Vulvar Squamous Cell Carcinoma: A Retrospective Analysis of Epidemiologic Characteristics, HPV Status, and Surgical Outcomes in 35 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
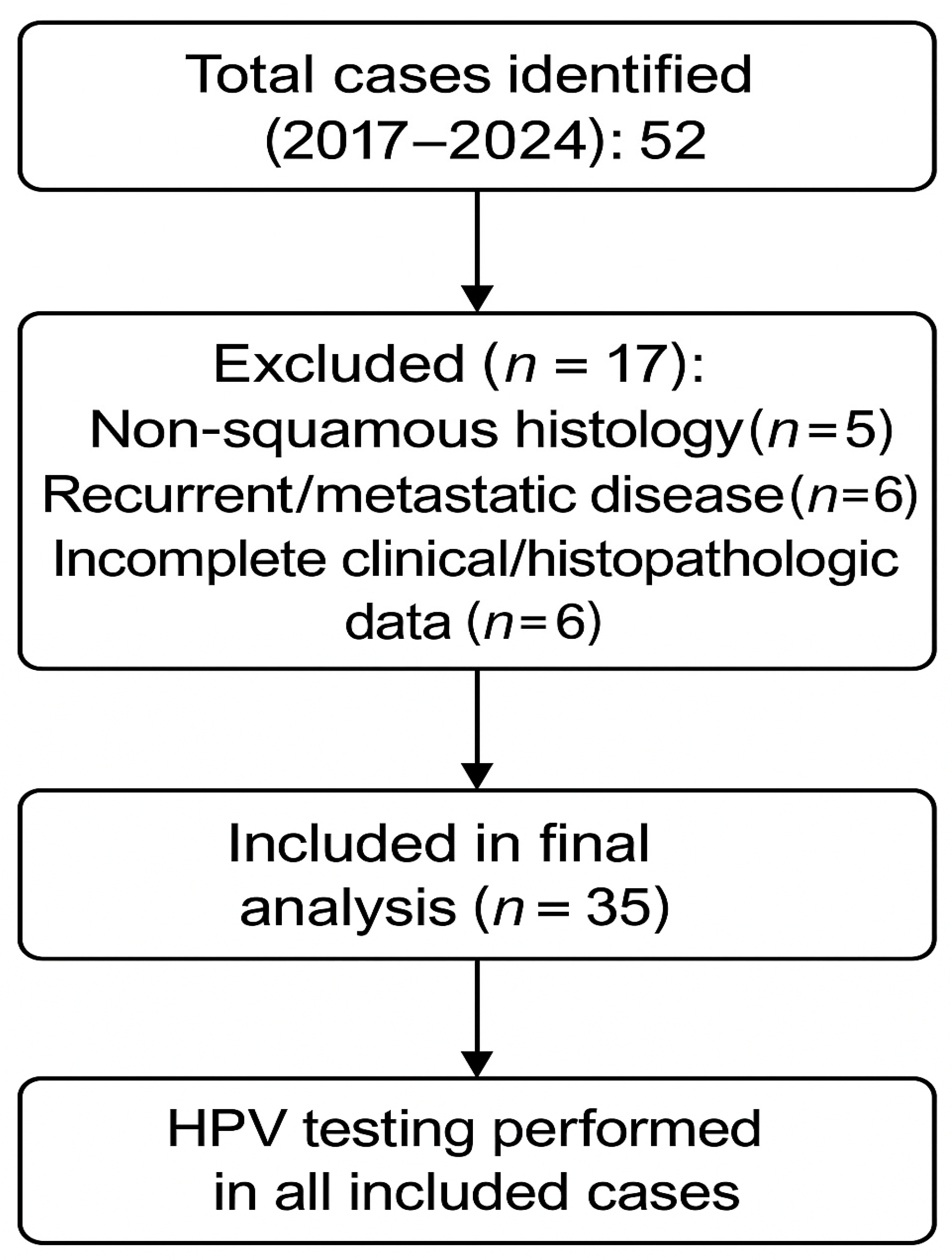
2.2. Inclusion and Exclusion Criteria
2.3. Clinicopathologic and Histopathologic Evaluation
2.4. HPV Detection and Genotyping
2.5. Surgical and Postoperative Management
2.6. Statistical Analysis
2.7. Ethical Approval
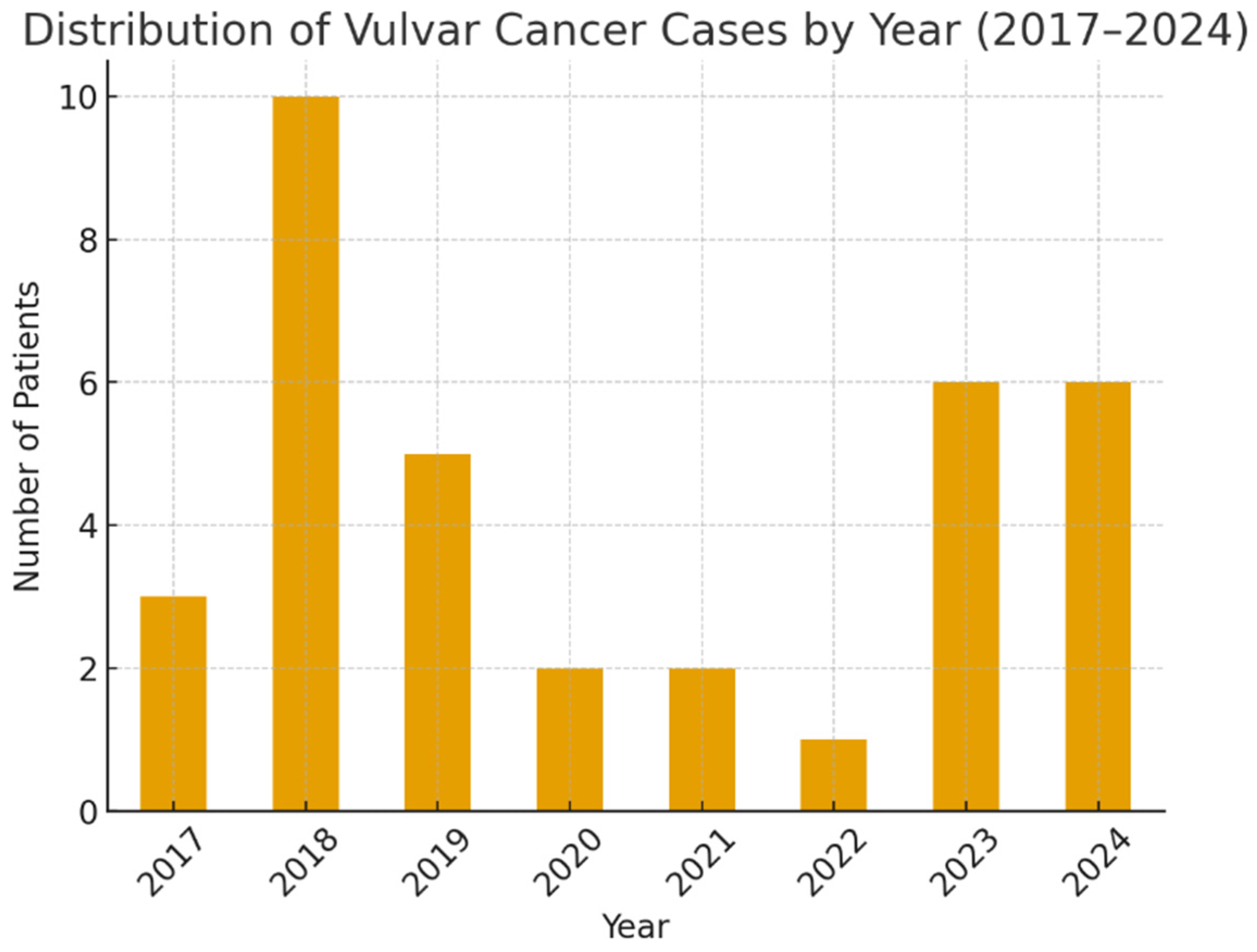
3. Results
- Grading distribution across groups: χ2 test, p = 1.00
- Complication rates across groups: χ2 test, p = 1.00
4. Discussion
4.1. Epidemiology and HPV Association
4.2. Surgical Management
4.2.1. Evolution of Surgical Management—Literature Perspective
4.2.2. Separate-Incision Techniques and Hemivulvectomy
4.2.3. Sentinel Lymph Node Biopsy (SLNB)
4.2.4. Minimally Invasive Inguinofemoral Lymphadenectomy (VEIL/Robotic)
4.2.5. Morbidity and Strategies for Complication Prevention
4.2.6. Training and Surgical Expertise
4.2.7. Vulvoperineal Reconstruction and Quality of Life
4.2.8. Advanced and Recurrent Disease
4.3. Adjuvant Therapies and Multimodal Treatment
4.4. Complications and Quality of Life
4.5. Future Perspectives
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olawaiye, A.B.; Cuello, M.A.; Rogers, L.J. Cancer of the vulva: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 7–18. [Google Scholar] [CrossRef]
- Jones, R.W.; Baranyai, J.; Stables, S. Trends in squamous cell carcinoma of the vulva: The influence of vulvar intraepithelial neoplasia. Obstet. Gynecol. 1997, 90, 448–452. [Google Scholar] [CrossRef]
- Judson, P.L.; Habermann, E.B.; Baxter, N.N.; Durham, S.B.; Virnig, B.A. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet. Gynecol. 2006, 107, 1018–1022. [Google Scholar] [CrossRef]
- Watson, M.; Saraiya, M.; Wu, X. Update of HPV-associated female genital cancers in the United States, 1999–2004. J. Womens Health 2009, 18, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Gallio, N.; Preti, M.; Jones, R.W.; Borella, F.; Woelber, L.; Bertero, L.; Urru, S.; Micheletti, L.; Zamagni, F.; Bevilacqua, F.; et al. Differentiated vulvar intraepithelial neoplasia long-term follow up and prognostic factors: An analysis of a large historical cohort. Acta Obstet. Gynecol. Scand. 2024, 103, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, L.; Pizzato, M.; Rosso, S.; Ferretti, S. New Insights into the Epidemiology of Vulvar Cancer: Systematic Literature Review for an Update of Incidence and Risk Factors. Cancers 2022, 14, 389. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012, 30 (Suppl. 5), F12–F23. [Google Scholar] [CrossRef]
