Evaluating White Worm (Enchytraeus sp.) Culture Conditions and Zeolite Supplementation for Aquaculture Live Feed
Abstract
1. Introduction
2. Materials and Methods
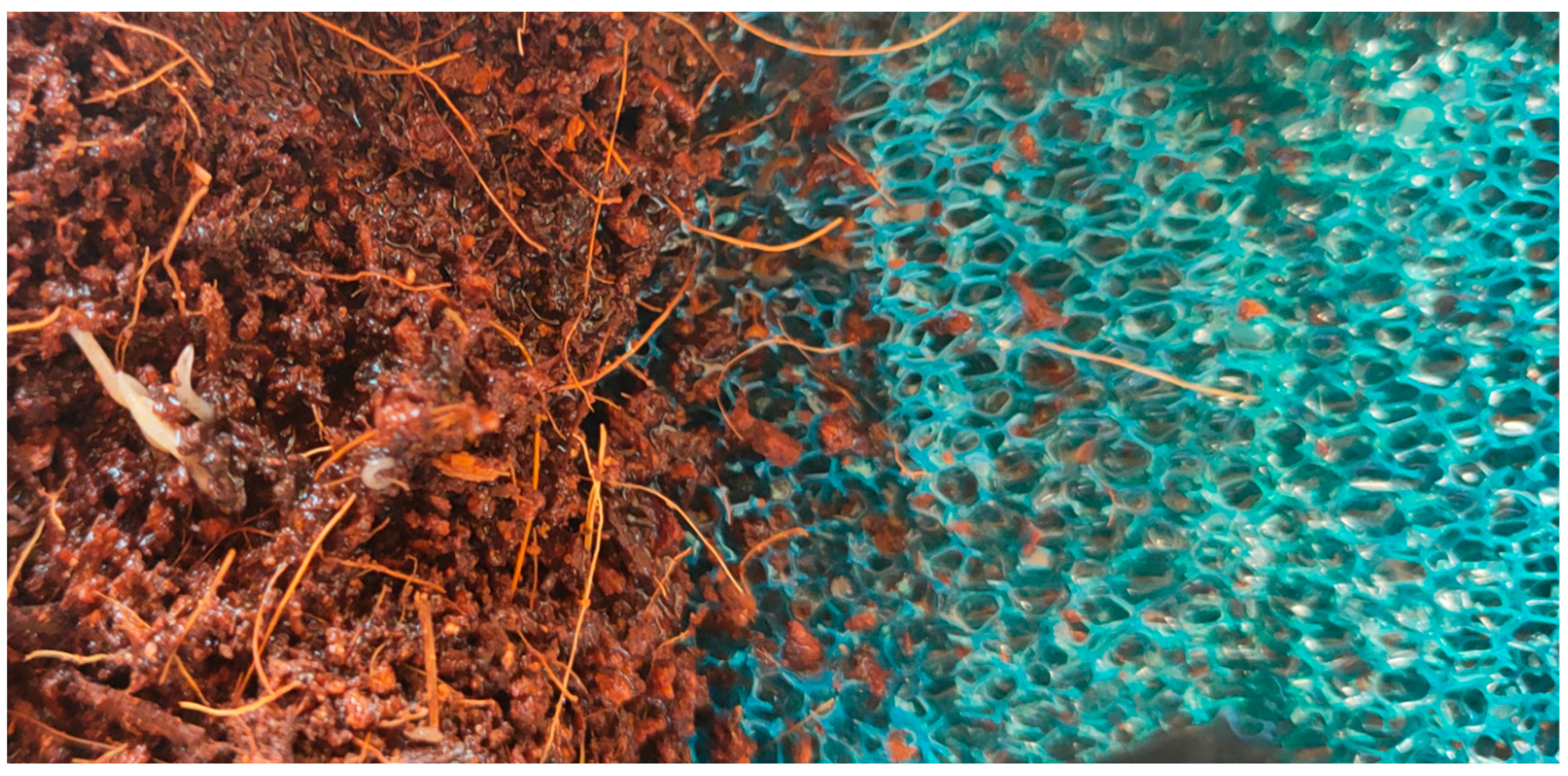
2.1. Experimental Organism and Culture Set Up
2.2. Culture and Measurement Instruments
2.3. Innovative Practices
2.4. Experimental Design
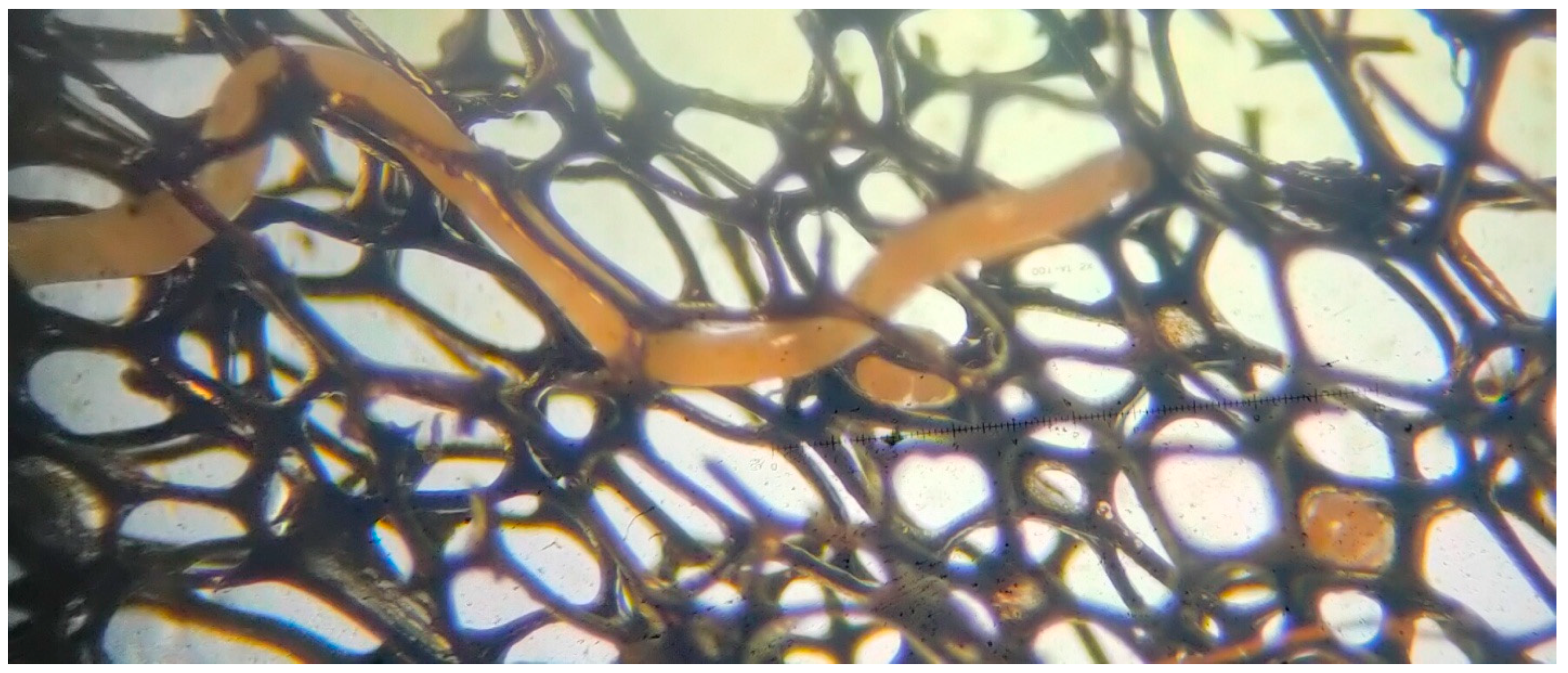
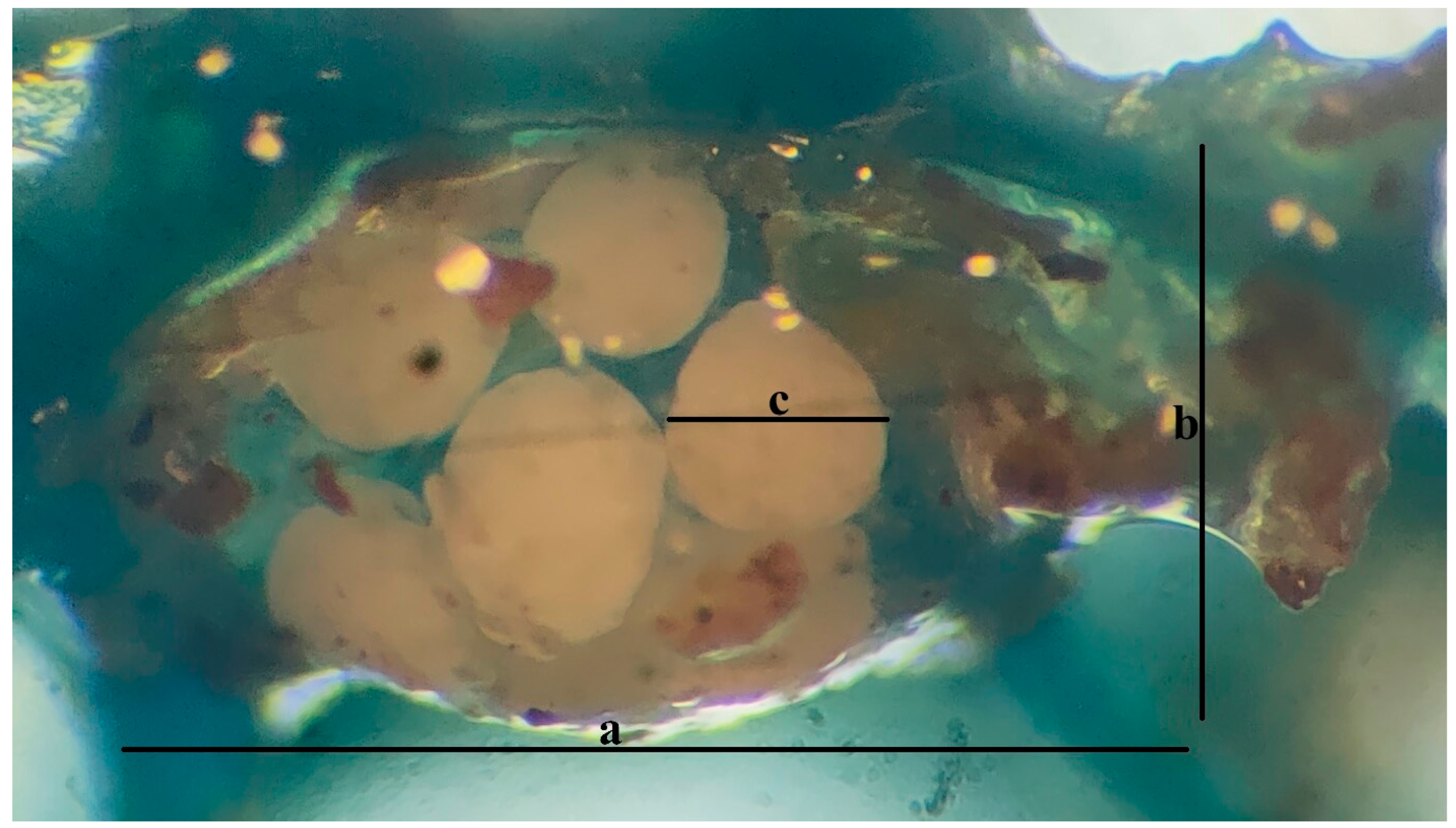
2.5. Reproductive Monitoring
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, M.; Pernetta, A.; Crooks, N. Size matters: A review of live feeds used in the culture of marine ornamental fish. Asian Fish. Sci. 2020, 33, 161–174. [Google Scholar] [CrossRef]
- Ghosh, A.; Mahapatra, B.K.; Datta, N.C. Ornamental fish farming—Successful small scale aqua business in India. Aquac. Asia 2003, 8, 14–16. [Google Scholar]
- Tappin, A.R. Rainbowfishes, Their Care, Keeping in Captivity; Electronic publication: Amsterdam, The Netherlands, 2010; 93p. [Google Scholar]
- Mugwanya, M.; Dawood, M.A.O.; Sewilam, H. Update on terrestrial and aquatic worms of the subclass oligochaeta in larviculture and aquaculture nutrition: Biomass production, benefits, potential risks, and management strategies. Aquac. Int. 2025, 33, 454. [Google Scholar] [CrossRef]
- Gerlich, H.S.; Schøn, M.L.; Grantland, D.; Slotsbo, S.M. Optimizing Enchytraeus albidus Production with Agri-FoodWaste: A Nutritional Landscape Approach. Aquac. Res. 2025, 2025, 5632761. [Google Scholar] [CrossRef]
- Dai, W.; Slotsbo, S.; Holmstrup, M. Thermal optimum for mass production of the live feed organism Enchytraeus albidus. J. Therm. Biol. 2021, 97, 9. [Google Scholar] [CrossRef] [PubMed]
