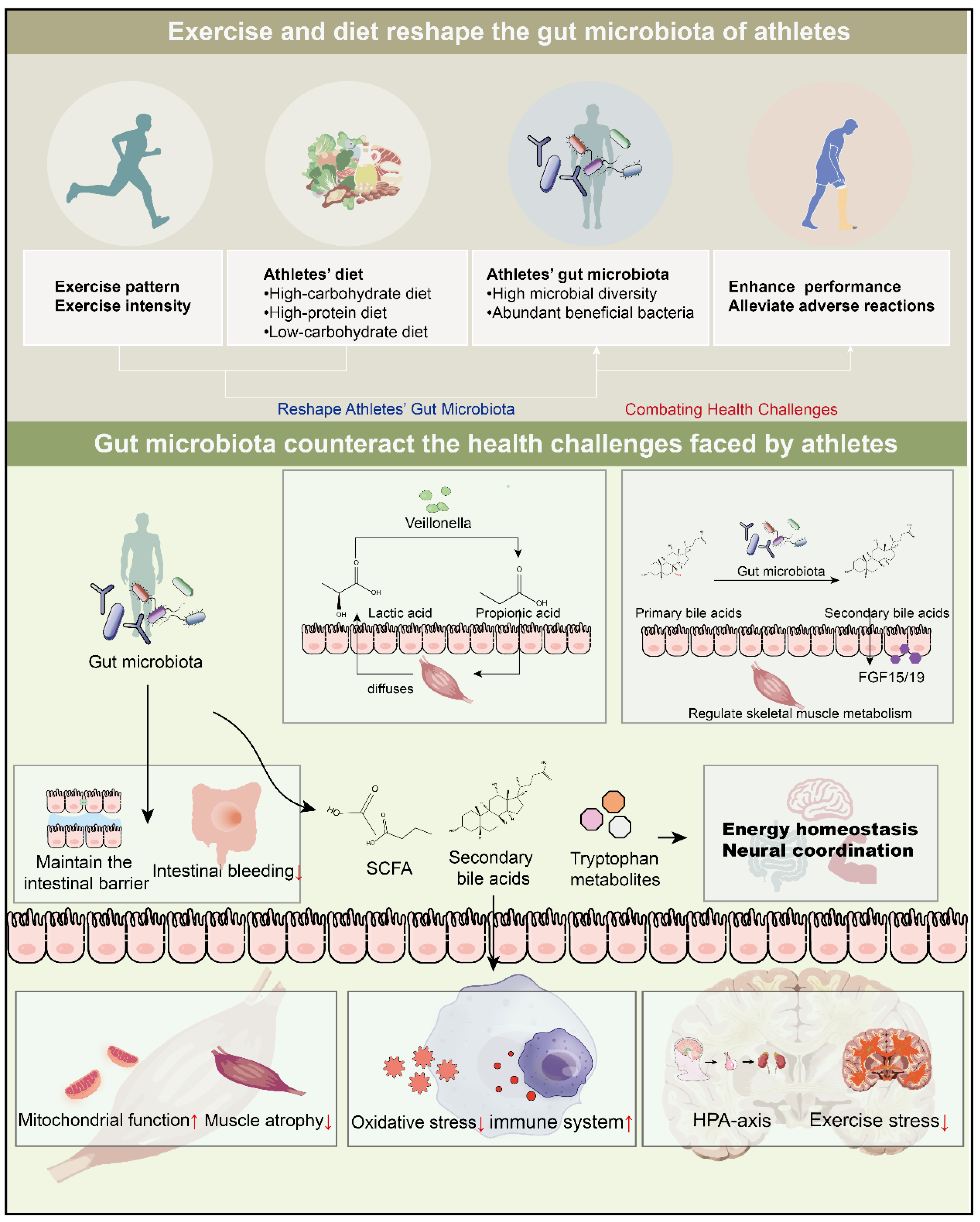
Exercise and Diet Reshape Athletes’ Gut Microbiota: Countering Health Challenges in Athletes
Abstract
1. Introduction
2. Exercise-Driven Shifts in Gut Microbiota Composition
3. Exercise Characteristics Shape the Gut Microbiota of Athletes
3.1. Exercise Patterns
3.2. Exercise Intensity
4. Athletes’ Diets Deeply Participate in the Construction of the Gut Microbiota Ecosystem
4.1. High-Carbohydrate Diet
4.2. High-Protein Diet
4.3. Low-Carbohydrate Diet
5. Improvement of Athletic Performance
5.1. Enhance Exercise Endurance
5.2. Improvement of Glycogen Storage
6. Energy Homeostasis and Nerve Coordination
6.1. Impact of Gut Microbiota on Host Energy, Appetite, and Metabolism
6.2. Gut Microbiota, Gut–Brain Axis, and Athletes’ Performance
7. Gut Microbiota Alleviates Adverse Effects of Exercise
7.1. Amelioration of Intestinal Hemorrhage
7.2. Regulating Athletes’ Post-Training Stress
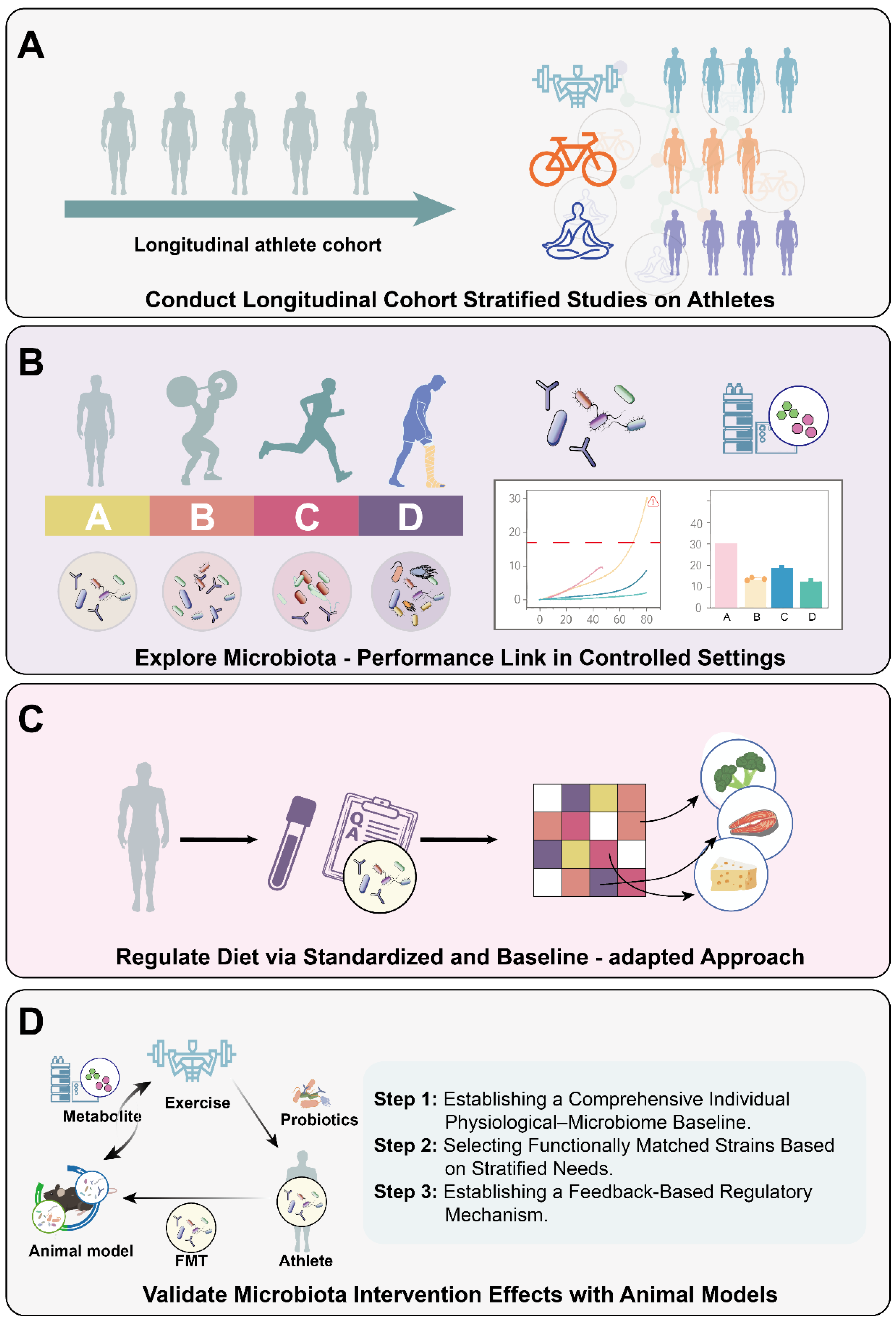
8. Limitations and Future Study
8.1. Current Challenges and Foundational Research Directions
8.2. A Closed-Loop Framework for Personalized Probiotic/Prebiotic Intervention
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, J.; Wang, Y.; Zhang, L.; Ren, J.; Zhang, Z.; Chen, B.; Zhang, K.; Zhu, B.; Liu, W.; et al. Lifestyle patterns influence the composition of the gut microbiome in a healthy Chinese population. Sci. Rep. 2023, 13, 14425. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Toledo, M.; Martínez-Martínez, S.; Van Hul, M.; Laudo, B.; Eyre, E.; Pelicaen, R.; Puel, A.; Altirriba, J.; Gómez-Valadés, A.G.; Inderhees, J.; et al. Rapid modulation of gut microbiota composition by hypothalamic circuits in mice. Nat. Metab. 2025, 7, 1123–1135. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, M.; Zha, Y.; Yang, K.; Tong, Y.; Wang, S.; Lu, Q.; Ning, K. Gut microbiota and inflammation patterns for specialized athletes: A multi-cohort study across different types of sports. mSystems 2023, 8, e00259-00223. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The human gut microbiome of athletes: Metagenomic and metabolic insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Kulecka, M.; Fraczek, B.; Mikula, M.; Zeber-Lubecka, N.; Karczmarski, J.; Paziewska, A.; Ambrozkiewicz, F.; Jagusztyn-Krynicka, K.; Cieszczyk, P.; Ostrowski, J. The composition and richness of the gut microbiota differentiate the top Polish endurance athletes from sedentary controls. Gut Microbes 2020, 11, 1374–1384. [Google Scholar] [CrossRef]
- Cullen, J.M.A.; Shahzad, S.; Dhillon, J. A systematic review on the effects of exercise on gut microbial diversity, taxonomic composition, and microbial metabolites: Identifying research gaps and future directions. Front. Physiol. 2023, 14, 1292673. [Google Scholar] [CrossRef]
