Isolation and Characterization of Integrin α9 Positive Extracellular Vesicles Derived from Human Corneoscleral Rings
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Limbal Tissue-Derived Extracellular Vesicles
2.2. Extracellular Vesicles Characterization
2.3. Transmission Electron Microscopy (TEM)
2.4. Nanoparticle Tracking Analysis (NTA)
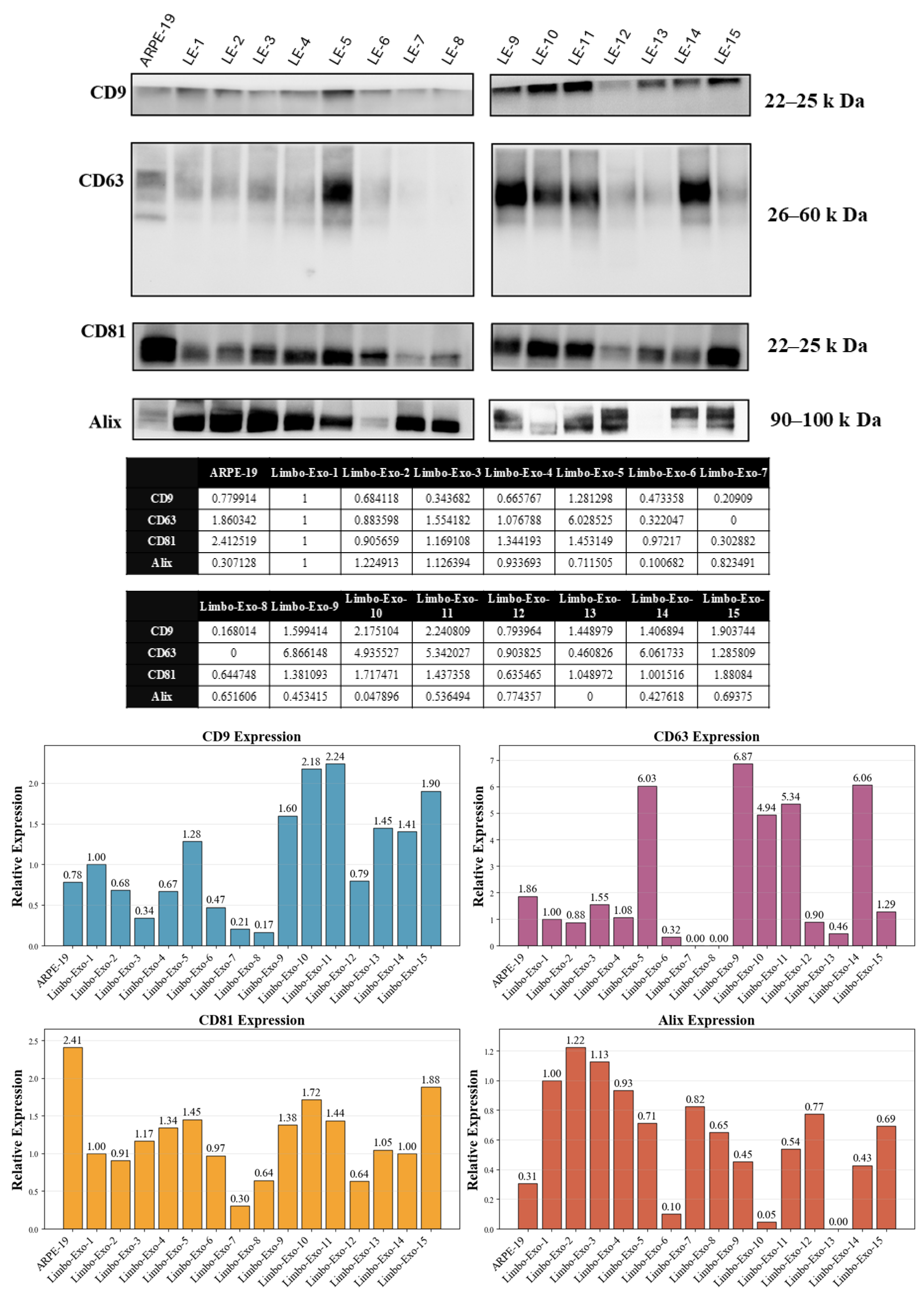
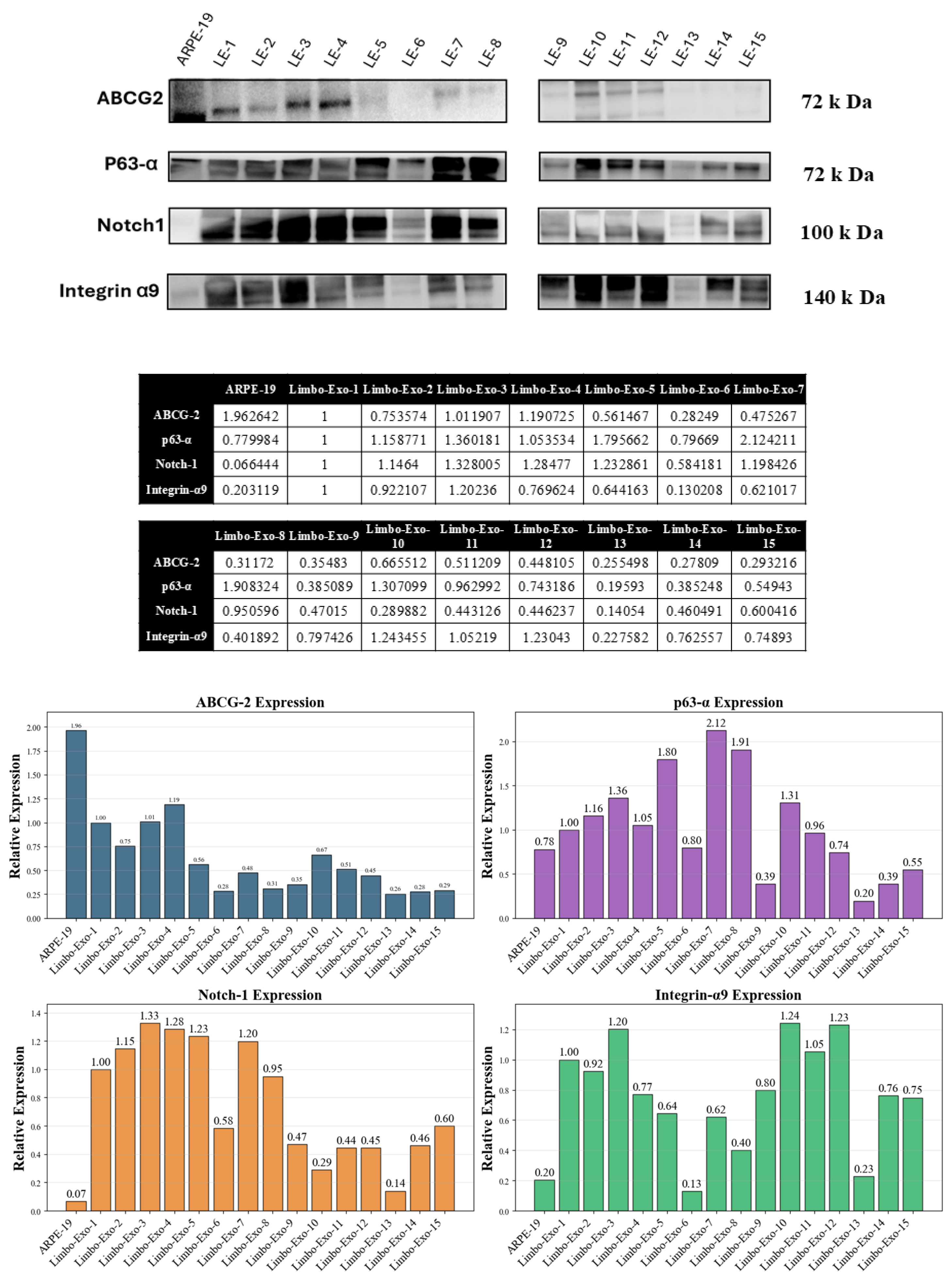
2.5. Western Blotting
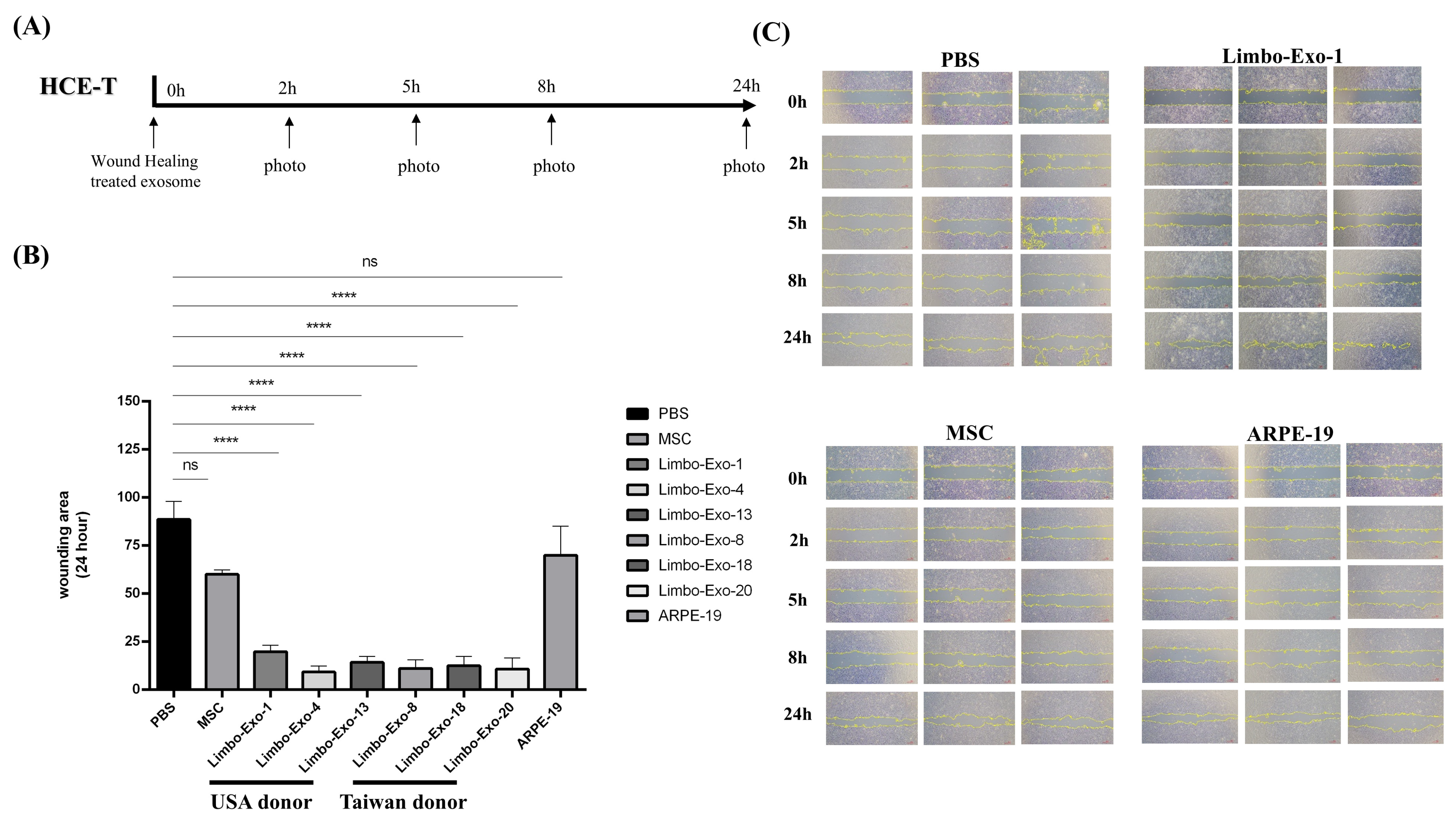
2.6. Wound Healing Test with HCE-T Cell Lines
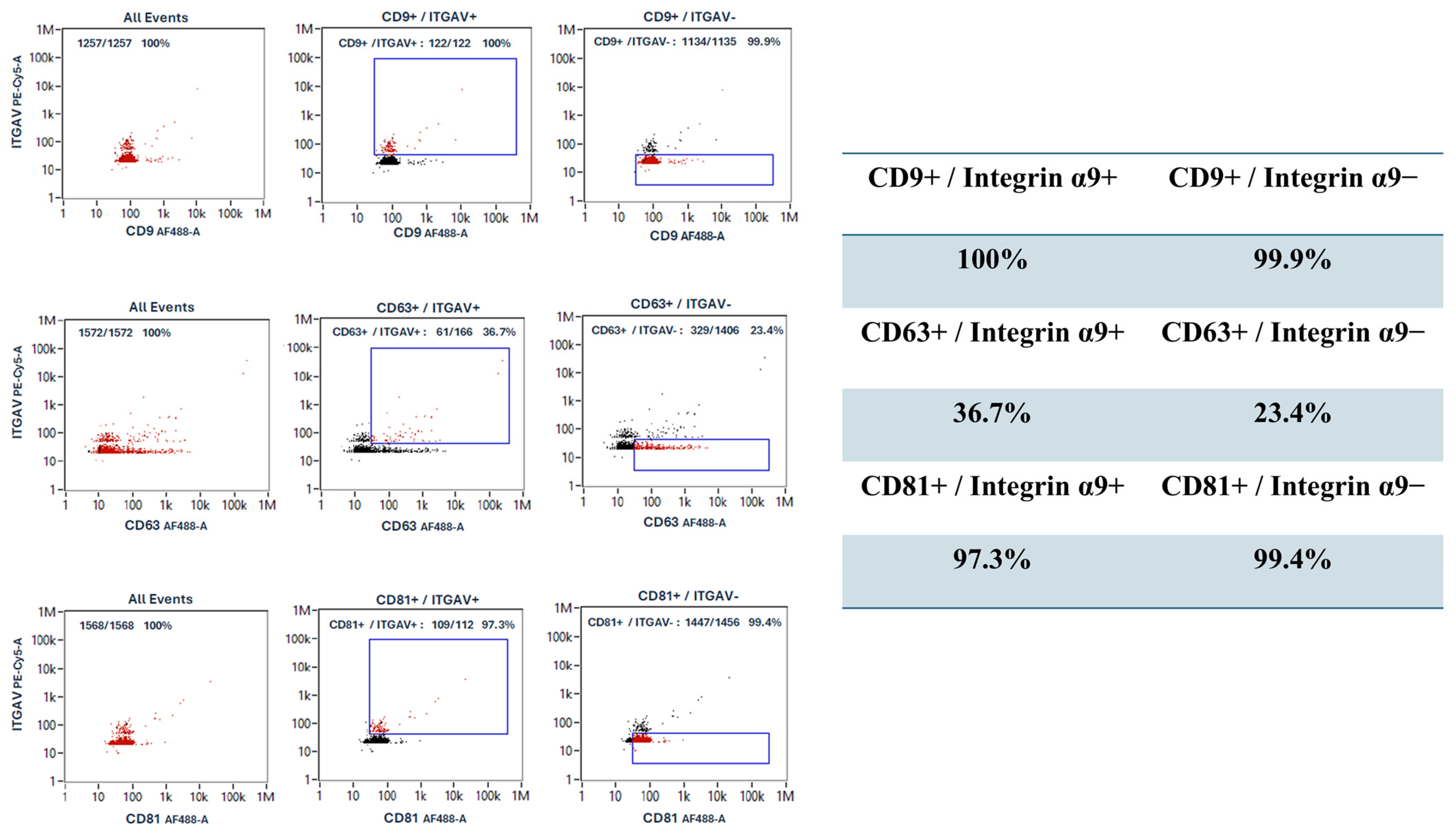
2.7. Nanoparticle Flow Cytometry (NanoFCM)