- Öz, M.; Bahtiyar, M.; Şahin, D.; Karslı, Z.; Öz, Ü. Using white worm (Enchytraeus spp.) as a live feed in aquarium fish culture. J. Acad. Doc. Fish. Aquac. 2015, 1, 165–168. [Google Scholar]
- Ruby, P.; Antony, C.; Ahilan, B.; Selvaraj, S.; Ramya, N.; Wino, A.J. White worms Enchytraeus albidus as a live feed and formulated aquafeeds. Vigyan Varta 2024, 5, 165–167. [Google Scholar]
- Christensen, B. Studies on Enchytraeidae 6. technique for culturing Enchytraeidae, with notes on cocoon types. OIKOS 1956, 7, 302–307. [Google Scholar] [CrossRef]
- Bell, A.W. The Anatomy of the Oligochaete Enchytraeus albidus, with a Key to the Species of the Genus Enchytraeus; The American Museum of Natural History: New York, NY, USA, 1958. [Google Scholar]
- Jamieson, B.G.; Ferraguti, M. Non-leech clitellata. Reprod. Biol. Phylogeny Annelida 2006, 4, 235–392. [Google Scholar]
- Hönemann, L.; Nentwig, W. Are survival and reproduction of Enchytraeus albidus (Annelida: Enchytraeidae) at risk by feeding on Bt-maize litter? Eur. J. Soil Biol. 2009, 45, 351–355. [Google Scholar] [CrossRef]
- Fairchild, E.A.; Bergman, A.M.; Trushenski, J.T. Production and nutritional composition of white worms Enchytraeus albidus fed different low-cost feeds. Aquaculture 2017, 481, 16–24. [Google Scholar] [CrossRef]
- Coleman, D.C.S.; Geiseny, S.; Wall, D.H. Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. Soil Microbiol. Ecol. Biochem. 2024, 5, 131–159. [Google Scholar]
- Eryalçın, K.M.; Tınkır, M. Live Feeds in, aquaculture. In Present-Day Turkish Aquaculture and Trends in International Research; Istanbul University Press: Istanbul, Turkey, 2024; Volume 9, pp. 229–257. [Google Scholar]
- Holmstrup, M.; Touzot, M.; Slotsbo, S. Characterization of the thermal death time landscape for Enchytraeus albidus. Pedobiol.-J. Soil Ecol. 2023, 97–98, 150876. [Google Scholar] [CrossRef]