- Morrison, J.; Baldwin, P.; Hanna, L.; Andreou, A.; Buckley, L.; Durrant, L.; Edey, K.; Faruqi, A.; Fotopoulou, C.; Ganesan, R.; et al. British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: An update on recommendations for practice 2023. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 210–238. [Google Scholar] [CrossRef]
- Rogers, L.J. Management of Advanced Squamous Cell Carcinoma of the Vulva. Cancers 2021, 14, 167. [Google Scholar] [CrossRef]
- Khairkhah, N.; Bolhassani, A.; Najafipour, R. Current and future direction in treatment of HPV-related cervical disease. J. Mol. Med. 2022, 100, 829–845. [Google Scholar] [CrossRef]
- Barton, D.P. The prevention and management of treatment related morbidity in vulval cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 683–701. [Google Scholar] [CrossRef]
- Soliman, A.A.; Heubner, M.; Kimmig, R.; Wimberger, P. Morbidity of inguinofemoral lymphadenectomy in vulval cancer. Sci. World J. 2012, 2012, 341253. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Schmeusser, B.N.; Nabavizadeh, R.; Master, V.A. Minimally invasive techniques to reduce complications of inguinal lymphadenectomy. AME Med. J. 2023, 8, 16. [Google Scholar] [CrossRef]
- Sommariva, A.; Pasquali, S.; Cona, C.; Ciccarese, A.A.; Saadeh, L.; Campana, L.G.; Meroni, M.; Rossi, C.R. Videoscopic ilioinguinal lymphadenectomy for groin lymph node metastases from melanoma. Br. J. Surg. 2016, 103, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.E.; Ghasemi, H.; Najafi, M.; Yekta, Z.; Nejadghaderi, S.A. Incidence Trends of Vulvar Cancer in the United States: A 20-Year Population-Based Study. Cancer Rep. 2024, 7, e2120. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Cuello, M.A.; Beriwal, S.; Rogers, L.J. Cancer of the vulva: 2025 update: FIGO Cancer Report 2025. Int. J. Gynaecol. Obstet. 2025, 171 (Suppl. 1), 36–47. [Google Scholar] [CrossRef]
- Lai, J.; Elleray, R.; Nordin, A.; Hirschowitz, L.; Rous, B.; Gildea, C.; Poole, J. Vulval cancer incidence, mortality and survival in England: Age-related trends. BJOG 2014, 121, 728–738, discussion 739. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Q.; Zhang, Y.; Ji, Y.; Li, J.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; Zhang, Y.; et al. Global burden of cervical cancer: Current estimates, temporal trend and future projections based on the GLOBOCAN 2022. J. Natl. Cancer Cent. 2025, 5, 322–329. [Google Scholar] [CrossRef]
