The Potential of a Robot Presence in Close Relationship to Influence Human Responses to Experimental Pain
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.1.1. Control Condition
2.1.2. Robot Condition
- Day 1: The Robot condition concluded with a Recovery Session, during which participants casually chatted with Moffuly for 30 min before providing the final blood sample and completing the questionnaires.
- Day 2: The Robot condition concluded with a Control Session, during which participants remained seated and relaxed for 30 min before providing the final blood sample and completing the questionnaires.
2.2. Participants
2.3. Pain Stimulation and Measures
2.3.1. Experimental Pain Stimulation and Subjective Assessment of Pain
2.3.2. Hormonal Measurement
- T1: before the first heat pain stimulation (baseline),
- T2: after the first heat pain stimulation without a robot hug (Control condition),
- T3: after habituation with the robot and before heat pain stimulation with a robot hug (Robot condition),
- T4: after the second heat pain stimulation with a robot hug (Robot condition),
- T5: at the end, after the Recovery Session (Day 1) or Control Session (Day 2).
2.3.3. Mood and Mental Status Assessment Questionnaires
2.4. Statistical Analyses
3. Results
3.1. Pain Perception Changes Induced by Robot Hug
3.1.1. VAS Results
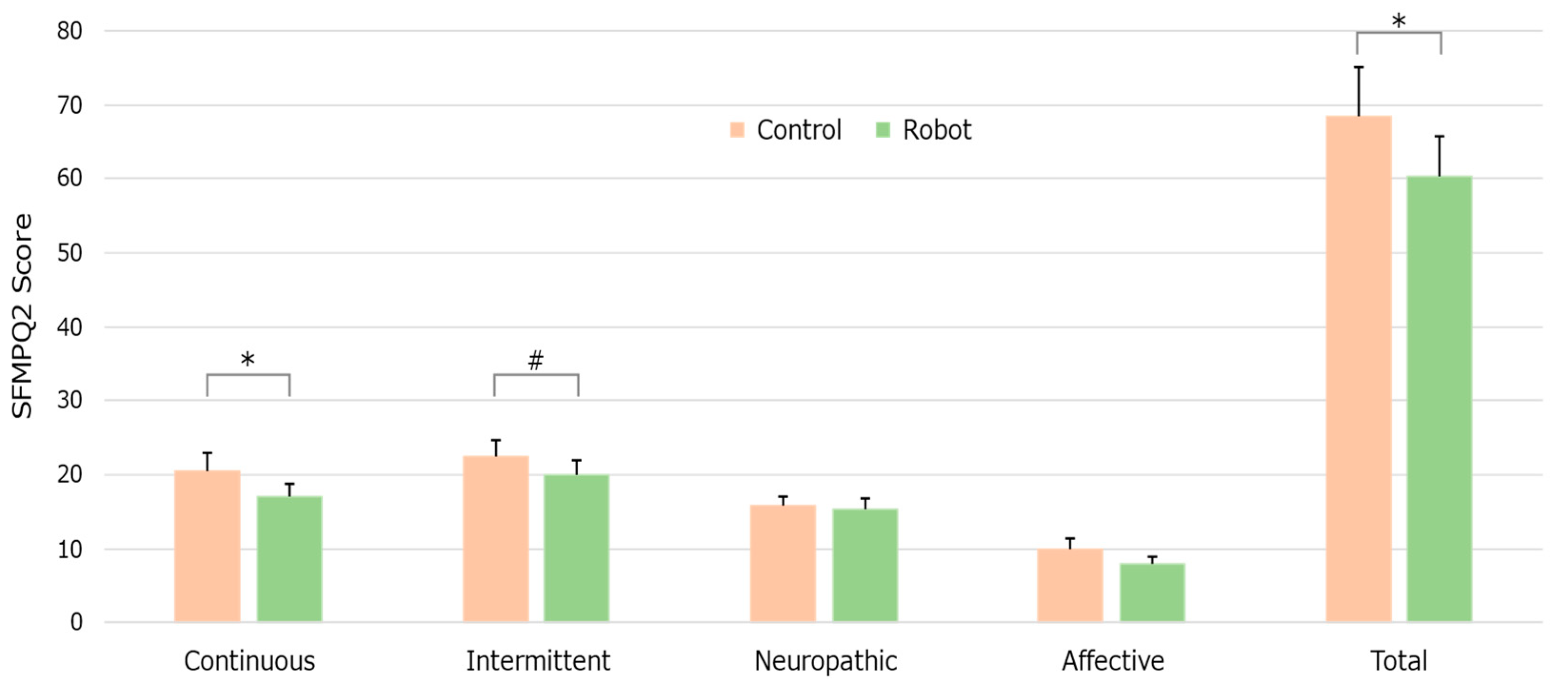
3.1.2. SF-MPQ-2 Responses
3.2. Hormone Level Changes
3.2.1. Growth Hormone Levels
3.2.2. Oxytocin Levels
3.2.3. Cortisol Levels
3.2.4. DHEA-S Levels
3.2.5. Estrogen Levels
3.2.6. Testosterone Levels
3.3. Mood and Mental Status Responses
3.3.1. POMS-2
3.3.2. HADS
3.3.3. SDS
3.4. Recovery Session-Related Results
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AH | Anger hostility |
| CB | Confusion bewilderment |
| DD | Depression dejection |
| DHEA-S | Dehydroepiandrosterone-Sulfate |
| FI | Fatigue inertia |
| GH | Growth Hormone |
| HADS | Hospital Anxiety and Depression Scale |
| HADS-A | HADS for anxiety |
| HADS-D | HADS for depression |
| OT | Oxytocin |
| POMS-2 | Profile of Mood States 2nd Edition |
| SDS | Self-Rating Depression Scale |
| SEM | Standard error of the mean |
| SF-MPQ-2 | The Short-Form McGill Pain Questionnaire |
| TA | Tension anxiety |
| TMD | Total mood disturbance |
| VA | Vigor activity |
| VAS | Visual Analogue Scale |
References
- Brennan, F.; Carr, D.B.; Cousins, M. Pain management: A fundamental human right. Anesth. Analg. 2007, 105, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D.; Zeidler, H.; Haglund, U.; Carr, A.J.; Chaussade, S.; Cucinotta, D.; Veale, D.J.; Martin-Mola, E. Musculoskeletal pain in Europe: Its impact and a comparison of population and medical perceptions of treatment in eight European countries. Ann. Rheum. Dis. 2004, 63, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef]
- Loeffler, A.; Steptoe, A. Bidirectional longitudinal associations between loneliness and pain, and the role of inflammation. Pain 2021, 162, 930–937. [Google Scholar] [CrossRef]
