Calliphoridae and Mesembrinellidae (Insect: Diptera) Across Different Environments of Rio de Janeiro, Brazil: Synanthropy and Potential Bioindicators, with Notes on Bait Preference
Abstract
1. Introduction
2. Materials and Methods
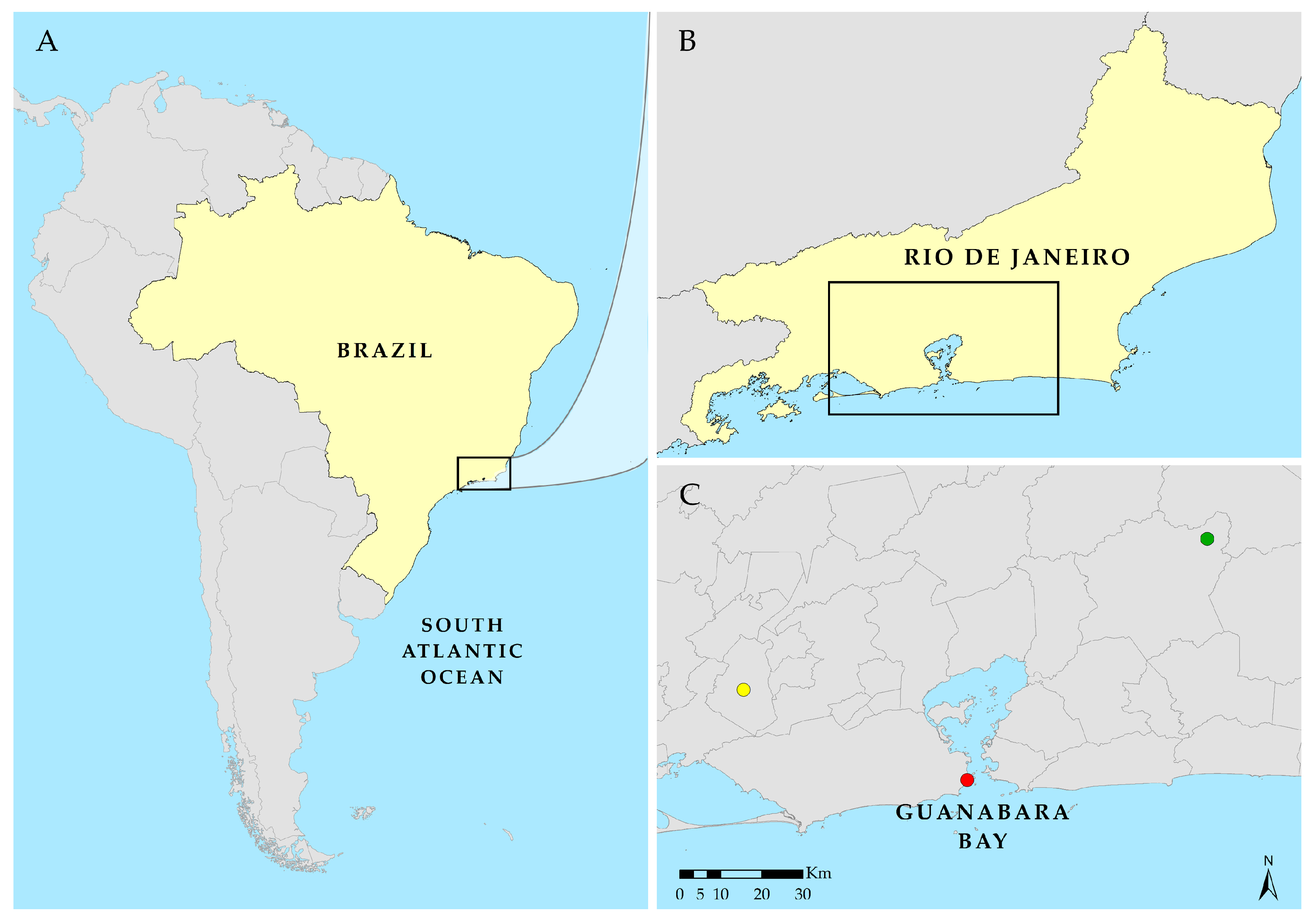
2.1. Forest Area—Três Picos State Park (PETP)
2.2. Rural Area—Federal Rural University of Rio de Janeiro (UFRRJ)
2.3. Urban Area—Federal University of the State of Rio de Janeiro (UNIRIO)
2.4. Sampling Method
2.5. Synanthropy Study
- a = percentage of individuals of a given species collected in the urban area;
- b = percentage of individuals of a given species collected in the rural area;
- c = percentage of individuals of a given species collected in the wilderness area.