- Tabone, M.; Bressa, C.; García-Merino, J.A.; Moreno-Pérez, D.; Van, E.C.; Castelli, F.A.; Fenaille, F.; Larrosa, M. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci. Rep. 2021, 11, 3558. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- O’Donovan, C.M.; Madigan, S.M.; Garcia-Perez, I.; Rankin, A.; Sullivan, O.O.; Cotter, P.D. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J. Sci. Med. Sport 2020, 23, 63–68. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Bycura, D.; Santos, A.C.; Shiffer, A.; Kyman, S.; Winfree, K.; Sutliffe, J.; Pearson, T.; Sonderegger, D.; Cope, E.; Caporaso, J.G. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports 2021, 9, 14. [Google Scholar] [CrossRef]
- Akazawa, N.; Nakamura, M.; Eda, N.; Murakami, H.; Nakagata, T.; Nanri, H.; Park, J.; Hosomi, K.; Mizuguchi, K.; Kunisawa, J.; et al. Gut microbiota alternation with training periodization and physical fitness in Japanese elite athletes. Front. Sports Act. Living 2023, 5, 1219345. [Google Scholar] [CrossRef] [PubMed]
- Shalmon, G.; Ibrahim, R.; Israel-Elgali, I.; Grad, M.; Shlayem, R.; Shapira, G.; Shomron, N.; Youngster, I.; Scheinowitz, M. Differential Gut Microbiome Profiles in Long-Distance Endurance Cyclists and Runners. Life 2024, 14, 1703. [Google Scholar] [CrossRef]
- Usta-Gorgun, B.; Yilmaz-Ersan, L. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Electron. J. Biotechnol. 2020, 47, 29–35. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Sex differences in energy metabolism: Natural selection, mechanisms and consequences. Nat. Rev. Nephrol. 2024, 20, 56–69. [Google Scholar] [CrossRef]
- Toro-Román, V.; Muñoz, A.; Zoido, A.; Sánchez-Alcaraz, B.J.; Grijota, F.; Muñoz, D. Type of Diet and Sports Supplements in Padel Players According to Level of Competition and Sex. Nutrients 2023, 15, 3633. [Google Scholar] [CrossRef]
- Mata, F.; Domínguez, R.; López-Samanes, Á.; Sánchez-Gómez, Á.; Jodra, P.; Sánchez-Oliver, A.J. Analysis of the consumption of sports supplements in elite fencers according to sex and competitive level. BMC Sports Sci. Med. Rehabil. 2021, 13, 50. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Snowberg, L.K.; Hirsch, P.E.; Lauber, C.L.; Org, E.; Parks, B.; Lusis, A.J.; Knight, R.; Caporaso, J.G.; Svanbäck, R. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat. Commun. 2014, 5, 4500. [Google Scholar] [CrossRef]
- Hokanson, K.C.; Hernández, C.; Deitzler, G.E.; Gaston, J.E.; David, M.M. Sex shapes gut–microbiota–brain communication and disease. Trends Microbiol. 2024, 32, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, H.; Li, Y.; Shi, Z.; Ren, H.; Zhang, Z.; Zhou, X.; Tang, S.; Han, X.; Lin, Y.; et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat. Aging 2021, 1, 87–100. [Google Scholar] [CrossRef]