- Kotwal, A.A.; Cenzer, I.S.; Waite, L.J.; Covinsky, K.E.; Perissinotto, C.M.; Boscardin, W.J.; Hawkley, L.C.; Dale, W.; Smith, A.K. The epidemiology of social isolation and loneliness among older adults during the last years of life. J. Am. Geriatr. Soc. 2021, 69, 3081–3091. [Google Scholar] [CrossRef]
- Lavin, P.; Lesage, M.; Monroe, E.; Kanevsky, M.; Gruber, J.; Cinalioglu, K.; Rej, S.; Sekhon, H. Humanoid robot intervention vs. treatment as usual for loneliness in long-term care homes: Study protocol for a pilot randomized controlled trial. Front. Psychiatry 2022, 13, 1003881. [Google Scholar] [CrossRef]
- Latikka, R.; Rubio-Hernández, R.; Lohan, E.S.; Rantala, J.; Nieto Fernández, F.; Laitinen, A.; Oksanen, A. Older adults’ loneliness, social isolation, and physical information and communication technology in the era of ambient assisted living: A systematic literature review. J. Med. Internet Res. 2021, 23, e28022. [Google Scholar] [CrossRef]
- Li, D.; Rau, P.L.P.; Li, Y. A Cross-cultural Study: Effect of Robot Appearance and Task. Int. J. Soc. Robot. 2010, 2, 175–186. [Google Scholar] [CrossRef]
- Bartneck, C.; Nomura, T.; Kanda, T.; Suzuki, T.; Kato, K. Cultural Differences in Attitudes Towards Robots. In Proceedings of the Symposium on Robot Companions: Hard Problems and Open Challenges in Robot-Human Interaction, Hatfield, UK, 12–15 April 2005; Society for the Study of Artificial Intelligence and the Simulation of Behaviour (SSAISB): Hatfield, UK, 2005. [Google Scholar]
- Haring, K.S.; Mougenot, C.; Ono, F.; Watanabe, K. Cultural Differences in Perception and Attitude towards Robots. Int. J. Affect. Eng 2014, 13, 149–157. [Google Scholar] [CrossRef]
- De Graaf, M.M.A.; Ben Allouch, S. Exploring influencing variables for the acceptance of social robots. Rob. Auton. Syst. 2013, 61, 1476–1486. [Google Scholar] [CrossRef]
- Sumioka, H.; Nakae, A.; Kanai, R.; Ishiguro, H. Huggable communication medium decreases cortisol levels. Sci. Rep. 2013, 3, 3034. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Moyle, W.; Jones, C.; Petsky, H. A social robot intervention on depression, loneliness, and quality of life for Taiwanese older adults in long-term care. Int. Psychogeriatr. 2020, 32, 981–991. [Google Scholar] [CrossRef]
- Gasteiger, N.; Loveys, K.; Law, M.; Broadbent, E. Friends from the Future: A Scoping Review of Research into Robots and Computer Agents to Combat Loneliness in Older People. Clin. Interv. Aging 2021, 16, 941–971. [Google Scholar] [CrossRef]
- Robinson, H.; Macdonald, B.; Kerse, N.; Broadbent, E. The psychosocial effects of a companion robot: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2013, 14, 661–667. [Google Scholar] [CrossRef]
- Quartana, P.J.; Buenaver, L.F.; Edwards, R.R.; Klick, B.; Haythornthwaite, J.A.; Smith, M.T. Pain catastrophizing and salivary cortisol responses to laboratory pain testing in temporomandibular disorder and healthy participants. J. Pain 2010, 11, 186–194. [Google Scholar] [CrossRef]