- Römbke, J.; Moser, T. Validating the Enchytraeid reproduction test: Organization and results of an international ring test. Chemosphere 2002, 46, 1117–1140. [Google Scholar] [CrossRef]
- Holmstrup, M.E.; Gadeberg, S.F.; Engell-Sørensen, K.; Slotsbo, S.; Holmstrup, M. A new strategy in rearing of European flounder: Using live Enchytraeus albidus to enhance juvenile growth. J. Insects Food Feed 2022, 8, 1333–1341. [Google Scholar] [CrossRef]
- Amorim, M.J.B.; Novais, S.; Römbke, J.; Soares, A.M.V.M. Avoidance test with Enchytraeus albidus (Enchytraeidae): Effects of different exposure time and soil proporties. Environ. Pollut. 2008, 155, 112–116. [Google Scholar] [CrossRef]
- Şahin, D.; Öz, M.; Karslı, Z.; Aral, O.; Bahtiyar, M. Effect of frozen White worm (Enchytraeus sp.) on growth of platy (Xiphophorus maculatus Günther, 1866). Turk. J. Aquat. Sci. 2017, 32, 71–75. [Google Scholar] [CrossRef]
- Bahrioğlu, E.; Isa, M.H.; Cengiz, S.; Ercan, E. The effect of fish meal and plant-based diets on the growth and nutritional composition of white worms (Enchytraeus albidus Henle, 1837) in various substrates. Pak. J. Zool. 2023, 5, 563–570. [Google Scholar] [CrossRef]
- Tizkar, B.; Alinezhad, S.; Zoughi Shalmani, A.; Hafezieh, M. Intraction effects of substrate and different protein diets on the growth and reproduction of white worm (Enchytraeus albidus Henle 1837). J. Aquac. Dev. JAD 2024, 18, 1–12. [Google Scholar]