- Tan, A.; Bieber, A.K.; Stein, J.A.; Pomeranz, M.K. Diagnosis and management of vulvar cancer: A review. J. Am. Acad. Dermatol. 2019, 81, 1387–1396. [Google Scholar] [CrossRef]
- Akhtar-Danesh, N.; Elit, L.; Lytwyn, A. Trends in incidence and survival of women with invasive vulvar cancer in the United States and Canada: A population-based study. Gynecol. Oncol. 2014, 134, 314–318. [Google Scholar] [CrossRef]
- Baandrup, L.; Varbo, A.; Munk, C.; Johansen, C.; Frisch, M.; Kjaer, S.K. In situ and invasive squamous cell carcinoma of the vulva in Denmark 1978-2007-a nationwide population-based study. Gynecol. Oncol. 2011, 122, 45–49. [Google Scholar] [CrossRef]
- Barlow, E.L.; Kang, Y.J.; Hacker, N.F.; Canfell, K. Changing Trends in Vulvar Cancer Incidence and Mortality Rates in Australia Since 1982. Int. J. Gynecol. Cancer 2015, 25, 1683–1689. [Google Scholar] [CrossRef]
- Buttmann-Schweiger, N.; Klug, S.J.; Luyten, A.; Holleczek, B.; Heitz, F.; du Bois, A.; Kraywinkel, K. Incidence patterns and temporal trends of invasive nonmelanotic vulvar tumors in Germany 1999-2011. A population-based cancer registry analysis. PLoS ONE 2015, 10, e0128073. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Fung, Y.C.; Pang, W.S.; Mak, F.Y.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., 3rd; Xu, W.; et al. Global incidence, risk factors and trends of vulvar cancer: A country-based analysis of cancer registries. Int. J. Cancer 2023, 153, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Barišić, I.; Čukelj, P.; Brkić Biloš, I.; Šekerija, M. Epidemiology of vulvar cancer in Croatia. Croat. Med. J. 2023, 64, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Moberg, L.; Sundqvist, A.; Holmberg, E.; Dickman, P.W.; Borgfeldt, C. Vulvar cancer incidence and net survival in Sweden 1960 to 2019: A population-based national study. Acta Obstet. Gynecol. Scand. 2024, 103, 561–571. [Google Scholar] [CrossRef]
- Belda, F.J.; Aguilera, L.; García de la Asunción, J.; Alberti, J.; Vicente, R.; Ferrándiz, L.; Rodríguez, R.; Company, R.; Sessler, D.I.; Aguilar, G.; et al. Supplemental perioperative oxygen and the risk of surgical wound infection: A randomized controlled trial. JAMA 2005, 294, 2035–2042. [Google Scholar] [CrossRef]