2.6. Bioindicator Analysis
2.7. Bait Preference
3. Results
3.1. Bait Preference
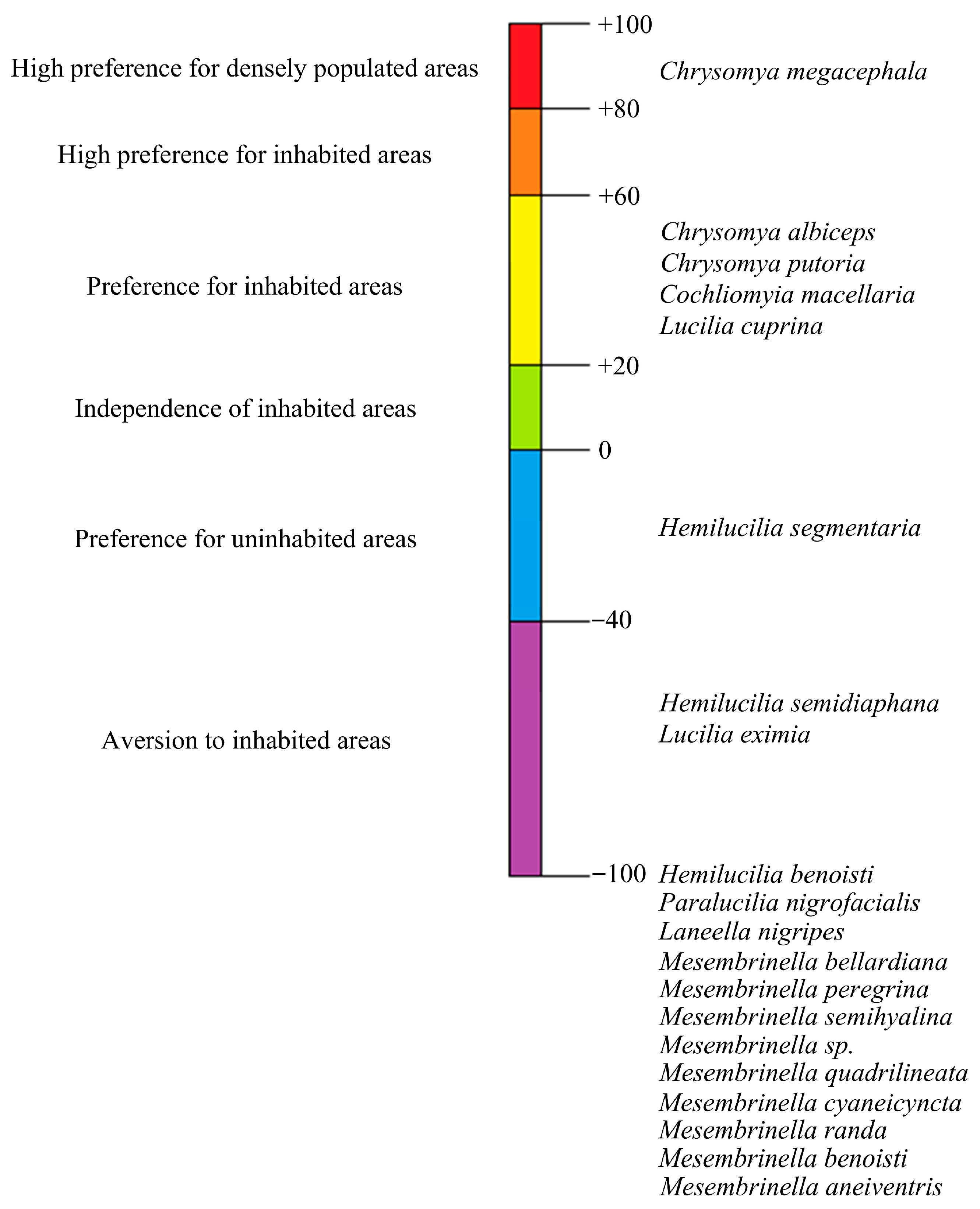
3.2. Study of Synanthropy
3.3. Bioindicator Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UNIRIO | Universidade Federal do Estado do Rio de Janeiro |
| UFRRJ | Universidade Federal Rural do Rio de Janeiro |
References
- CEPF—Critical Ecosystem Partnership Fund: Perfil do Ecossistema Mata Atlântica, Hotspot de Biodiversidade, Brasil. 29p. Available online: https://www.cepf.net/sites/default/files/atlantic-forest-ecosystem-profile-2001-portuguese.pdf (accessed on 7 September 2023).
- INEA—Instituto Nacional de Ecologia e Ambiente: Parque Estadual dos Três Picos. Available online: https://www.inea.rj.gov.br/biodiversidade-territorio/conheca-as-unidades-de-conservacao/parque-estadual-dos-tres-picos/ (accessed on 10 September 2023).
- Turner, R.M.; Collet, R.T. The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol. Evol. 1996, 11, 330–333. [Google Scholar] [CrossRef]
- Gadelha, B.Q.; Ribeiro, A.C.; Aguiar, V.M.; Mello-Patiu, C.A. Edge effects on the blowfly fauna (Diptera, Calliphoridae) of the Tijuca National Park, Rio de Janeiro, Brazil. Braz. J. Biol. 2015, 75, 999–1007. [Google Scholar] [CrossRef]
- ICMBIO—Instituto Chico Mendes de Conservação da Biodiversidade: Mata Atlântica. Available online: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/mata-atlantica (accessed on 13 September 2023).