- Yun, S.; Seo, Y.; Lee, Y.; Lee, D.T. Gut microbiome related to metabolic diseases after moderate-to-vigorous intensity exercise. J. Exerc. Sci. Fit. 2024, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, S.; Yang, T.; Guo, J.; Zhu, X.; Dong, Y. Impact of exercise-induced alterations on gut microbiota diversity and composition: Comparing effects of different training modalities. Cell Regen. 2025, 14, 28. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, S.; Peng, X.; Yang, W.; Xu, Y.; Wu, P.; Chen, J.; Cai, Y.; Zhou, J. Characteristics of the gut microbiota in professional martial arts athletes: A comparison between different competition levels. PLoS ONE 2019, 14, e0226240. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Takami, M.; Aoi, W.; Matsumoto, K.; Kato, Y.; Kobayashi, Y.; Kuwahata, M. High-intensity exercise impairs intestinal barrier function by generating oxidative stress. J. Clin. Biochem. Nutr. 2024, 74, 136–140. [Google Scholar] [CrossRef]
- Bonomini-Gnutzmann, R.; Plaza-Díaz, J.; Jorquera-Aguilera, C.; Rodríguez-Rodríguez, A.; Rodríguez-Rodríguez, F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9518. [Google Scholar] [CrossRef]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Sato, M.; Suzuki, Y. Alterations in intestinal microbiota in ultramarathon runners. Sci. Rep. 2022, 12, 6984. [Google Scholar] [CrossRef]
- Lewis, G.; Reczek, S.; Omozusi, O.; Hogue, T.; Cook, M.D.; Hampton-Marcell, J. Machine Learning Reveals Microbial Taxa Associated with a Swim across the Pacific Ocean. Biomedicines 2024, 12, 2309. [Google Scholar] [CrossRef]
- Hawley, J.A.; Forster, S.C.; Giles, E.M. Exercise, the Gut Microbiome and Gastrointestinal Diseases: Therapeutic Impact and Molecular Mechanisms. Gastroenterology 2025, 169, 48–62. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Wardenaar, F.; Brinkmans, N.; Ceelen, I.; Van Rooij, B.; Mensink, M.; Witkamp, R.; De Vries, J. Macronutrient Intakes in 553 Dutch Elite and Sub-Elite Endurance, Team, and Strength Athletes: Does Intake Differ between Sport Disciplines? Nutrients 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, K.; Xu, M.; Zhang, Y.; Weng, X.; Luo, J.; Li, Y.; Mao, Y.H. Dietary Patterns, Gut Microbiota and Sports Performance in Athletes: A Narrative Review. Nutrients 2024, 16, 1634. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Turner, N.D.; Lupton, J.R. Dietary fiber. Adv. Nutr. 2011, 2, 151–152. [Google Scholar] [CrossRef]
- Kay, R.M. Dietary fiber. J. Lipid Res. 1982, 23, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Furber, M.J.W.; Young, G.R.; Holt, G.S.; Pyle, S.; Davison, G.; Roberts, M.G.; Roberts, J.D.; Howatson, G.; Smith, D.L. Gut Microbial Stability is Associated with Greater Endurance Performance in Athletes Undertaking Dietary Periodization. mSystems 2022, 7, e0012922. [Google Scholar] [CrossRef]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef] [PubMed]