- Ashpole, N.M.; Sanders, J.E.; Hodges, E.L.; Yan, H.; Sonntag, W.E. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015, 68, 76–81. [Google Scholar] [CrossRef]
- Xu, J.; Casserly, E.; Yin, Y.; Cheng, J. A systematic review of growth hormone in pain medicine: From rodents to humans. Pain Med. 2020, 21, 21–31. [Google Scholar] [CrossRef]
- Boll, S.; Almeida de Minas, A.C.; Raftogianni, A.; Herpertz, S.C.; Grinevich, V. Oxytocin and pain perception: From animal models to human research. Neuroscience 2018, 387, 149–161. [Google Scholar] [CrossRef]
- Li, Y.-X.; An, H.; Wen, Z.; Tao, Z.-Y.; Cao, D.-Y. Can oxytocin inhibit stress-induced hyperalgesia? Neuropeptides 2020, 79, 101996. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.A.; Aguirre-Camacho, A.; Campbell, T.S. Oxytocin and pain: A systematic review and synthesis of findings. Clin. J. Pain 2014, 30, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-H.; Zhang, W.-X.; Xu, Q.; Wu, H.; Jiao, C.-C.; Chen, X.-Z. Estrogen modulation of visceral pain. J. Zhejiang Univ. Sci. B 2019, 20, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.M.; Newhouse, P.A. Estrogen, stress, and depression: Cognitive and biological interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef]
- Shiomi, M.; Nakata, A.; Kanbara, M.; Hagita, N. Robot Reciprocation of Hugs Increases Both Interacting Times and Self-disclosures. Int. J. Soc. Robot. 2021, 13, 353–361. [Google Scholar] [CrossRef]
- Sorrentino, A.; Mancioppi, G.; Coviello, L.; Cavallo, F.; Fiorini, L. Feasibility study on the role of personality, emotion, and engagement in socially assistive robotics: A cognitive assessment scenario. Informatics 2021, 8, 23. [Google Scholar] [CrossRef]
- Nakae, A.; Bu-Omer, H.M.; Chang, W.-C.; Kishimoto, C.; Sumioka, H. Exploring the psychological and physiological effects of operating a telenoid: The preliminary assessment of a minimal humanoid robot for mediated communication. Sensors 2024, 24, 7541. [Google Scholar] [CrossRef]
- Cross, E.S.; Riddoch, K.A.; Pratts, J.; Titone, S.; Chaudhury, B.; Hortensius, R. A neurocognitive investigation of the impact of socializing with a robot on empathy for pain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180034. [Google Scholar] [CrossRef]
- Van Neerven, S.G.A.; Mouraux, A. Capsaicin-Induced Skin Desensitization Differentially Affects A-Delta and C-Fiber-Mediated Heat Sensitivity. Front. Pharmacol. 2020, 11, 615. [Google Scholar] [CrossRef]
- Kong, J.-T.; Schnyer, R.N.; Johnson, K.A.; Mackey, S. Understanding central mechanisms of acupuncture analgesia using dynamic quantitative sensory testing: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 187182. [Google Scholar] [CrossRef]
- (Medoc Ltd. 2005). PATHWAY Pain & Sensory Evaluation System. [apparatus and software]. Available online: https://www.medoc-web.com/pathway (accessed on 28 January 2025).