- Zhao, L.; Ma, G. Reproductive potential and population growth of theworm Enchytraeus buchholzi (Clitellata: Enchytraeidae) under laboratory conditions as well as regression models. Biology 2025, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Felipe, M.C.; Bernegossi, A.C.; Pinheiro, F.R.; Castro, G.B.; Moura, L.; Zaiat, M.; Corbi, J.J. Counting Enchytraeus crypticus juveniles in chronic exposures: An alternative method for ecotoxicity studies using tropical artificial soil. Bull. Environ. Contam. Toxicol. 2021, 107, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Memiş, D.; Çelikkale, M.S.; Ercan, E. The effect of different diets on the white worm (Enchytraeus albidus Henle, 1837) reproduction. Turk. J. Fish. Aquat. Sci. 2004, 4, 05–07. [Google Scholar]
- Rodriguez, P.; Arrate, J.A.; Martinez-Madrid, M. Life history of the oligochaeta Enchytraeus coronatus (Annelida, Enchytraeidae) in agar culture. Invertebr. Biol. 2002, 121, 350–356. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for the Testing of Chemicals; Test No 220 Adopted: 29 July 2016; Available online: www.oecd.org/content/dam/oecd/en/publications/reports/2016/07/test-no-220-enchytraeid-reproduction-test_g1g6ecfe/9789264264472-en.pdf (accessed on 1 October 2025).
- Şahin, D.; Öz, M.; Öz, Ü. The Assessment of natural biomineral leonardite on growth and pigmentation of goldfish, Carassius auratus. Life 2025, 15, 74. [Google Scholar] [CrossRef]
- Lock, L.; Janssen, C.R. Effect of clay and organic matter type on the ecotoxicity of zinc and cadmiyum to the potworm Enchytraeus albidus. Chemosphere 2001, 44, 1669–1672. [Google Scholar] [CrossRef]
- Walsh, M.L.; Fairchild, E.A.; Rennels, N.; Howell, W.H. The effects of live and artifical diets on feeding performance of winter flounder, Pseudopleuronectes americanus, in the hatchery. J. World Aquac. Soc. 2015, 46, 61–68. [Google Scholar] [CrossRef]
- Schmelz, R.M.; Collado, R. A guide to European terrestrial and freshwater species of Enchytraeidae (Oligochaeta). Soil Org. 2010, 82, 1–176. [Google Scholar]