- Prins, P.J.; Noakes, T.D.; Buga, A.; D’Agostino, D.P.; Volek, J.S.; Buxton, J.D.; Heckman, K.; Jones, D.W.; Tobias, N.E.; Grose, H.M.; et al. Low and high carbohydrate isocaloric diets on performance, fat oxidation, glucose and cardiometabolic health in middle age males. Front. Nutr. 2023, 10, 1084021. [Google Scholar] [CrossRef]
- Gao, Y.; Hua, R.; Hu, K.; Wang, Z. Carbohydrates deteriorate fatty liver by activating the inflammatory response. Nutr. Res. Rev. 2022, 35, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Hochrein, S.M.; Wu, H.; Eckstein, M.; Arrigoni, L.; Herman, J.S.; Schumacher, F.; Gerecke, C.; Rosenfeldt, M.; Grün, D.; Kleuser, B.; et al. The glucose transporter GLUT3 controls T helper 17 cell responses through glycolytic-epigenetic reprogramming. Cell Metab. 2022, 34, 516–532.e511. [Google Scholar] [CrossRef]
- Hecht, A.L.; Harling, L.C.; Friedman, E.S.; Tanes, C.; Lee, J.; Firrman, J.; Hao, F.; Tu, V.; Liu, L.; Patterson, A.D.; et al. Dietary carbohydrates regulate intestinal colonization and dissemination of Klebsiella pneumoniae. J. Clin. Investig. 2024, 134, e174726. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Sung-Ho Kim, J.; Dijk, S.I.; Mahmood, R.; Ogungbola, O.; et al. Unfermented β-fructan Fibers Fuel Inflammation in Select Inflammatory Bowel Disease Patients. Gastroenterology 2023, 164, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shang, M.; Yu, S.; Liu, Y.; Zhang, H.; Zhou, Y.; Wang, M.; Wang, T.; Li, H.; Liu, Z.; et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell. Mol. Immunol. 2022, 19, 1414–1424. [Google Scholar] [CrossRef]
- Watford, M.; Wu, G. Protein. Adv. Nutr. 2018, 9, 651–653. [Google Scholar] [CrossRef]
- Tipton, K.D.; Wolfe, R.R. Exercise, protein metabolism, and muscle growth. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 109–132. [Google Scholar] [CrossRef]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Ramirez-Campillo, R.; Arazi, H.; de Villarreal, E.S. The effects of maturation on jumping ability and sprint adaptations to plyometric training in youth soccer players. J. Sports Sci. 2018, 36, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, X.; Yang, A.; Huang, Y.; Tan, Y.; Qian, Y.; Lv, F.; Si, H. Effects of Diets with Different Protein Levels on Lipid Metabolism and Gut Microbes in the Host of Different Genders. Front. Nutr. 2022, 9, 940217. [Google Scholar] [CrossRef]
- Sultan, Z.H.; Speelman, D. A Systematic Review of the Effects of Low-Carbohydrate Diet on Athletic Physical Performance Parameters. Cureus 2025, 17, e79166. [Google Scholar] [CrossRef]