- Dworkin, R.H.; Turk, D.C.; Revicki, D.A.; Harding, G.; Coyne, K.S.; Peirce-Sandner, S.; Bhagwat, D.; Everton, D.; Burke, L.B.; Cowan, P.; et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009, 144, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Nakae, A.; Maeda, L.; Shi, K.; Takahashi, K.; Morris, S.; Hosomi, K.; Kanatani, H.; Matsuzaki, T.; Saitoh, Y. Validity, reliability, and assessment sensitivity of the Japanese version of the short-form McGill pain questionnaire 2 in Japanese patients with neuropathic and non-neuropathic pain. Pain Med. 2014, 15, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Speroff, L.; Marc, A.M.D.F. Clinical Gynecologic Endocrinology and Infertility, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; p. 1334. ISBN 0781747953. [Google Scholar]
- Russell, N.; Grossmann, M. Mechanisms in endocrinology: Estradiol as a male hormone. Eur. J. Endocrinol. 2019, 181, R23–R43. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Davis, S.R. Minireview: Aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology 2001, 142, 4589–4594. [Google Scholar] [CrossRef]
- Heuchert, J.P.; McNair, D.M. Profile of Mood States Second Edition (POMS 2); Multi-Health Systems: North Tonawanda, NY, USA, 2012. [Google Scholar]
- Yokoyama, K.; Watanabe, K. Japanese Translation of POMS 2: Profile of Mood States Second Edition; Kaneko Shobo: Tokyo, Japan, 2015. [Google Scholar]
- Kitamura, T. Hospital anxiety and depression scale. Arch. Psyciatric. Diagn. Clin. Eval. 1993, 4, 371–372. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Zung, W.W. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New Jersey, NJ, USA, 1988; pp. 79–81. ISBN 9780203771587. [Google Scholar]
- Pu, L.; Moyle, W.; Jones, C.; Todorovic, M. The Effectiveness of Social Robots for Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. Gerontologist 2019, 59, e37–e51. [Google Scholar] [CrossRef]
- Morgan, A.A.; Abdi, J.; Syed, M.A.Q.; Kohen, G.E.; Barlow, P.; Vizcaychipi, M.P. Robots in healthcare: A scoping review. Curr. Robot. Rep. 2022, 3, 271–280. [Google Scholar] [CrossRef]
- Jibb, L.A.; Birnie, K.A.; Nathan, P.C.; Beran, T.N.; Hum, V.; Victor, J.C.; Stinson, J.N. Using the MEDiPORT humanoid robot to reduce procedural pain and distress in children with cancer: A pilot randomized controlled trial. Pediatr. Blood Cancer 2018, 65, e27242. [Google Scholar] [CrossRef]
- Trost, M.J.; Chrysilla, G.; Gold, J.I.; Matarić, M. Socially-Assistive Robots Using Empathy to Reduce Pain and Distress during Peripheral IV Placement in Children. Pain Res. Manag. 2020, 2020, 7935215. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Sivakumar, M.; Beran, T.; Scott, S.D.; Vandermeer, B.; Curtis, S.; Jou, H.; Hartling, L. Study protocol for a randomised controlled trial of humanoid robot-based distraction for venipuncture pain in children. BMJ Open 2018, 8, e023366. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Manaloor, R.; Ma, K.; Sivakumar, M.; Beran, T.; Scott, S.D.; Vandermeer, B.; Beirnes, N.; Graham, T.A.D.; Curtis, S.; et al. A randomized trial of robot-based distraction to reduce children’s distress and pain during intravenous insertion in the emergency department. Can. J. Emerg. Med. 2021, 23, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Beran, T.N.; Ramirez-Serrano, A.; Vanderkooi, O.G.; Kuhn, S. Reducing children’s pain and distress towards flu vaccinations: A novel and effective application of humanoid robotics. Vaccine 2013, 31, 2772–2777. [Google Scholar] [CrossRef]