- Figueiredo, J.C.G.; Negreiros, D.; Ramos, L.; Paiva, D.C.; Oki, Y.; Justino, W.S.; Santos, R.M.; Aguilar, R.; Nunes, Y.R.F.; Fernandes, G.W. Reference sites of threatened riverine atlantic forest in upper rio doce watershed. Nat. Conserv. Res. 2024, 9, 58–71. [Google Scholar] [CrossRef]
- Ferraz, A.C.P.; Gadelha, B.Q.; Aguiar-Coelho, V.M. Influência climática e antrópica na abundância e riqueza de Calliphoridae (Diptera) em fragmento florestal da Reserva Biológica do Tinguá, RJ. Neotrop. Entomol. 2010, 39, 476–485. [Google Scholar] [CrossRef]
- Battán-Horenstein, B.L.M. Diversity of necrophagous blowfly (Diptera: Calliphoridae) of medical and veterinary importance in urban environments in Córdoba, Argentina. Caldasia 2016, 38, 189–195. [Google Scholar] [CrossRef]
- Mendes, T.P.; Esposito, M.C.; Carvalho-Filho, F.S.; Juen, L.; Alvarado, S.T.; Sousa, J.R.P. Necrophagous flies (Diptera: Calliphoridae and Sarcophagidae) as indicators of the conservation or anthropization of environments in eastern Amazonia, Brazil. J. Insect Conserv. 2021, 25, 719–732. [Google Scholar] [CrossRef]
- Dufek, M.I.; Oscherov, E.L.; Damborsky, M.P.; Mulieri, P.R. Calliphoridae (Diptera) in human-transformed and wild habitats: Diversity and seasonal fluctuations in the humid chaco ecoregion of south America. J. Med. Entomol. 2019, 56, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Serra-Freire, N.M.; Mello, R.P. Entomologia e Acarologia na Medicina Veterinária; L. F. Livros de Veterinária Ltd.a.: Rio de Janeiro, Brazil, 2006; 200p. [Google Scholar]
- Borghesan, T.C.; Campaner, M.; Matsumoto, T.E.; Espinosa, O.A.; Razafindranaivo, V.; Paiva, F.; Carranza, J.C.; Añez, N.; Neves, L.; Teixeira, M.M.G.; et al. Genetic diversity and phylogenetic relationships of coevolving symbiont-harboring insect trypanosomatids; their neotropical dispersal by invader African blowflies (Calliphoridae). Front. Microbiol. 2018, 9, 131. [Google Scholar] [CrossRef]
- Figueiredo, A.L.; Carvalho, R.P.; Azevedo, W.T.A.; Teixeira, M.L.F.; Rebello, M.T.; Ramos, A.C.d.C.; Lessa, C.S.S.; Aguiar, V.M. Faunistic analysis of the families Calliphoridae and Mesembrinellidae (Diptera) at Jardim Botânico do Rio de Janeiro, Brazil. J. Med. Entomol. 2018, 55, 1527–1535. [Google Scholar] [CrossRef]
- Skevington, J.H.; Dang, P.T. Exploring the diversity of flies (Diptera). Biodiversity 2002, 3, 3–27. [Google Scholar] [CrossRef]
- Beltran, Y.T.P.; Segura, N.A.; Bello, F.J. Synanthropy of Calliphoridae and Sarcophagidae (Diptera) in Bogotá, Colombia. Neotrop. Entomol. 2012, 41, 237–242. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esn, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef] [PubMed]
- Borror, D.J.; Triplehorn, C.A.; Johnson, N.F. An Introduction to the Study of Insects, 7th ed.; Saunders College Publishing: Philadelphia, PA, USA, 2005; 818p. [Google Scholar]
- Lenko, K.; Papavero, N. Insetos no Folclore, 2nd ed.; Plêiade Fapesp: São Paulo, Brazil, 1996; 468p. [Google Scholar]
- Vargas, J.; Wood, D.M. Calliphoridae. In Manual of Central American Diptera; Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., Zumbado, M.A., Eds.; NCR Research Press: Ottawa, ON, USA, 2010; pp. 1297–1304. [Google Scholar]
- Archer, M.S.; Elgar, M.A. Female breeding-site preferences and larval feeding strategies of carrion-breeding Calliphoridae and Sarcophagidae (Diptera): A quantitative analysis. Aust. J. Zool. 2003, 51, 165–174. [Google Scholar] [CrossRef]