- McSwiney, F.T.; Doyle, L.; Plews, D.J.; Zinn, C. Impact of Ketogenic Diet on Athletes: Current Insights. Open Access J. Sports Med. 2019, 10, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.T.; Bogatyrev, S.R.; Ismagilov, R.F. A quantitative sequencing framework for absolute abundance measurements of mucosal and lumenal microbial communities. Nat. Commun. 2020, 11, 2590. [Google Scholar] [CrossRef]
- Hengist, A.; Davies, R.G.; Walhin, J.P.; Buniam, J.; Merrell, L.H.; Rogers, L.; Bradshaw, L.; Moreno-Cabañas, A.; Rogers, P.J.; Brunstrom, J.M.; et al. Ketogenic diet but not free-sugar restriction alters glucose tolerance, lipid metabolism, peripheral tissue phenotype, and gut microbiome: RCT. Cell Rep. Med. 2024, 5, 101667. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e1216. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Rapoport, B.I. Metabolic Factors Limiting Performance in Marathon Runners. PLoS Comput. Biol. 2010, 6, e1000960. [Google Scholar] [CrossRef]
- Rachoin, J.S.; Weisberg, L.S.; McFadden, C.B. Treatment of lactic acidosis: Appropriate confusion. J. Hosp. Med. 2010, 5, E1–E7. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Lee, M.-C.; Hsu, Y.-J.; Ho, H.-H.; Hsieh, S.-H.; Kuo, Y.-W.; Sung, H.-C.; Huang, C.-C. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef]
- Liu, S.; Rodriguez, J.S.; Munteanu, V.; Ronkowski, C.; Sharma, N.K.; Alser, M.; Andreace, F.; Blekhman, R.; Błaszczyk, D.; Chikhi, R.; et al. Analysis of metagenomic data. Nat. Rev. Methods Primers 2025, 5, 5. [Google Scholar] [CrossRef]
- Mohr, A.E.; Pyne, D.B.; Leite, G.S.F.; Akins, D.; Pugh, J. A systematic scoping review of study methodology for randomized controlled trials investigating probiotics in athletic and physically active populations. J. Sport Health Sci. 2024, 13, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Bonneau, M.; Orfila, L.; Horeau, M.; Hazon, M.; Demay, R.; Lecommandeur, E.; Boumpoutou, R.; Guillotel, A.; Guillemot, P.; et al. Atypical gut microbial ecosystem from athletes with very high exercise capacity improves insulin sensitivity and muscle glycogen store in mice. Cell Rep. 2025, 44, 115448. [Google Scholar] [CrossRef]
- Arnoldini, M.; Sharma, R.; Moresi, C.; Chure, G.; Chabbey, J.; Slack, E.; Cremer, J. Quantifying the varying harvest of fermentation products from the human gut microbiota. Cell 2025, 188, 5332–5342.e5316. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Brüning, J.C.; Fenselau, H. Integrative neurocircuits that control metabolism and food intake. Science 2023, 381, eabl7398. [Google Scholar] [CrossRef]
- Hong, D.; Zhang, C.; Wu, W.; Lu, X.; Zhang, L. Modulation of the gut-brain axis via the gut microbiota: A new era in treatment of amyotrophic lateral sclerosis. Front. Neurol. 2023, 14, 1133546. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Cintado, E.; Muela, P.; Martín-Rodríguez, L.; Alcaide, I.; Tezanos, P.; Vlckova, K.; Valderrama, B.; Bastiaanssen, T.F.S.; Rodríguez-Muñoz, M.; de Ceballos, M.L.; et al. Gut microbiota regulates exercise-induced hormetic modulation of cognitive function. eBioMedicine 2025, 119, 105876. [Google Scholar] [CrossRef]