- Farrier, C.E.; Pearson, J.D.R.; Beran, T.N. Children’s fear and pain during medical procedures: A quality improvement study with a humanoid robot. Can. J. Nurs. Res. 2020, 52, 328–334. [Google Scholar] [CrossRef]
- Blanchard, A.; Nguyen, S.M.; Devanne, M.; Simonnet, M.; Le Goff-Pronost, M.; Rémy-Néris, O. Technical Feasibility of Supervision of Stretching Exercises by a Humanoid Robot Coach for Chronic Low Back Pain: The R-COOL Randomized Trial. Biomed Res. Int. 2022, 2022, 5667223. [Google Scholar] [CrossRef]
- Abdi, J.; Al-Hindawi, A.; Ng, T.; Vizcaychipi, M.P. Scoping review on the use of socially assistive robot technology in elderly care. BMJ Open 2018, 8, e018815. [Google Scholar] [CrossRef]
- Fattal, C.; Cossin, I.; Pain, F.; Haize, E.; Marissael, C.; Schmutz, S.; Ocnarescu, I. Perspectives on usability and accessibility of an autonomous humanoid robot living with elderly people. Disabil. Rehabil. Assist. Technol. 2022, 17, 418–430. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Hawkley, L.C.; Thisted, R.A. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol. Aging 2010, 25, 453–463. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef]
- Williams, A.C.d.C.; Craig, K.D. Updating the definition of pain. Pain 2016, 157, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Young, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg. Med. J. 2001, 18, 205–207. [Google Scholar] [CrossRef]
- Wang, J.; Mann, F.; Lloyd-Evans, B.; Ma, R.; Johnson, S. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry 2018, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Birken, M.; Chipp, B.; Shah, P.; Olive, R.R.; Nyikavaranda, P.; Hardy, J.; Chhapia, A.; Barber, N.; Lee, S.; Pearce, E.; et al. Exploring the experiences of loneliness in adults with mental health problems: A participatory qualitative interview study. PLoS ONE 2023, 18, e0280946. [Google Scholar] [CrossRef]
- Allen, S.F.; Gilbody, S.; Atkin, K.; van der Feltz-Cornelis, C. The associations between loneliness, social exclusion and pain in the general population: A N=502,528 cross-sectional UK Biobank study. J. Psychiatr. Res. 2020, 130, 68–74. [Google Scholar] [CrossRef]
- Coker, A.L.; Smith, P.H.; Bethea, L.; King, M.R.; McKeown, R.E. Physical health consequences of physical and psychological intimate partner violence. Arch. Fam. Med. 2000, 9, 451–457. [Google Scholar] [CrossRef]
- Crouch, J.L.; Milner, J.S.; Skowronski, J.J.; Farc, M.M.; Irwin, L.M.; Neese, A. Automatic encoding of ambiguous child behavior in high and low risk for child physical abuse parents. J. Fam. Viol. 2010, 25, 73–80. [Google Scholar] [CrossRef]
- Tsur, N. Chronic pain personification following child abuse: The imprinted experience of child abuse in later chronic pain. J. Interpers. Violence 2022, 37, NP2516–NP2537. [Google Scholar] [CrossRef]