- Lupi, M.; Tsokani, S.; Howell, A.-M.; Ahmed, M.; Brogden, D.; Tekkis, P.; Kontovounisios, C.; Mills, S. Anogenital HPV-Related Cancers in Women: Investigating Trends and Sociodemographic Risk Factors. Cancers 2024, 16, 2177. [Google Scholar] [CrossRef]
- Barbu, L.A.; Vasile, L.; Ţenea-Cojan, T.Ş.; Mogoş, G.F.R.; Şurlin, V.; Vîlcea, I.D.; Cercelaru, L.; Mogoantă, S.Ş.; Mărgăritescu, N.D. Endometrioid adenofibroma of ovary—A literature review. Rom. J. Morphol. Embryol. 2025, 66, 39–49. [Google Scholar] [CrossRef]
- Sturgeon, S.R.; Brinton, L.A.; Devesa, S.S.; Kurman, R.J. In situ and invasive vulvar cancer incidence trends (1973 to 1987). Am. J. Obstet. Gynecol. 1992, 166, 1482–1485. [Google Scholar] [CrossRef]
- Madsen, B.S.; Jensen, H.L.; van den Brule, A.J.; Wohlfahrt, J.; Frisch, M. Risk factors for invasive squamous cell carcinoma of the vulva and vagina--population-based case-control study in Denmark. Int. J. Cancer 2008, 122, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, M.S.; van den Einden, L.C.; Massuger, L.F.; Kiemeney, L.A.; van der Aa, M.A.; de Hullu, J.A. Trends in incidence and survival of Dutch women with vulvar squamous cell carcinoma. Eur. J. Cancer. 2013, 49, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Somoye, G.O.; Mocroft, A.; Olaitan, A. Analysis of the incidence and mortality of vulval cancer in women in South East England 1960–1999. Arch. Gynecol. Obstet. 2009, 279, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S. Modern treatment of vulvar cancer. Radiol. Oncol. 2020, 54, 371–376. [Google Scholar] [CrossRef]
- Rodrigues, G.J.; Guglielmetti, G.B.; Orvieto, M.; Seetharam Bhat, K.R.; Patel, V.R.; Coelho, R.F. Robot-assisted endoscopic inguinal lymphadenectomy: A review of current outcomes. Asian J. Urol. 2021, 8, 20–26. [Google Scholar] [CrossRef]
- Hacker, N.F.; Barlow, E.L. Conservative Management of Vulvar Cancer-Where Should We Draw the Line? Cancers 2024, 16, 2991. [Google Scholar] [CrossRef]
- Brincat, M.R.; Muscat Baron, Y. Sentinel Lymph Node Biopsy in the Management of Vulvar Carcinoma: An Evidence-Based Insight. Int. J. Gynecol. Cancer 2017, 27, 1769–1773. [Google Scholar] [CrossRef]
- Selcuk, İ.; Aktaş Akdemir, H.; Ersak, B.; Tatar, İ.; Sargon, M.F.; Güngör, T. Inguinofemoral lymphadenectomy and femoral dissection: Cadaveric educational video. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 155–157. [Google Scholar] [CrossRef]
- Cetin Avci, N.; Hatipoglu, F.; Alacacıoglu, A.; Bayar, E.E.; Bural, G.G. FDG PET/CT and Conventional Imaging Methods in Cancer of Unknown Primary: An Approach to Overscanning. Nucl. Med. Mol. Imaging 2018, 52, 438–444. [Google Scholar] [CrossRef]