- Romaní-Pérez, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Liébana-García, R.; Olivares, M.; Sanz, Y. The Microbiota and the Gut-Brain Axis in Controlling Food Intake and Energy Homeostasis. Int. J. Mol. Sci. 2021, 22, 5830. [Google Scholar] [CrossRef]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C.; et al. A microbiome-dependent gut–brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Fu, P.; Wang, C.; Zheng, S.; Qiao, L.; Gao, W.; Gong, L. Connection of pre-competition anxiety with gut microbiota and metabolites in wrestlers with varying sports performances based on brain-gut axis theory. BMC Microbiol. 2024, 24, 147. [Google Scholar] [CrossRef]
- Tofani, G.S.S.; Leigh, S.J.; Gheorghe, C.E.; Bastiaanssen, T.F.S.; Wilmes, L.; Sen, P.; Clarke, G.; Cryan, J.F. Gut microbiota regulates stress responsivity via the circadian system. Cell Metab. 2025, 37, 138–153.e135. [Google Scholar] [CrossRef] [PubMed]
- Ter Steege, R.W.; Kolkman, J.J. Review article: The pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment. Pharmacol. Ther. 2012, 35, 516–528. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.K.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Cohrs, J.; Salmonson, C.; Fryer, J.D.; Nehra, V.; Hale, V.L.; Kashyap, P.; White, B.A.; Woods, J.A. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes 2018, 9, 115–130. [Google Scholar] [CrossRef]
- Park, J.-H.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y. Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of short-chain fatty acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Vijay, A.; Kouraki, A.; Gohir, S.; Turnbull, J.; Kelly, A.; Chapman, V.; Barrett, D.A.; Bulsiewicz, W.J.; Valdes, A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes 2021, 13, 1997559. [Google Scholar] [CrossRef]
- Aykut, M.N.; Erdoğan, E.N.; Çelik, M.N.; Gürbüz, M. An Updated View of the Effect of Probiotic Supplement on Sports Performance: A Detailed Review. Curr. Nutr. Rep. 2024, 13, 251–263. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- Murphy, C.; Newsholme, P. Importance of glutamine metabolism in murine macrophages and human monocytes to L-arginine biosynthesis and rates of nitrite or urea production. Clin. Sci. 1998, 95, 397–407. [Google Scholar] [CrossRef]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical Exercise and the Gut Microbiome: A Bidirectional Relationship Influencing Health and Performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Wang, Y.; Chen, J.; Liu, Y. The Athlete Gut Microbiome: A Narrative Review of Multi-Omics Insights and Next-Generation Probiotic Strategies. Nutrients 2025, 17, 3260. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, H.A.; Savidge, T.C. Gut microbiome metagenomics in clinical practice: Bridging the gap between research and precision medicine. Gut Microbes 2025, 17, 2569739. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.; Reitmeier, S.; Haller, D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhu, S.; Xue, M.-Y.; Chen, H.; Xu, J.; Song, M.; Tang, Y.; Liu, X.; Tao, Y.; Zhang, T.; et al. Single-cell transcriptomics across 2534 microbial species reveals functional heterogeneity in the rumen microbiome. Nat. Microbiol. 2024, 9, 1884–1898. [Google Scholar] [CrossRef]
- Yu, G.; Xu, C.; Zhang, D.; Ju, F.; Ni, Y. MetOrigin: Discriminating the origins of microbial metabolites for integrative analysis of the gut microbiome and metabolome. iMeta 2022, 1, e10. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Chen, Y.; Cao, Z.; Liu, C.; Bao, R.; Wang, Y.; Huang, S.; Pan, S.; Qin, L.; et al. Akkermansia muciniphila supplementation in patients with overweight/obese type 2 diabetes: Efficacy depends on its baseline levels in the gut. Cell Metab. 2025, 37, 592–605.e596. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Lin, S.; Jiang, S.; Zhou, X.; Li, J.; Lu, W.; Zhang, J. Probiotic-induced enrichment of Adlercreutzia equolifaciens increases gut microbiome wellness index and maps to lower host blood glucose levels. Gut Microbes 2025, 17, 2520407. [Google Scholar] [CrossRef]
- Niamah, A.K.; Gddoa Al-Sahlany, S.T.; Abdul-Sada, H.K.; Prabhakar, P.; Tripathy, S.; Dadrwal, B.K.; Singh, S.; Verma, D.K.; Gupta, A.K.; Shukla, R.M.; et al. Phytophagous probiotic foods: Exploring the intersection of characteristics, quality implications, health benefits, and market dynamics. Trends Food Sci. Technol. 2024, 154, 104795. [Google Scholar] [CrossRef]
- Carletti, M.; Pandit, J.; Gadaleta, M.; Chiang, D.; Delgado, F.; Quartuccio, K.; Fernandez, B.; Raygoza Garay, J.A.; Torkamani, A.; Miotto, R.; et al. Multimodal AI correlates of glucose spikes in people with normal glucose regulation, pre-diabetes and type 2 diabetes. Nat. Med. 2025, 31, 3121–3127. [Google Scholar] [CrossRef]
- Silvestri, N.J.; Wolfe, G.I. Asymptomatic/pauci-symptomatic creatine kinase elevations (hyperckemia). Muscle Nerve 2013, 47, 805–815. [Google Scholar] [CrossRef]
- Xie, H.; Mao, X.; Wang, Z. Effect of high-intensity interval training and moderate-intensity continuous training on blood lactate clearance after high-intensity test in adult men. Front. Physiol. 2024, 15, 1451464. [Google Scholar] [CrossRef]
| Type | Influencing Factors | Phenomena | Conclusions |
|---|---|---|---|