- Lindfeld, A.; Lang, C.; Knop, E.; Nentwing, W. Hard to digest or a piece of cake? does GM wheat affect survival and reproduction of Enchytraeus albidus (Annelida:Enchytraidae). Appl. Soil Ecol. 2011, 47, 51–58. [Google Scholar] [CrossRef]
- Haukka, J.K. Growth and survival of Eisenia fetida (Sav.) (Oligochaeta: Lumbricidae) in relation to temperature, moisture and presence of Enchytraues albidus (Henle) (Enchytraeidae). Biol. Fertil. Soils 1987, 3, 99–102. [Google Scholar] [CrossRef]
- Römbke, J. Entwicklung Eines Reproduktionstests an Bodenorganismen Enchytraeen; Abschlußbericht des Battelle-Instituts e.V. Frankfurt für das Umweltbundesamt (Berlin), FEVorhaben 106 03 051/01; Umwelt-bundesamt: Berlin, Germany, 1989. [Google Scholar]
- Ivleva, I.V. Enchytraeus albidus. In Mass Cultivation of Invertebrates: Biology and Methods; The Israel Program for Scientific Translations; National Marine Fisheries Service, National Oceanic and Atmospheric Admin-istration: Washington, DC, USA, 1973; pp. 8–38. (In Russian) [Google Scholar]
- Learner, M.A. Laboratory studies on the life-histories of four enchytraeid worms (Oligochaeta) which inhabit sewage percolating filters. Ann. Appl. Biol. 1972, 70, 251–266. [Google Scholar] [CrossRef]
- Römbke, J. Ecotoxicological laboratory tests with enchytraeids: A review. Pedobiologia 2003, 47, 607–616. [Google Scholar] [CrossRef]
- Adl, S.M. Enchytraeids, in Soil Sampling and Methods of Analysis. In Chapter 35 E.G. Gregorich/Soil Sampling and Methods of Analysis3586_C035; 2007; pp. 445–453. Available online: https://www.researchgate.net/publication/310828675_Enchytraeids_in_Soil_Sampling_and_Methods_of_Analysis (accessed on 1 October 2025).