- Baumhover, A.H. Erradication of the screw worm fly, an agent of myiasis. JAMA 1966, 166, 240–248. [Google Scholar] [CrossRef]
- Azevedo, W.T.A.; Figueiredo, A.L.; Carvalho, R.P.; Lemos, G.A.; Silva, P.F.; Miranda, T.A.; Lessa, C.S.; Aguiar, V.M. Record of the first cases of human myiasis by Lucilia cuprina (Diptera: Calliphoridae), Rio de Janeiro, Brazil. J. Med. Entomol. 2015, 52, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Costa, J. Entomologia Forense: Quando os Insetos São os Vestígios, 3rd ed.; Millenium: Campinas, Brazil, 2011; 513p. [Google Scholar]
- Carvalho, R.P.; Azevedo, W.T.A.; Figueiredo, A.L.; Lessa, C.S.S.; Aguiar, V.M. Dipterofauna associated with rat carcasses in the Atlantic Forest, Southeastern Brazil. J. Med. Entomol. 2017, 54, 1498–1509. [Google Scholar] [CrossRef]
- Azevedo, W.T.A.; Carvalho, R.P.; Figueiredo, A.L.; Ross, S.D.; Lessa, C.S.S.; Fortes, R.D.R.; Aguiar, V.M. Calliphoridae (Diptera) associated with Rattus rattus carcasses in the Tijuca National Park, Rio de Janeiro, Brazil. J. Med. Entomol. 2018, 55, 915–922. [Google Scholar] [CrossRef]
- Marinho, M.A.T.; Wolff, M.; Ramos-Pastrana, Y.; Azeredo-Espin, A.M.L.; Amorim, D.S. The first phylogenetic study of Mesembrinellidae (Diptera: Oestroidea) based on molecular data: Clades and congruence with morphological characters. Cladistics 2017, 33, 134–152. [Google Scholar] [CrossRef]
- Gadelha, B.Q.; Silva, A.B.; Ferraz, A.C.P.; Aguiar, V.M. Mesembrinellinae (Diptera: Calliphoridae) to edge effects in the Tinguá Biological Reserve, Rio de Janeiro, Brazil. Braz. J. Biol. 2015, 75, S196–S205. [Google Scholar] [CrossRef]
- Guimarães, J.H.A. A systematic revision of the Mesembrinellidae, stat. nov. (Diptera, Cyclorrhapha). Arq. Zool. 1977, 29, 1–109. [Google Scholar] [CrossRef]
- Klegarth, A.Y. Synanthropy. In The International Encyclopedia of Primatology, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–5. [Google Scholar]
- Gomes, P.M.S.; Santos, A.M.M. Moscas sinantrópicas nocivas, um desafio atual: Musca domestica L. (Muscidae) e Chrysomya megacephala (Fabricius) (Calliphoridae). Sustinere 2015, 3, 89–106. [Google Scholar] [CrossRef]
- Mota-Filho, F.O.; Pereira, E.C.; Silva, R.A.; Xavier-Filho, L. Líquens: Bioindicadores ou Biomonitores? Portal Biomonitor, 6p. Available online: http://biomonitor.ist.utl.pt/biomonitor (accessed on 27 October 2023).
- Kim, H.; Sun, Y.; Kim, T.-Y.; Moon, M.-J. Biodiversity monitoring for selection of insect and spider bioindicators at local organic agricultural habitats in South Korea. Entomol. Res. 2020, 50, 13. [Google Scholar] [CrossRef]
- Rohyani, I.S. Community structure analysis of soil insects and their potential role as bioindicators in various ecosystem types in Lombok, West Nusa Tenggara, Indonesia. Biodiversitas 2020, 21, 4221–4227. [Google Scholar] [CrossRef]
- Cagni, G.S.; Prazeres, J.A.; Leal, D.; Constantin, P.P.; Sousa, C.; Silva, C.R.; Conte, H. Organismos bioindicadores de metais pesados: Uma revisão. Rev. Ibero-Am. Ciênc Ambient. 2022, 13, 180–194. [Google Scholar] [CrossRef]
- UFRRJ—Universidade Federal Rural do Rio de Janeiro. A UFRRJ. Available online: https://portal.ufrrj.br/institucional/ (accessed on 19 October 2023).
- UNIRIO—Universidade Federal do Estado do Rio de Janeiro. História. Available online: https://www.unirio.br/instituicao/historia (accessed on 19 October 2023).