- Wendelspiess, S.; Kouba, L.; Stoffel, J.; Speck, N.; Appenzeller-Herzog, C.; Gahl, B.; Montavon, C.; Heinzelmann-Schwarz, V.; Lariu, A.; Schaefer, D.J.; et al. Perforator versus Non-Perforator Flap-Based Vulvoperineal Reconstruction-A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2213. [Google Scholar] [CrossRef]
- Barbu, L.A.; Vasile, L.; Cercelaru, L.; Șurlin, V.; Mogoantă, S.-Ș.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Mărgăritescu, N.-D.; Iordache, M.P.; Buliman, A. Aggressiveness in Well-Differentiated Small Intestinal Neuroendocrine Tumors: A Rare Case and Narrative Literature Review. J. Clin. Med. 2025, 14, 5821. [Google Scholar] [CrossRef]
- Barbu, L.A.; Cercelaru, L.; Șurlin, V.; Mogoantă, S.-S.; Țenea Cojan, T.S.; Mărgăritescu, N.-D.; Țenea Cojan, A.-M.; Popescu, M.; Căluianu, V.; Mogoș, G.F.R.; et al. Serous Papillary Adenofibroma Cyst of the Ovary in a Young Woman: Case Report and Literature Review. Life 2025, 15, 1601. [Google Scholar] [CrossRef] [PubMed]
- Barbu, L.A.; Vasile, L.; Cercelaru, L.; Vîlcea, I.-D.; Șurlin, V.; Mogoantă, S.-S.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Mărgăritescu, N.-D. Fused Ischiorectal Phlegmon with Pre- and Retroperitoneal Extension: Case Report and Narrative Literature Review. J. Clin. Med. 2025, 14, 4959. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Dieguez, N.; Guerrero, J.; Rodrigo-Calvo, M.T.; Ribera-Cortada, I.; Trias, I.; Jares, P.; López Del Campo, R.; Saco, A.; Munmany, M.; Marimon, L.; et al. Molecular Landscape of Vulvar Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 7069. [Google Scholar] [CrossRef] [PubMed]
- Accorona, R.; D’Onghia, A.; Pignataro, L.; Capaccio, P. Head and neck robotic surgery combined with sentinel lymph node biopsy. Fascinating, but feasible? Oral Oncol. 2020, 111, 104939. [Google Scholar] [CrossRef]
- Cakir, M.O.; Kayhan, G.; Yilmaz, B.; Ozdogan, M.; Ashrafi, G.H. Emerging Therapeutic Strategies for HPV-Related Cancers: From Gene Editing to Precision Oncology. Curr. Issues Mol. Biol. 2025, 47, 759. [Google Scholar] [CrossRef]
- Eremia, I.A.; Ciobanu, M.; Tenea, T.; Comănescu, M.V.; Crăiţoiu, S. Invasive papillary carcinoma of the mammary gland: Histopathologic and immunohistochemical aspects. Rom. J. Morphol. Embryol. 2012, 53 (Suppl. 3), 811–815. [Google Scholar]