- Mateju, V.; Vosáhlová, S.; Kyclt, R.; Janoch, T.; Šedivcová, G. The reproduction of Enchytraeus sp.—Technical improvement for the counting of juveniles. Environ. Monit. Assess. 2014, 186, 711–718. [Google Scholar] [CrossRef]
- Springett, J.A. A method for culturing Enchytraeidae. Oikos 1964, 15, 175–177. [Google Scholar] [CrossRef]
- Westheide, W.; Müller, M.C. Cinematographic documentation of Enchytraeid morphology and reproductive biology. Hidrobiologia 1996, 334, 263–267. [Google Scholar] [CrossRef]
- Windarto, S.; Elfitasari, T.; Darmanto, Y.S.; Anggraeni, N.; Herawati, V.E. Effect of substrate media on the growth, amino acids and fatty acids profiles of the marine worm (Nereis virens). AACL Bioflux 2023 2023, 16, 1177-1185. [Google Scholar]
- İbrahim, H.M.; Alghamdi, A.G. Effect of the particle size of clinoptilolite zeolite on water content and soil water storage in a loamy sand soil. Water 2021, 13, 607. [Google Scholar] [CrossRef]
- Amorim, M.J.; Ro¨mbke, J.; Soares, A.M.V.M. Effect of different soil types on the enchytraeids Enchytraeus albidus and Enchytraeus luxuriosus using the herbicide Phenmedipham. Chemosphere 2005, 61, 1102e1114. [Google Scholar] [CrossRef] [PubMed]
- Röembke, J.; Knacker, T. Aquatic toxicity test for enchytraeids. Hydrobiologia 1989, 180, 235–242. [Google Scholar] [CrossRef]
- Szatanik-Kloc, A.; Szerement, J.; Adamczuk, A.; Józefaciuk, G. Effect of low zeolite doses on plants and soil physicochemical properties. Materials 2021, 14, 2617. [Google Scholar] [CrossRef] [PubMed]

| Fish Feed | Soil | |
|---|---|---|
| G1 | None | None |
| G2 | + | None |
| G3 | None | + |