- Mello, R.S.; Queiroz, M.M.C.; Aguiar-Coelho, V.M. Population fluctuations of calliphorid species (Diptera, Calliphoridae) in the Biological Reserve of Tinguá, state of Rio de Janeiro, Brazil. Iheringia Série Zool. 2007, 97, 481–485. [Google Scholar] [CrossRef]
- Mello, R.P. Chave para a identificação das formas adultas das espécies da família Calliphoridae (Diptera, Brachycera, Cyclorrhapha) encontradas no Brasil. Entomol. Vectores 2003, 10, 255–268. [Google Scholar]
- Kosmann, C.; Mello, R.P.; Harterreiten-Souza, E.S.; Pujol-Luz, J.R. A list of current valid blow fly names (Diptera: Calliphoridae) in the Americas South of Mexico with a key to the Brazilian species. Entomo Bras. 2013, 6, 74–85. [Google Scholar] [CrossRef]
- Nuorteva, P. Synanthropy of blowflies (Diptera Calliphoridae) in Finland. An. Entomol. Fenn. 1963, 29, 1–49. [Google Scholar]
- Oliveira, D.L.; Soares, T.F.; Vasconcelos, S.D. Effect of bait decomposition on the attractiveness to species of Diptera of veterinary and forensic importance in a rainforest fragment in Brazil. Parasitol. Res. 2016, 115, 449–455. [Google Scholar] [CrossRef]
- Carmo, R.F.R.; Oliveira, D.L.; Barbosa, T.M.; Soares, T.F.; Souza, J.R.B.; Vasconcelos, S.D. Visitors versus colonizers: An empirical study on the use of vertebrate carcasses by necrophagous Diptera in a Rainforest fragment. Ann. Entomol. Soc. Am. 2017, 110, 492–500. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Vasconcelos, S.D. Diversity, daily flight activity and temporal occurrence of necrophagous Diptera associated with decomposing carcasses in a semi-arid environment. Neotrop. Entomol. 2018, 47, 470–477. [Google Scholar] [CrossRef]
- Nunes, M.P.; Azevedo, W.T.A.; Silva, A.S.; Lessa, C.S.S.; Alencar, J.A.; Aguiar, V.M. Synanthropy and ecological aspects of Calliphoridae and Mesembrinellidae (Diptera: Oestroidea) in three ecological areas in Rio de Janeiro State, Brazil. PLoS ONE 2023, 18, e0285844. [Google Scholar] [CrossRef]
- Vasconcelos, S.D.; Salgado, R.L.; Barbosa, T.M.; Souza, J.R.B. Diptera of medico-legal importance associated with pig carrion in a tropical dry forest. J. Med. Entomol. 2017, 53, 1131–1139. [Google Scholar] [CrossRef]
- Ruiz, R.A. Aspectos da Biologia Larval de Chrysomya megacephala (F.) (Diptera: Calliphoridae): Curva de Crescimento e Período de Mais Rápido Desenvolvimento Larval. Master′s Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 13 March 2008. [Google Scholar]
- Aguiar-Coelho, V.M.; Azevedo, E.M.V.M. Relações intraespecíficas de Chrysomya albiceps (Wiedemann), Chrysomya megacephala (Fabricius) e Cochliomyia macellaria (Fabricius) (Diptera: Calliphoridae), sob condições experimentais. Rev. Bras. Entomol. 1996, 40, 35–40. [Google Scholar]
- Ururahy-Rodrigues, A.; Rafael, J.A.; Pujol-Luz, J.R. Temporal distribution of blowflies of forensic importance (Diptera: Calliphoridae), in man-size domestic pigs carcasses, in the forest reserve Adolpho Ducke, Manaus, Amazonas, Brazil. Entomo Bras. 2013, 6, 9–22. [Google Scholar] [CrossRef]
- Vasconcelos, S.D.; Barbosa, T.M.; Oliveira, T.P.B. Diversity of forensically-important dipteran species in different environments in northeastern Brazil, with notes on the attractiveness of animal baits. Fla. Entomol. 2015, 98, 770–775. [Google Scholar] [CrossRef]
- Souza, J.R.P.; Carvalho-Filho, F.S.; Juen, L.; Esposito, M.C. The effects of cattle ranching on the communities of necrophagous flies (Diptera: Calliphoridae, Mesembrinellidae and Sarcophagidae) in northeastern Brazil. J. Insect Conserv. 2020, 24, 705–717. [Google Scholar] [CrossRef]