- Barbu, L.A.; Vasile, L.; Cercelaru, L.; Șurlin, V.; Mogoantă, S.S.; Mogoș, G.F.R.; Țenea Cojan, T.S.; Mărgăritescu, N.D.; Buliman, A. Non-Variceal Upper Gastrointestinal Bleeding: A Retrospective Cohort of 364 Cases, Historical Comparison, and Updated Management Algorithm. Life 2025, 15, 1320. [Google Scholar] [CrossRef]
| Criterion Type | Details |
|---|---|
| Inclusion | Primary histologically confirmed VSCC |
| FIGO stage I–IV | |
| Age ≥ 18 years | |
| Complete clinicopathologic and surgical data | |
| FFPE tissue blocks available for HPV testing | |
| Treated at the same institution | |
| Exclusion | Non-squamous histology (melanoma, sarcoma, Paget, adnexal) |
| Recurrent/metastatic disease | |
| Prior treatment elsewhere | |
| Missing or incomplete documentation |
| Variable | n (%) or Value |
|---|---|
| Age (years) | Mean 68.4 ± 10.0 (range 43–83) |
| Tumor grade | |
| G1 | 17 (48.6) |
| G2 | 14 (40.0) |
| G3 | 4 (11.4) |
| Histological type | Squamous cell carcinoma—35 (100.0) |
| HPV status | |
| HPV-positive (total) | 11 (31.4) |
| – HPV genotypes 16/18/33 | 11 (31.4) |
| HPV-negative | 24 (68.6) |
| Median age by HPV status (years) | |
| HPV-positive | 58.0 (55.0–65.5) |
| HPV-negative | 72.5 (67.8–79.0) |
| Surgical procedure | |
| Hemivulvectomy ± ipsilateral lymph node dissection ± adjuvant RT | 12 (34.3) |
| Radical total vulvectomy ± adjuvant RT/bilateral LN dissection | 6 (17.1) |
| Radical vulvectomy with separate incisions and bilateral LN dissection | 17 (48.6) |
| Hospital stay (days) | Mean 8.8 ± 2.9 (range 5–15) |
| Drainage duration (days) | Mean 11.7 ± 6.6 (range 4–29) |
| Early complications | |
| Neuropathic pain/paresthesia | 11 (31.4) |
| Lymphocysts | 6 (17.1) |
| Postoperative hematoma | 3 (8.6) |
| Wound infection | 2 (5.7) |
| Complete wound dehiscence | 1 (2.9) |
| No early complications | 12 (34.3) |
| Late complications | |
| Lymphedema (mons pubis/lower limbs) | 25 (71.4) |
| Local recurrence | 1 (2.9) |
| No late complications | 9 (25.7) |
| Variable | Category | n | Complications n/N (%) | Late Complications n/N (%) | Recurrence n/N (%) | Hospital Stay Median [Mean] | Drainage Duration Median [Mean] |
|---|---|---|---|---|---|---|---|
| Overall complications | Early complications (total) | 35 | 23/35 (65.7) | — | — | — | — |
| Late complications (total) | 35 | — | 28/35 (80.0) | — | — | — | |
| Tumor grade | G1–G2 | 31 | 19/31 (61.3) | 22/31 (71.0) | 0 (0.0) | 8.0 (7.0–10.0) [8.5] | 9.0 (8.0–12.0) [11.1] |
| G3 | 4 | 4/4 (100.0) | 4/4 (100.0) | 1 (25.0) | 10.5 (7.0–14.2) [10.8] | 17.0 (10.8–22.8) [16.5] | |