- Long, P.A.; Freeman, H. Patients in pain: The effects of oxytocin on trust and decision making. Proc. Int. Symp. Hum. Factors Ergon. Health Care 2019, 8, 164–166. [Google Scholar] [CrossRef]
- Olza, I.; Uvnas-Moberg, K.; Ekström-Bergström, A.; Leahy-Warren, P.; Karlsdottir, S.I.; Nieuwenhuijze, M.; Villarmea, S.; Hadjigeorgiou, E.; Kazmierczak, M.; Spyridou, A.; et al. Birth as a neuro-psycho-social event: An integrative model of maternal experiences and their relation to neurohormonal events during childbirth. PLoS ONE 2020, 15, e0230992. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Muhtz, C.; Rodriguez-Raecke, R.; Hinkelmann, K.; Moeller-Bertram, T.; Kiefer, F.; Wiedemann, K.; May, A.; Otte, C. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med. 2013, 14, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Leitao, C.B. Laughter as medicine: A systematic review and meta-analysis of interventional studies evaluating the impact of spontaneous laughter on cortisol levels. PLoS ONE 2023, 18, e0286260. [Google Scholar] [CrossRef] [PubMed]
- Kökönyei, G.; Galambos, A.; Kocsel, N.; Szabó, E.; Édes, A.E.; Gecse, K.; Baksa, D.; Pap, D.; Kozák, L.R.; Bagdy, G.; et al. Inter-individual differences in pain anticipation and pain perception in migraine: Neural correlates of migraine frequency and cortisol-to-dehydroepiandrosterone sulfate (DHEA-S) ratio. PLoS ONE 2021, 16, e0261570. [Google Scholar] [CrossRef]
- Ritsner, M.S. The clinical and therapeutic potentials of dehydroepiandrosterone and pregnenolone in schizophrenia. Neuroscience 2011, 191, 91–100. [Google Scholar] [CrossRef]
- Athnaiel, O.; Cantillo, S.; Paredes, S.; Knezevic, N.N. The Role of Sex Hormones in Pain-Related Conditions. Int. J. Mol. Sci. 2023, 24, 1866. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Sadana, N.; Chen, X. Estrogen receptors in pain modulation: Cellular signaling. Biol. Sex Differ. 2021, 12, 22. [Google Scholar] [CrossRef]
- Sripada, R.K.; Marx, C.E.; King, A.P.; Rajaram, N.; Garfinkel, S.N.; Abelson, J.L.; Liberzon, I. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology 2013, 38, 1798–1807. [Google Scholar] [CrossRef]
- Karachaliou, F.-H.; Karavanaki, K.; Simatou, A.; Tsintzou, E.; Skarakis, N.S.; Kanaka-Gatenbein, C. Association of growth hormone deficiency (GHD) with anxiety and depression: Experimental data and evidence from GHD children and adolescents. Hormones 2021, 20, 679–689. [Google Scholar] [CrossRef]
- Algahtany, M.; Sharma, S.; Fahoum, K.; Jing, R.; Zhang, S.; Kovacs, K.; Rotondo, F.; Lee, J.; Vanek, I.; Cusimano, M.D. The role of growth hormone in depression: A human model. Front. Neurosci. 2021, 15, 661819. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Matsushita, H.; Tomizawa, K.; Matsui, H. Oxytocin: A therapeutic target for mental disorders. J. Physiol. Sci. 2012, 62, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Colloca, L.; Benedetti, F. Placebo analgesia induced by social observational learning. Pain 2009, 144, 28–34. [Google Scholar] [CrossRef]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef]