- Amat, E.; Marinho, M.A.T.; Rafael, J.A. A survey of necrophagous blowflies (Diptera: Oestridea) in the Amazonas-Negro interfluvial region (Brazilian Amazon). Rev. Bras. Entomol. 2016, 60, 57–62. [Google Scholar] [CrossRef]
- Faria, L.S.; Paseto, M.L.; Couri, M.S.; Mello-Patiu, C.A.; Mendes, J. Insects associated with pig carrion in two environments of the Brazilian savanna. Neotrop. Entomol. 2018, 47, 181–198. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Mello, R.P.; D’Almeida, J.M. Dípteros muscóides como vetores mecânicos de ovos de helmintos em jardim zoológico, Brasil. RSP 2002, 36, 614–620. [Google Scholar] [CrossRef]
- Luz, R.T.; Azevedo, W.T.A.; Silva, A.S.; Lessa, C.S.S.; Maia, V.C.; Aguiar, V.M. Diversity of Calliphoridae and Mesembrinellidae (Diptera: Oestroidea) in a mangrove, restinga; forest landscapes from a lagoon complex on an Atlantic forest coastline (Rio de Janeiro, Brazil). J. Med. Entomol. 2020, 57, 1758–1767. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 1st ed.; Springer Nature: Berlin, Germany, 2011; 306p. [Google Scholar] [CrossRef]
- Yu, H.; Yang, M.; Lu, Z.; Wang, W.; Yu, F.; Zhang, Y.; Yin, X.; Yu, H.; Hu, J.; Deane, D.C. A phylogenetic approach identifies patterns of beta diversity and floristic subregions of the Qinghai-Tibet Plateau. Plant Divers. 2024, 46, 59–69. [Google Scholar] [CrossRef]
- Buss, D.F.; Batista, D.F.; Nessimian, J.L. Bases conceituais para a aplicação de biomonitoramento em programas de avaliação da qualidade da água de rios. CSP 2003, 19, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.M.; Carmo, R.F.R.; Silva, L.P.; Sales, R.G.; Vasconcelos, S.D. Diversity of sarcosaprophagous Calyptrate (Diptera) on sandy beaches exposed to increasing levels of urbanization in Brazil. Environ. Entomol. 2017, 46, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.S.; Pepinelli, M.; Almeida, E.C.; Ochoa-Quintero, J.M.; Roque, F.O. Blow flies from forest fragments embedded in different land uses: Implications for selecting indicators in forensic entomology. J. Forensic Sci. 2016, 61, 93–98. [Google Scholar] [CrossRef]
- Gadelha, B.Q.; Ferraz, A.C.P.; Coelho, V.M.A. A importância dos mesembrinelíneos (Diptera: Calliphoridae) e seu potencial como indicadores de preservação ambiental. Oecol Bras. 2009, 13, 661–665. [Google Scholar]
- Silva, A.B.; Gadelha, B.Q.; Ribeiro, A.C.; Ferraz, A.C.P.; Aguiar, V.M. Entomofauna capturada em armadilha para dípteros na Reserva Biológica do Tinguá, Nova Iguaçu, Rio de Janeiro. Bioikos 2014, 28, 11–23. [Google Scholar]
- Graham, S.M.; Sawyer, S.J.; Crozier, O.; Denton, K.; Tomberlin, J.K. Nutrition and water deprivation negatively impacts adult longevity of Lucilia eximia (Diptera: Calliphoridae). JCHS 2021, 6, 44. [Google Scholar] [CrossRef]
- Ridall, A.; Ingels, J. Suitability of free-living marine nematodes as bioindicators: Status and future considerations. Front. Mar. Sci. 2021, 8, 16. [Google Scholar] [CrossRef]
- Oberprieler, S.K.; Andersen, A.N. The importance of sampling intensity when assessing ecosystem restoration: Ants as bioindicators in northern Australia. Restor. Ecol. 2020, 28, 5. [Google Scholar] [CrossRef]
- Moreira, E.A.; Pinto, G.S.; Neves, L.C.R.S.; Martins, C.A. Fauna de dípteros necrófagos e suas respostas à complexidade vegetal. Rev. Iniciaç Cient. Univ. Val. Rio Verde 2014, 12, 444–454. [Google Scholar]