| Surgical procedure | Hemivulvectomy ± ipsilateral LN dissection/adjuvant RT | 12 | 8/12 (66.7) | 9/12 (75.0) | 0 (0.0) | 8.0 (6.0–9.2) [8.2] | 9.5 (7.0–11.2) [11.3] |
| Radical total vulvectomy ± adjuvant RT/bilateral LN dissection | 6 | 5/6 (83.3) | 5/6 (83.3) | 0 (0.0) | 10.5 (9.0–13.5) [11.0] | 14.0 (12.0–20.5) [16.5] | |
| Radical vulvectomy with separate incisions and bilateral LN dissection | 17 | 10/17 (58.8) | 14/17 (82.4) | 1 (5.9) | 8.0 (7.0–10.0) [8.4] | 9.0 (8.0–12.0) [10.3] | |
| Age group | <65 years | 8 | 5 (62.5) | 6 (75.0) | 0 (0.0) | 8.3 | 10.6 |
| ≥65 years | 27 | 23 (85.2) | 22 (81.5) | 1 (3.7) | 8.9 | 12.0 | |
| Diabetes | Yes | 12 | 11 (91.7) | 10 (83.3) | 0 (0.0) | 9.5 | 13.7 |
| No | 23 | 17 (73.9) | 18 (78.3) | 1 (4.3) | 8.4 | 10.7 | |
| Early vs. late complications | No early complications | 12 | — | 5 (41.7) | 0 (0.0) | 8.3 | 10.8 |
| With early complications | 23 | — | 23 (100.0) | 1 (4.3) | 9.0 | 12.2 | |
| No late complications | 7 | 0 (0.0) | — | 0 (0.0) | 7.9 | 10.0 | |
| With late complications | 28 | 23 (82.1) | — | 1 (3.6) | 9.0 | 12.1 | |
| Specific early complications | Neuropathic pain/paresthesia | 11 | 11/35 (31.4) | — | — | — | — |
| Lymphocyst | 6 | 6/35 (17.1) | — | — | — | — | |
| Hematoma | 3 | 3/35 (8.6) | — | — | — | — | |
| Wound infection | 2 | 2/35 (5.7) | — | — | — | — | |
| Wound dehiscence | 1 | 1/35 (2.9) | — | — | — | — | |
| Specific late complications | Lymphedema of mons pubis/lower limbs | 25 | — | 25/35 (71.4) | — | — | — |
| Local recurrence | 1 | — | 1/35 (2.9) | 1/35 (2.9) | — | — |
| Variable | Category | n | Complications n (%) | Recurrence n (%) | Lymphedema n (%) | Mean Hospital Stay (Days ± SD) | Mean Drainage (Days ± SD) | Median (Range) |
|---|---|---|---|---|---|---|---|---|
| Hospitalization | — | 35 | — | — | — | 8.8 ± 2.9 | — | 8 (5–15) |
| Drainage duration | — | 35 | — | — | — | — | 11.7 ± 6.6 | 10 (4–29) |
| Cardiovascular disease | Yes | 32 | 25 (78.1) | — | — | 8.9 | 11.9 | — |
| No | 3 | 3 (100.0) | — | — | 7.7 | 9.7 | — | |
| Chronic kidney disease | Yes | 1 | 1 (100.0) | — | — | 7.0 | 12.0 | — |
| No | 34 | 27 (79.4) | — | — | 8.8 | 11.7 | — | |
| Smoking | Yes | 4 | 3 (75.0) | — | — | 8.8 | 11.5 | — |
| No | 31 | 25 (80.6) | — | — | 8.8 | 11.8 | — | |
| Time period | Pre-2020 | 18 | 13 (72.2) | — | — | 8.6 | 10.8 | — |
| Post-2020 | 17 | 15 (88.2) | — | — | 9.0 | 12.6 | — | |
| Drainage duration group | Short (<10 days) | 17 | — | 1 (5.9) | 12 (70.6) | 6.9 | 7.3 | — |