| Culture 2 Parameters * | Group 1 (Control) | Group 2 | Group 3 |
|---|---|---|---|
| Temperature (°C) | 23.44 ± 0.28 | 23.39 ± 0.26 | 23.39 ± 0.22 |
| pH | 6.89 ± 0.05 b | 7.03 ± 0.05 b | 7.22 ± 0.05 a |
| Moisture (%) | 42.22 ± 1.73 | 41.67 ± 1.67 | 40.00 ± 1.40 |
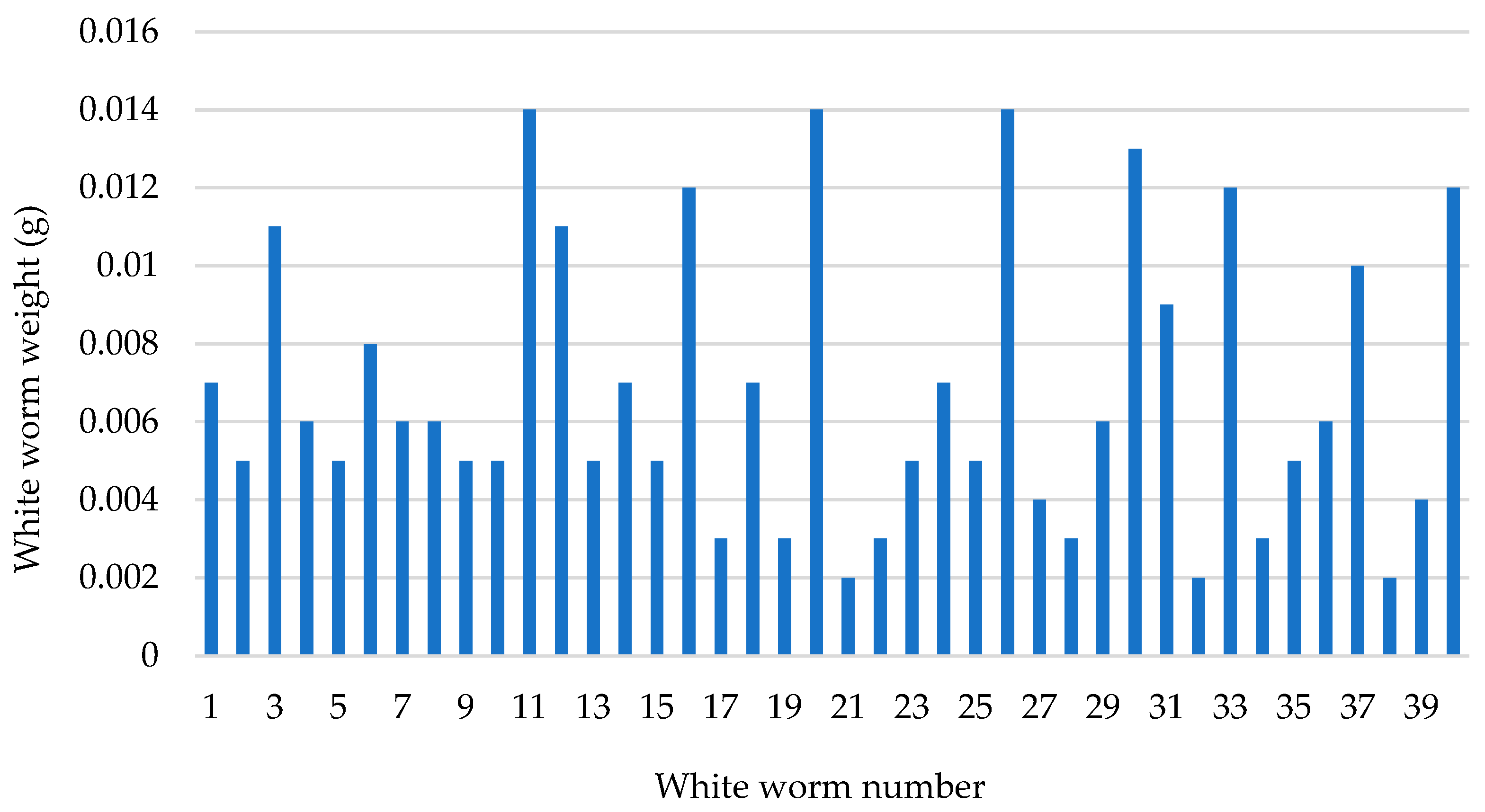
| Adult Worms Mean Length (mm) (n = 10) | Adult Worms Mean Weight (g) (n = 10) | Total Weight (g) of Individuals (n = 424) on Day 70 | Numbers of New Individuals Hatched from Cocoons on Day 90 (Number) * |
|---|---|---|---|
| 24.30 ± 1.56 | 0.0123 ± 0.00 | 2.54 | 327 |
| Experimental Groups * | Mean Number of Individuals at the End of the Experiment |
|---|---|
| Group 1 | 58.67 ± 4.67 b |
| Group 2 | 71.67 ± 2.96 b |
| Group 3 | 134 ± 3.79 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öz, M.; Bahtiyar, M. Evaluating White Worm (Enchytraeus sp.) Culture Conditions and Zeolite Supplementation for Aquaculture Live Feed. Life 2025, 15, 1813. https://doi.org/10.3390/life15121813
Öz M, Bahtiyar M. Evaluating White Worm (Enchytraeus sp.) Culture Conditions and Zeolite Supplementation for Aquaculture Live Feed. Life. 2025; 15(12):1813. https://doi.org/10.3390/life15121813
Chicago/Turabian StyleÖz, Meryem, and Mehmet Bahtiyar. 2025. "Evaluating White Worm (Enchytraeus sp.) Culture Conditions and Zeolite Supplementation for Aquaculture Live Feed" Life 15, no. 12: 1813. https://doi.org/10.3390/life15121813
APA StyleÖz, M., & Bahtiyar, M. (2025). Evaluating White Worm (Enchytraeus sp.) Culture Conditions and Zeolite Supplementation for Aquaculture Live Feed. Life, 15(12), 1813. https://doi.org/10.3390/life15121813