- Pereira, A.J.; Centeno, N.D.; Nuñez-Vázquez, C. Effects of population variations and temperature on Chrysomya megacephala (Diptera: Calliphoridae) development: Implications for estimating the postmortem interval. Int. J. Leg. Med. 2023, 138, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.M.; Barbosa, T.M.; Thyssen, P.J.; Vasconcelos, S.D. Diversity of Diptera species associated with pig carcasses in a Brazilian city exposed to high rates of homicide. Pap. Avulsos Zool. 2021, 61, e20216101. [Google Scholar] [CrossRef]
- Viana-Junior, A.B.; Souza, C.C.; Medeiros, H.F.; Carvalho-Filho, F.S. Diversity partitioning and distance-decay relationship of saprophytic flies (Insecta: Diptera) in the western Brazilian Amazon. Acta Oecol 2021, 112, 103768. [Google Scholar] [CrossRef]
- Façanha, B.L.B.; Esposito, M.C.; Juen, L. Trap and bait efficiency for catching Calliphoridae and Mesembrinellidae (Insecta, Diptera) at different heights. An. Acad. Bras. Ciênc. 2022, 94, e20210763. [Google Scholar] [CrossRef] [PubMed]
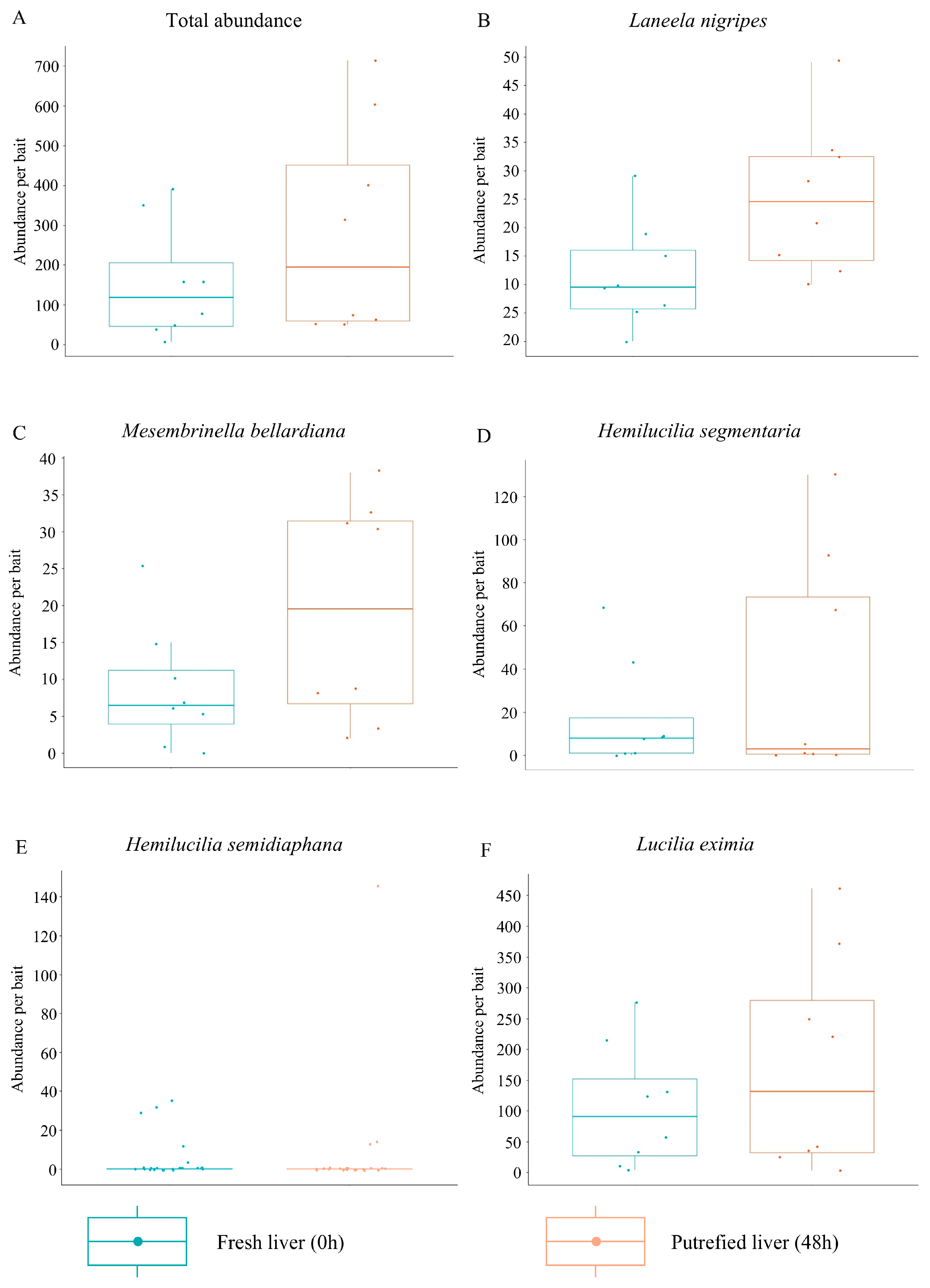
| Taxa | 0 h | 48 | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Calliphoridae | ||||||
| Luciliinae | ||||||
| Lucilia eximia (Wiedemann, 1819) | 1015 | 58.4 | 1488 | 60.4 | 2524 | 60.1 |
| Lucilia cuprina (Wiedemann, 1830) | 12 | 0.7 | 8 | 0.3 | 20 | 0.5 |
| Chrysomyinae | ||||||
| Chrysomya megacephala (Fabricius, 1794) | 33 | 1.9 | 26 | 1.1 | 59 | 1.4 |
| Chrysomya albiceps (Wiedemann, 1818) | 22 | 1.3 | 30 | 1.2 | 52 | 1.2 |
| Chrysomya putoria (Wiedemann, 1818) | - | - | 1 | <0.1 | 1 | <0.1 |
| Cochliomyia macellaria (Fabricius, 1775) | 16 | 0.9 | 29 | 1.2 | 45 | 1.1 |
| Hemilucilia segmentaria (Fabricius, 1805) | 353 | 20.3 | 313 | 12.7 | 666 | 15.9 |
| Hemilucilia semidiaphana (Rondani, 1850) | 111 | 6.4 | 172 | 7.0 | 283 | 6.7 |
| Hemilucilia benoisti (Séguy, 1925) | 1 | <0.1 | 3 | 0.1 | 4 | <0.1 |
| Paralucilia nigrofacialis (Mello, 1969) | 2 | 0.1 | - | - | 2 | <0.1 |
| Mesembrinellidae | ||||||
| Mesembrinellinae | ||||||
| Mesembrinella bellardiana (Aldrich, 1922) | 69 | 4.0 | 154 | 6.3 | 223 | 5.3 |
| Mesembrinella peregrina (Aldrich, 1922) | 1 | <0.1 | 4 | 0.2 | 5 | <0.1 |
| Mesembrinella semihyalina (Mello, 1967) | 2 | 0.1 | 8 | 0.3 | 10 | 0.2 |
| Mesembrinella currani (Guimarães, 1977) | 4 | 0.2 | 1 | <0.1 | 5 | 0.1 |
| Mesembrinella quadrilineata (Fabricius, 1805) | 1 | <0.1 | 11 | 0.5 | 12 | 0.3 |
| Mesembrinella cyaneicyncta (Surcouf, 1919) | - | - | 2 | <0.1 | 2 | <0.1 |
| Mesembrinella randa (Walker, 1849) | 1 | <0.1 | 3 | 0.1 | 4 | 0.1 |
| Mesembrinella benoisti (Séguy, 1925) | - | - | 1 | <0.1 | 1 | <0.1 |
| Mesembrinella aeneiventris (Wiedemann, 1830) | 1 | <0.1 | 5 | 0.2 | 6 | 0.1 |
| Mesembrinella purpurata (Aldrich, 1922) | 2 | 0.1 | 3 | 0.1 | 5 | 0.1 |
| Laneellinae | ||||||
| Laneella nigripes (Guimarães, 1977) | 93 | 5.3 | 201 | 8.2 | 294 | 7.0 |
| Total | 1739 | 100 | 2463 | 100 | 4202 | 100 |
| Environment | Forest | Rural | Urban |
|---|---|---|---|
| Forest | 16 | 2 | 3 |
| Rural | 0.174 | 7 | 4 |
| Urban | 0.286 | 0.667 | 5 |
| Taxa | Forest | Rural | Urban | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Calliphoridae | ||||||||
| Luciliinae | ||||||||
| Lucilia eximia | 2257 | 64.5 | 105 | 40.2 | 162 | 34.8 | 2524 | 58.9 |
| Lucilia cuprina | - | - | 20 | 7.7 | - | - | 20 | 0.5 |
| Chrysomyinae | ||||||||
| Chrysomya megacephala | - | - | 34 | 13.0 | 27 | 0.6 | 61 | 1.4 |
| Chrysomya albiceps | - | - | 49 | 18.8 | 3 | 0.1 | 52 | 1.2 |
