including 3 min WU) | CG: NA EG: at VT (app. 50% VO2max) | The color-word matching Stroop task | Inhibition | 15 Min. after |
| Stute et. al. [53] (2020) | Counterbalanced, randomized, and controlled | 69.18 ± 3.92 | Total: 42 (21F, 21M) | CG: Listening to an audio book EG: Cycling | CG: 15 min EG: 10 min cycling | CG: NA EG: 50% VO2max | N-back task | Working Memory | 15, 30, and 45 min after |
| Tsai et. al. [52] (2021) | Counterbalanced, randomized, and controlled | 61.15 ± 4.43 | Total: 20 (10F, 10M) | CG: Reading a book EG: Cycling | CG: 30 Min EG: 30 Min (including 4 min WU and 2 min CD) | CG: NA EG: 50% to 55% HRR | Saccadic Paradigm | Inhibition | 3–5 min after |
| Ji et al. [7] (2019) | Counterbalanced, randomized, and controlled | 65.60 ± 1.32 | Total: 20 (9F, 11M) | CG: Reading a book EG: Treadmill walking; cognitive exercise; cognitive + treadmill walking | CG: 25 min. EG: 25 Min (including 5 min WU and 5 min CD) | CG: NA EG: 65% HRmax | The Modified Stroop test | Inhibition | Immediately after |
| Won et al. [55] (2019) | Counter-balanced randomized controlled group post-test | 66.2 ± 7.3 | Total: 32 (24F, 8M) | CG: Resting EG: Cycling | CG: 35 Min EG: 35 Min (including 5 min WU; 5 min CD and 5 min recovery) | CG: NA EG: RPE of 15 | Flanker Task | Inhibition | 30–40 Min after |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çakaloğlu, E.; Yüksel, H.S.; Şahin, F.N.; Güler, Ö.; Arslanoğlu, E.; Yamak, B.; Aydoğmuş, M.; Yaşar, O.M.; Gürkan, A.C.; Söyler, M.; et al. The Acute Effects of Moderate-Intensity Aerobic Exercise on Core Executive Functions in Healthy Older Adults: A Systematic Review. Life 2025, 15, 230. https://doi.org/10.3390/life15020230
Çakaloğlu E, Yüksel HS, Şahin FN, Güler Ö, Arslanoğlu E, Yamak B, Aydoğmuş M, Yaşar OM, Gürkan AC, Söyler M, et al. The Acute Effects of Moderate-Intensity Aerobic Exercise on Core Executive Functions in Healthy Older Adults: A Systematic Review. Life. 2025; 15(2):230. https://doi.org/10.3390/life15020230
Chicago/Turabian StyleÇakaloğlu, Erdem, Hidayet Suha Yüksel, Fatma Neşe Şahin, Özkan Güler, Erkal Arslanoğlu, Bade Yamak, Mert Aydoğmuş, Onur Mutlu Yaşar, Alper Cenk Gürkan, Mehmet Söyler, and et al. 2025. "The Acute Effects of Moderate-Intensity Aerobic Exercise on Core Executive Functions in Healthy Older Adults: A Systematic Review" Life 15, no. 2: 230. https://doi.org/10.3390/life15020230
APA StyleÇakaloğlu, E., Yüksel, H. S., Şahin, F. N., Güler, Ö., Arslanoğlu, E., Yamak, B., Aydoğmuş, M., Yaşar, O. M., Gürkan, A. C., Söyler, M., Ceylan, L., & Küçük, H. (2025). The Acute Effects of Moderate-Intensity Aerobic Exercise on Core Executive Functions in Healthy Older Adults: A Systematic Review. Life, 15(2), 230. https://doi.org/10.3390/life15020230