2.8. Statistical Analysis
3. Results
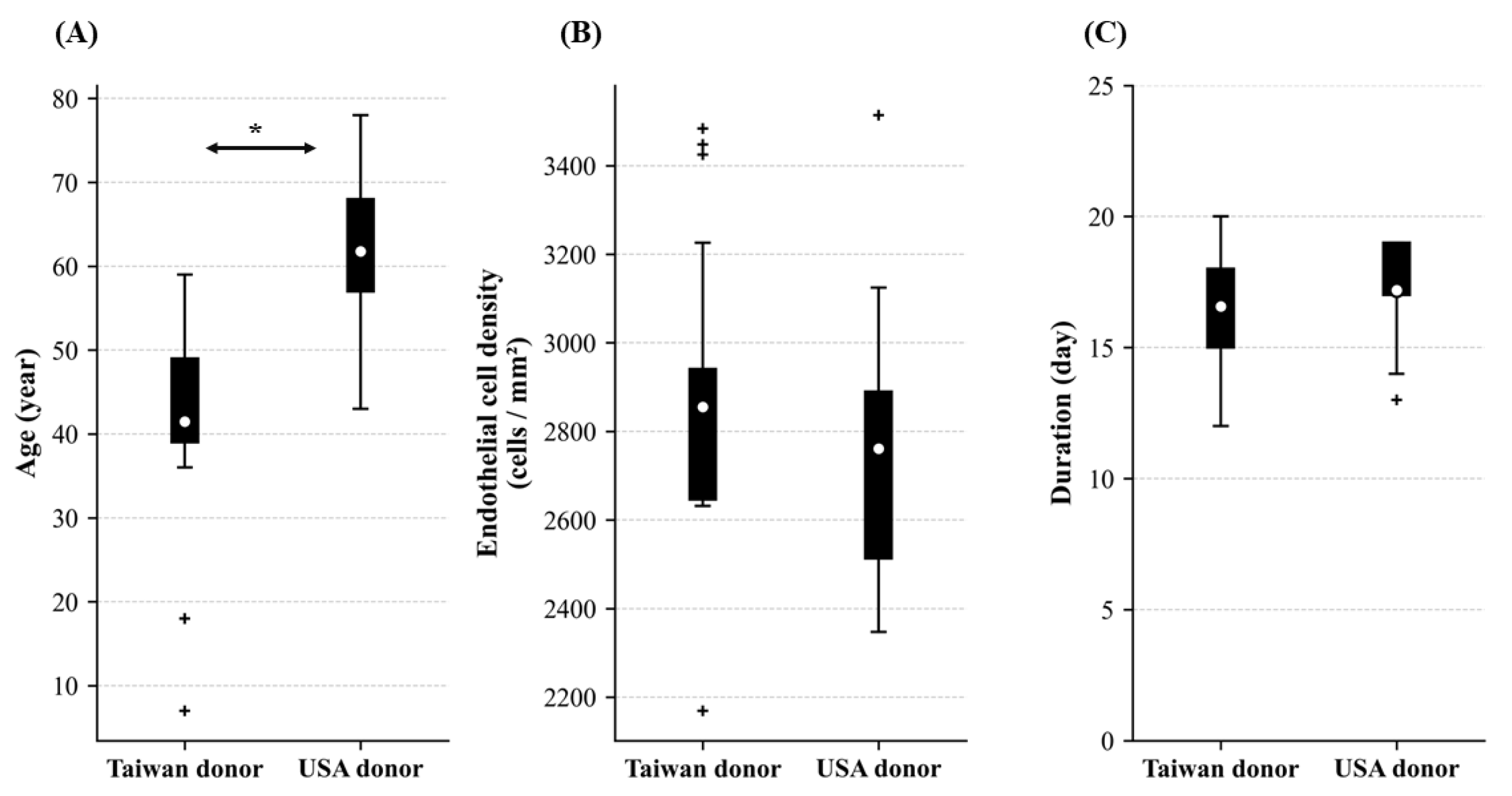
3.1. Baseline Information of Cornea Donors
3.2. The ECD and Extracellular Vesicle Extraction Duration in Donors from Taiwan and the USA
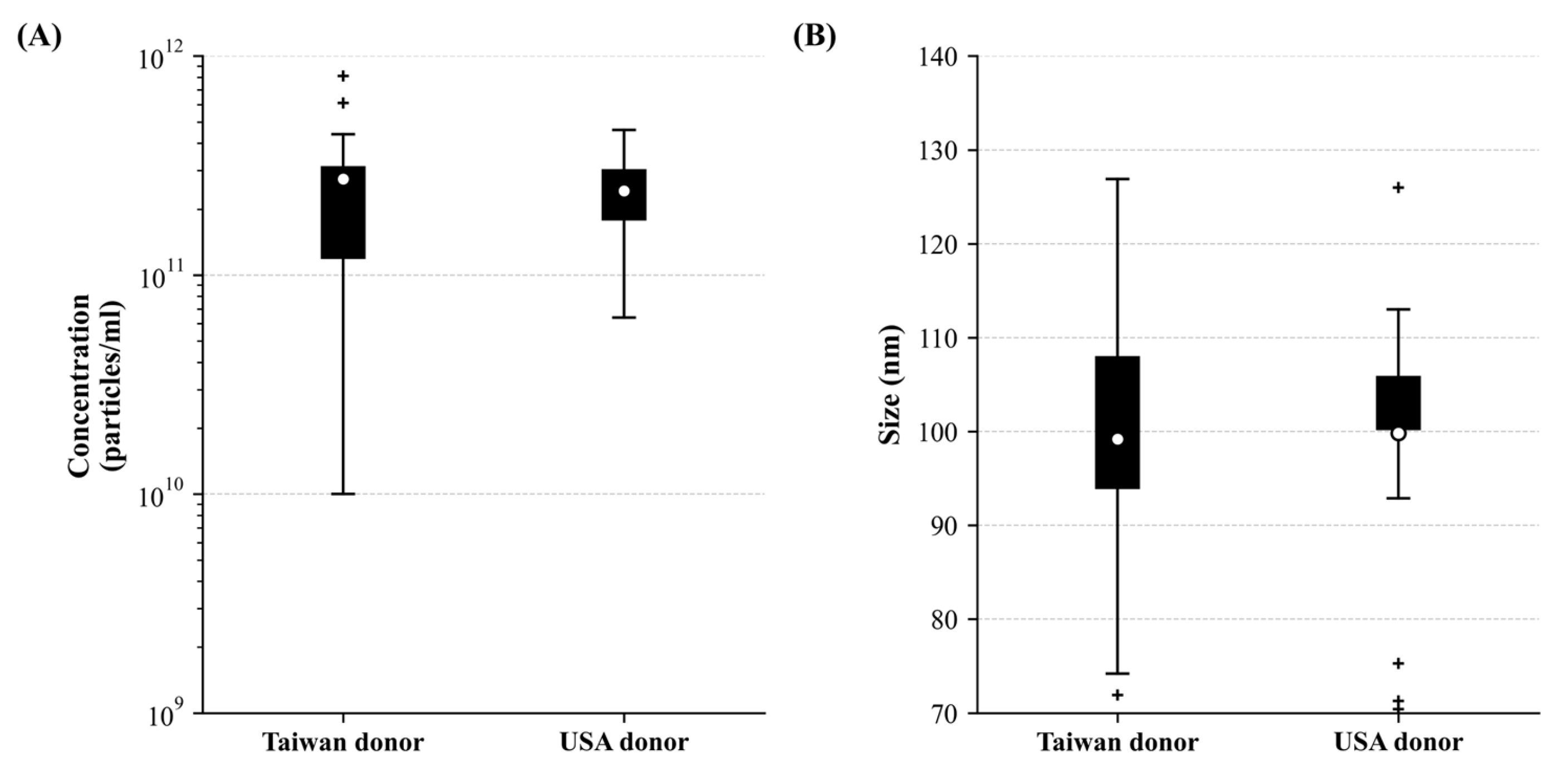
3.3. The High Yield of Extracellular Vesicles Derived from the Corneoscleral Rings
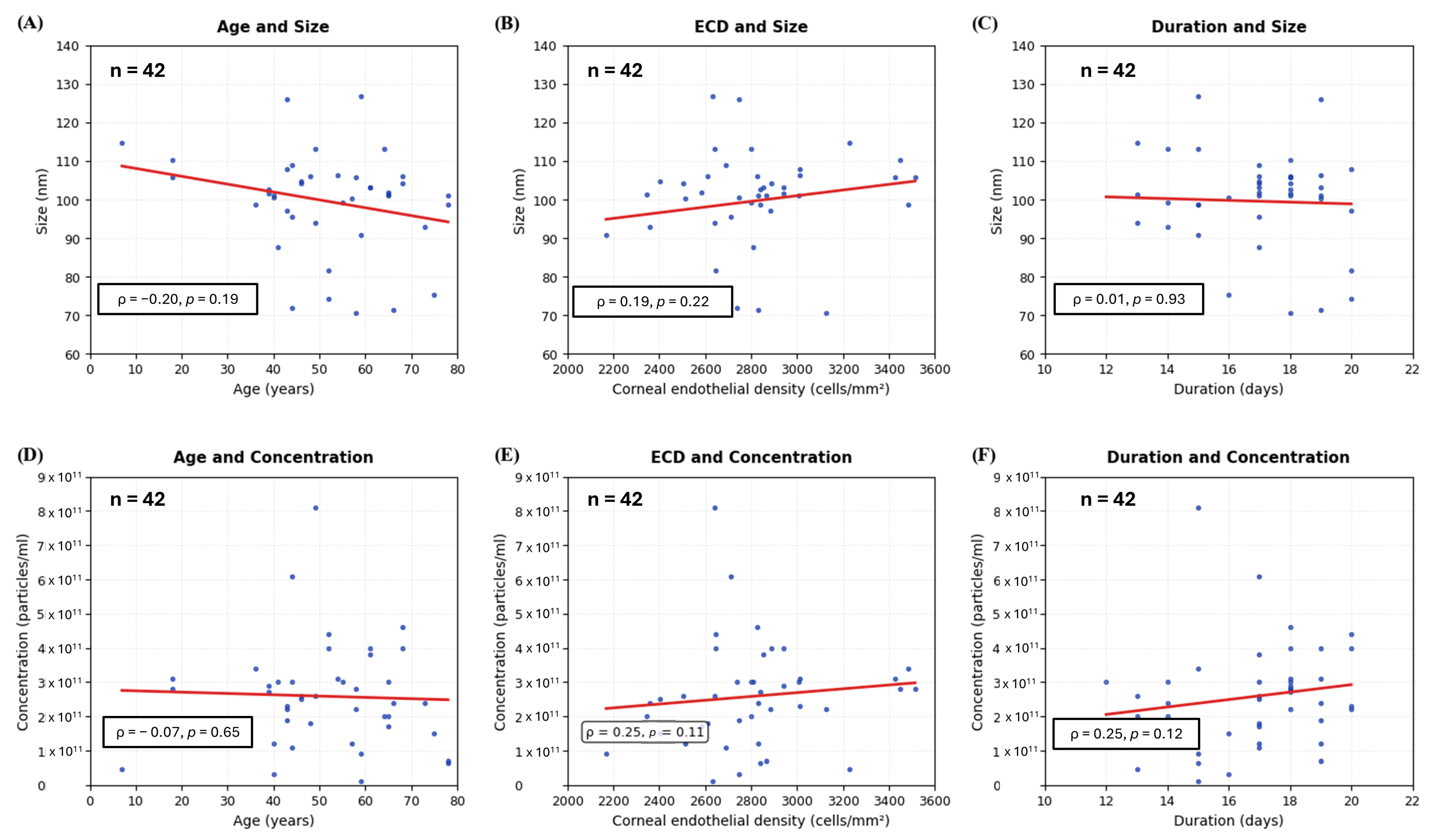
3.4. The Correlation and Coefficient of Donor Variables on Extracellular Vesicles Size/Concentration
3.5. Heterogeneity of Tetraspanin Expression on Corneoscleral-Ring-Derived Extracellular Vesicles from Taiwan and USA Donors