| Chrysomya putoria | - | - | 1 | <0.1 | - | - | 1 | <0.1 |
| Cochliomyia macellaria | - | - | 45 | 17.2 | - | - | 45 | 1.1 |
| Hemilucilia segmentaria | 435 | 12.4 | 7 | 0.3 | 227 | 48.7 | 669 | 15.6 |
| Hemilucilia semidiaphana | 236 | 6.7 | - | - | 47 | 10.1 | 283 | 6.6 |
| Hemilucilia benoisti | 4 | 0.1 | - | - | - | - | 4 | <0.1 |
| Paralucilia nigrofacialis | 2 | 0.1 | - | - | - | - | 2 | <0.1 |
| Mesembrinellidae | ||||||||
| Mesembrinellinae | ||||||||
| Mesembrinella bellardiana | 223 | 6.4 | - | - | - | - | 223 | 5.2 |
| Mesembrinella peregrina | 5 | 0.1 | - | - | - | - | 5 | <0.1 |
| Mesembrinella semihyalina | 10 | 0.3 | - | - | - | - | 10 | 0.2 |
| Mesembrinella currani | 5 | 0.1 | - | - | - | - | 5 | <0.1 |
| Mesembrinella quadrilineata | 12 | 0.3 | - | - | - | - | 12 | 0.3 |
| Mesembrinella cyaneicyncta | 2 | 0.1 | - | - | - | - | 2 | <0.1 |
| Mesembrinella randa | 4 | 0.1 | - | - | - | - | 4 | <0.1 |
| Mesembrinella benoisti | 1 | <0.1 | - | - | - | - | 1 | <0.1 |
| Mesembrinella aeneiventris | 6 | 0.2 | - | - | - | - | 6 | <0.1 |
| Mesembrinella purpurata | 5 | 0.1 | - | - | - | - | 5 | <0.1 |
| Laneellinae | ||||||||
| Laneella nigripes | 294 | 8.4 | - | - | - | - | 294 | 6.9 |
| Total | 3501 | 100 | 261 | 100 | 466 | 100 | 4228 | 100 |
| Environment | Species | Stat | p-Value |
|---|---|---|---|
| Forest | Laneella nigripes | 0.968 | 0.001 |
| Mesembrinella bellardiana | 0.968 | 0.001 | |
| Lucilia eximia | 0.946 | 0.001 | |
| Mesembrinella quadrilineata | 0.559 | 0.011 | |
| Hemilucilia segmentaria | 0.727 | 0.018 | |
| Mesembrinella randa | 0.500 | 0.020 | |
| Mesembrinella semihyalina | 0.500 | 0.027 | |
| Mesembrinella currani | 0.500 | 0.030 | |
| Rural | Cochliomyia macellaria | 0.707 | 0.001 |
| Lucilia cuprina | 0.661 | 0.002 | |
| Chrysomya albiceps | 0.543 | 0.019 | |
| Chrysomya megacephala | 0.590 | 0.036 | |
| Urban | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, W.T.d.A.; Nunes, M.d.P.; Machado, T.d.S.T.; Albuquerque, V.M.L.; Lessa, C.S.S.; Alencar, J.; Aguiar, V.M. Calliphoridae and Mesembrinellidae (Insect: Diptera) Across Different Environments of Rio de Janeiro, Brazil: Synanthropy and Potential Bioindicators, with Notes on Bait Preference. Life 2025, 15, 1818. https://doi.org/10.3390/life15121818
Azevedo WTdA, Nunes MdP, Machado TdST, Albuquerque VML, Lessa CSS, Alencar J, Aguiar VM. Calliphoridae and Mesembrinellidae (Insect: Diptera) Across Different Environments of Rio de Janeiro, Brazil: Synanthropy and Potential Bioindicators, with Notes on Bait Preference. Life. 2025; 15(12):1818. https://doi.org/10.3390/life15121818
Chicago/Turabian StyleAzevedo, Wellington Thadeu de Alcantara, Mariana dos Passos Nunes, Tomaz da Silva Telles Machado, Valmíria Moura Leôncio Albuquerque, Cláudia Soares Santos Lessa, Jeronimo Alencar, and Valéria Magalhães Aguiar. 2025. "Calliphoridae and Mesembrinellidae (Insect: Diptera) Across Different Environments of Rio de Janeiro, Brazil: Synanthropy and Potential Bioindicators, with Notes on Bait Preference" Life 15, no. 12: 1818. https://doi.org/10.3390/life15121818
APA StyleAzevedo, W. T. d. A., Nunes, M. d. P., Machado, T. d. S. T., Albuquerque, V. M. L., Lessa, C. S. S., Alencar, J., & Aguiar, V. M. (2025). Calliphoridae and Mesembrinellidae (Insect: Diptera) Across Different Environments of Rio de Janeiro, Brazil: Synanthropy and Potential Bioindicators, with Notes on Bait Preference. Life, 15(12), 1818. https://doi.org/10.3390/life15121818