- Gaffey, A.E.; Bergeman, C.S.; Clark, L.A.; Wirth, M.M. Aging and the HPA axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev. 2016, 68, 928–945. [Google Scholar] [CrossRef]
- Aguilera, G. HPA axis responsiveness to stress: Implications for healthy aging. Exp. Gerontol. 2011, 46, 90–95. [Google Scholar] [CrossRef]





| No. | Questions |
|---|---|
| 1 | What are you most passionate about right now? |
| 2 | What have you enjoyed lately? |
| 3 | What do you most want to do, but cannot right now? |
| 4 | What do you want right now? |
| 5 | If you could know one thing about the future, what would it be? |
| 6 | What is your favorite type of person? |
| 7 | What do you like to do? |
| 8 | What has made you very happy lately? |
| 9 | When was the last time you cried? Why did you cry? |
| 10 | Tell us what you have done well lately. |
| 11 | What is the most precious thing you remember? |
| 12 | What is something you have failed at recently? |
| 13 | Where would you go on a trip together? |
| 14 | If you went to the movies together, what kind of movie would you like to see? |
| 15 | What would you like to do for your friend? |
| Type of Pain | Control Mean ± SD | With Robot Mean ± SD | p-Value |
|---|---|---|---|
| Sharp pain | 6.00 ± 0.506 | 5.41 ± 0.546 | 0.0778 # |
| Cramping pain | 3.97 ± 0.474 | 3.03 ± 0.488 | 0.0494 * |
| Aching pain | 4.18 ± 0.616 | 3.38 ± 0.562 | 0.0172 * |
| Heavy pain | 2.45 ± 0.424 | 1.68 ± 0.358 | 0.0301 * |
| Splitting pain | 1.94 ± 0.424 | 1.03 ± 0.233 | 0.0108 * |
| Fearful | 3.21 ± 0.573 | 2.29 ± 0.446 | 0.0416 * |
| Sharp pain | 6.00 ± 0.506 | 5.41 ± 0.546 | 0.0778 # |
| Cramping pain | 3.97 ± 0.474 | 3.03 ± 0.488 | 0.0494 * |
| Sharp pain | 6.00 ± 0.506 | 5.41 ± 0.546 | 0.0778 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakae, A.; Bu-Omer, H.M.; Chang, W.-C.; Kishimoto, C.; Onishi, Y.; Sumioka, H.; Shiomi, M. The Potential of a Robot Presence in Close Relationship to Influence Human Responses to Experimental Pain. Life 2025, 15, 229. https://doi.org/10.3390/life15020229
Nakae A, Bu-Omer HM, Chang W-C, Kishimoto C, Onishi Y, Sumioka H, Shiomi M. The Potential of a Robot Presence in Close Relationship to Influence Human Responses to Experimental Pain. Life. 2025; 15(2):229. https://doi.org/10.3390/life15020229
Chicago/Turabian StyleNakae, Aya, Hani M. Bu-Omer, Wei-Chuan Chang, Chie Kishimoto, Yuya Onishi, Hidenobu Sumioka, and Masahiro Shiomi. 2025. "The Potential of a Robot Presence in Close Relationship to Influence Human Responses to Experimental Pain" Life 15, no. 2: 229. https://doi.org/10.3390/life15020229
APA StyleNakae, A., Bu-Omer, H. M., Chang, W.-C., Kishimoto, C., Onishi, Y., Sumioka, H., & Shiomi, M. (2025). The Potential of a Robot Presence in Close Relationship to Influence Human Responses to Experimental Pain. Life, 15(2), 229. https://doi.org/10.3390/life15020229