3.6. The Corneoscleral-Ring-Derived Extracellular Vesicles Presented Expression of LSC Markers
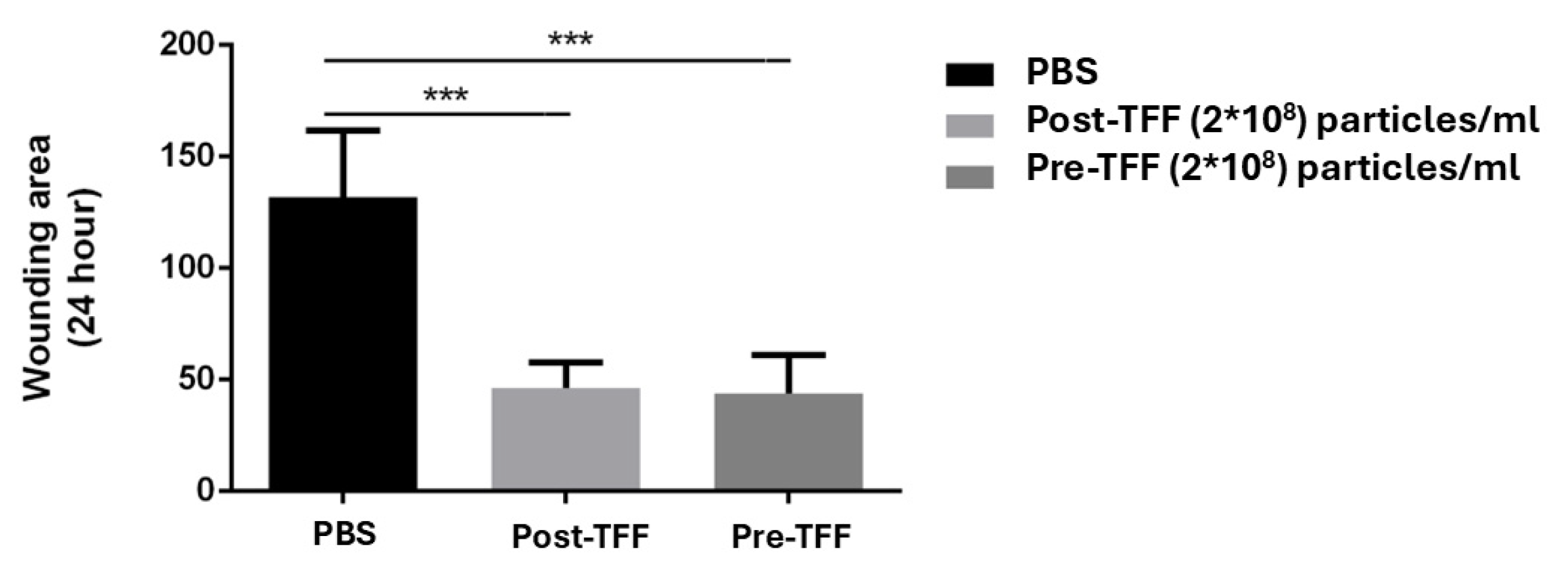
3.7. Wound Healing Promotion by Corneoscleral-Ring-Derived Extracellular Vesicles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| AMD | Age-related macular degeneration |
| DPBS | Dulbecco’s phosphate-buffered saline |
| DLS | Dynamic light scattering |
| dUC | Differential ultracentrifugation |
| ECD | Endothelial cell density |
| HCET | Human Corneal Epithelial-transformed cell |
| LSC | Limbal stem cell |
| MEM α | Minimum essential medium α |
| MSC | Mesenchymal stem cell |
| MVBs | Multivesicular Bodies |