| Exercise patterns. | Different Exercise Type Groupings | - Moderate dynamic: Streptococcus ↑ Anaerostipes hadrus ↑ - High dynamic–low static: Bifidobacterium animalis ↑ Enterococcus faecalis ↑ Lactobacillus acidophilus ↑ - High dynamic–high static: Bacteroides caccae ↑ | The exercise mode determines the unique composition of the gut microbiota, and different exercise types shape the unique gut microbiota of athletes. |
| Different Exercise Energy Metabolism and Metabolic Pathway Activation | Microbiota replacement | Exercise-induced microbiota diversity differences relate to energy metabolism and stress patterns. Different exercises activate distinct pathways, affecting gut microbiota. | |
| Differences in Specific Exercise Behaviors | Endurance runners: α-diversity ↑ | Specific exercise behaviors may affect the characteristics of gut microbiota. | |
| Gender Dependency | Male cyclists: Coriobacteriaceae ↑, ifidobacterium ↑, Pseudomonas ↑ Female cyclists: Clostridiaceae ↑, Lachnospiraceae ↑, Ruminococcaceae ↑, Mitsuokella ↑, Male runners: Catenibacterium ↑ Female runners: Methanosphaera ↑ | Exercise-induced gut microbiota shaping shows gender differences related to metabolic homeostasis, sex hormone signals, and diet–microbiota interactions. | |
| Exercise Intensity | Different Intensities of Exercise Training | Moderate-intensity exercise: Prevotella ↑ High-intensity exercise: Bacteroides ↑, Butyricimonas ↑, Odoribacter ↑, Alistipes ↑ | Different exercise intensities lead to different gut microbial characteristics among participants. |
| Different Training Frequencies for the Same Type of Exercise | Cyclists with high training frequencies: Prevotella ↑ Martial artists in the high-level group: Bacteroides ↑ | High-intensity exercise has a more positive impact on enhancing the diversity of gut microbiota. | |
| Vigorous and Prolonged Exercise | Bifidobacterium ↓, Ruminococcus ↓, F. prausnitzii ↓, Prevotella ↑ Haemophilus ↑, Mucispirillum ↑, Ruminococcus gnavus ↑ | Excessive exercise has a negative impact on gut microbiota. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Y.; Zhang, F.; Zhou, G. Exercise and Diet Reshape Athletes’ Gut Microbiota: Countering Health Challenges in Athletes. Life 2025, 15, 1812. https://doi.org/10.3390/life15121812
Zhang X, Li Y, Zhang F, Zhou G. Exercise and Diet Reshape Athletes’ Gut Microbiota: Countering Health Challenges in Athletes. Life. 2025; 15(12):1812. https://doi.org/10.3390/life15121812
Chicago/Turabian StyleZhang, Xiao’e, Yao Li, Fen Zhang, and Guicheng Zhou. 2025. "Exercise and Diet Reshape Athletes’ Gut Microbiota: Countering Health Challenges in Athletes" Life 15, no. 12: 1812. https://doi.org/10.3390/life15121812
APA StyleZhang, X., Li, Y., Zhang, F., & Zhou, G. (2025). Exercise and Diet Reshape Athletes’ Gut Microbiota: Countering Health Challenges in Athletes. Life, 15(12), 1812. https://doi.org/10.3390/life15121812