| Long (≥10 days) | 18 | — | 0 (0.0%) | 13 (72.2) | 10.6 | 15.9 |
| Group | n | G1 | G2 | G3 | Complications n (%) | Mean Hospital Stay (Days) | Mean Drainage (Days) |
|---|---|---|---|---|---|---|---|
| Vegetative/ulcerated (mixed) | 32 | 17 | 12 | 3 | 25 (78.1) | 8.6 | 11.6 |
| Infiltrative | 3 | 0 | 2 | 1 | 3 (100.0) | 10.7 | 14.0 |
| Outcome | HPV-Positive, n (%) (n = 11) | HPV-Negative, n (%) (n = 24) | Test/Effect Size (95% C) |
|---|---|---|---|
| Age (years) | Median 58.0 (IQR 55.0–65.5) | Median 72.5 (IQR 67.8–79.0) | Mann–Whitney U = 20.0, p < 0.001 |
| Tumor grade G3 (vs. G1–2) | 2/11 (18.2) | 2/24 (8.3) | Fisher exact p = 0.575 |
| Early complications (any) | 8/11 (72.7) | 15/24 (62.5) | Fisher p = 0.709; RR = 1.16 (0.72–1.87); RD = +10.2% (−35.4–+47.5) |
| Late complications (total) | 8/11 (72.7) | 18/24 (75.0) | Fisher p = 1.000; RR = 0.97 (0.63–1.49); RD = −2.3% (−44.6–+35.2) |
| Hospitalization days | Median 7.0 (IQR 6.0–10.5) | Median 8.5 (IQR 7.0–10.0) | Mann–Whitney U = 111.5, p = 0.474; RLM β(HPV+) = +0.41 days, p = 0.804 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinescu, D.; Barbu, L.A.; Țenea Cojan, T.S.; Tudorache, Ș.; Iliescu, D.; Marinescu, R.A.; Zorilă, L.G.; Șurlin, V. Vulvar Squamous Cell Carcinoma: A Retrospective Analysis of Epidemiologic Characteristics, HPV Status, and Surgical Outcomes in 35 Cases. Life 2025, 15, 1781. https://doi.org/10.3390/life15111781
Marinescu D, Barbu LA, Țenea Cojan TS, Tudorache Ș, Iliescu D, Marinescu RA, Zorilă LG, Șurlin V. Vulvar Squamous Cell Carcinoma: A Retrospective Analysis of Epidemiologic Characteristics, HPV Status, and Surgical Outcomes in 35 Cases. Life. 2025; 15(11):1781. https://doi.org/10.3390/life15111781
Chicago/Turabian StyleMarinescu, Daniela, Laurențiu Augustus Barbu, Tiberiu Stefăniță Țenea Cojan, Ștefania Tudorache, Dominic Iliescu, Răzvan Alexandru Marinescu, Lucian George Zorilă, and Valeriu Șurlin. 2025. "Vulvar Squamous Cell Carcinoma: A Retrospective Analysis of Epidemiologic Characteristics, HPV Status, and Surgical Outcomes in 35 Cases" Life 15, no. 11: 1781. https://doi.org/10.3390/life15111781
APA StyleMarinescu, D., Barbu, L. A., Țenea Cojan, T. S., Tudorache, Ș., Iliescu, D., Marinescu, R. A., Zorilă, L. G., & Șurlin, V. (2025). Vulvar Squamous Cell Carcinoma: A Retrospective Analysis of Epidemiologic Characteristics, HPV Status, and Surgical Outcomes in 35 Cases. Life, 15(11), 1781. https://doi.org/10.3390/life15111781