| NanoFCM | Nanoparticle flow cytometry |
| NTA | Nanoparticle tracking analysis |
| PBS | Phosphate-buffered saline |
| RPE | Retinal pigment epithelia |
| TEM | Transmission electron microscopy |
| TFF | Tangential flow filtration |
| UA | Uranyl acetate |
References
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Kramerov, A.A.; Svendsen, C.N.; Ljubimov, A.V. Concise Review: Stem Cells for Corneal Wound Healing. Stem Cells 2017, 35, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; González, S.; Roberts, J.S.; Robertson, S.Y.T.; Ruiz, M.; Zheng, J.; Deng, S.X. Human limbal epithelial stem cell regulation, bioengineering and function. Prog. Retin. Eye Res. 2021, 85, 100956. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Alcoholado, C.; Martín-Astorga, M.D.C.; Rico, G.; Fernández, V.; Becerra, J. Characterization of the secretory profile and exosomes of limbal stem cells in the canine species. PLoS ONE 2020, 15, e0244327. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Ou, S.; Zhu, C.; Lin, S.; Liu, X.; Liang, M.; Yu, J.; Wu, Y.; He, H.; Zong, R.; et al. Downregulation of p38 MAPK Signaling Pathway Ameliorates Tissue-Engineered Corneal Epithelium. Tissue Eng. Part A 2022, 28, 977–989. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Yin, J.; Hao, L.; Diao, Y.; Zhong, J. Exosomes and autophagy in ocular surface and retinal diseases: New insights into pathophysiology and treatment. Stem Cell Res. Ther. 2022, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W.; et al. miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hou, Y.; Li, X.; Song, Z.; Sun, B.; Li, X.; Zhang, H. Comparison of exosomes derived from induced pluripotent stem cells and mesenchymal stem cells as therapeutic nanoparticles for treatment of corneal epithelial defects. Aging 2020, 12, 19546–19562. [Google Scholar] [CrossRef] [PubMed]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Braunsperger, M.V.; Martin, G.; Herzig, T.; Kußberger, I.; Gießl, A.; Steimle, S.; Schlötzer-Schrehardt, U.; Schlunck, G.; Reinhard, T.; Polisetti, N. Proteomic Insights into Human Limbal Epithelial Progenitor-Derived Small Extracellular Vesicles. Stem Cell Rev. Rep. 2025, 21, 1578–1593. [Google Scholar] [CrossRef]
- Hynds, R.E.; Bonfanti, P.; Janes, S.M. Regenerating human epithelia with cultured stem cells: Feeder cells, organoids and beyond. EMBO Mol. Med. 2018, 10, 139–150. [Google Scholar] [CrossRef]
- Meyer-Blazejewska, E.A.; Kruse, F.E.; Bitterer, K.; Meyer, C.; Hofmann-Rummelt, C.; Wünsch, P.H.; Schlötzer-Schrehardt, U. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Investig. Ophthalmol. Vis. Sci. 2010, 51, 765–774. [Google Scholar] [CrossRef]
- Zekušić, M.; Bujić Mihica, M.; Skoko, M.; Vukušić, K.; Risteski, P.; Martinčić, J.; Tolić, I.M.; Bendelja, K.; Ramić, S.; Dolenec, T.; et al. New characterization and safety evaluation of human limbal stem cells used in clinical application: Fidelity of mitotic process and mitotic spindle morphologies. Stem Cell Res. Ther. 2023, 14, 368. [Google Scholar] [CrossRef]
- Sahoo, A.; Damala, M.; Jaffet, J.; Prasad, D.; Basu, S.; Singh, V. Expansion and characterization of human limbus-derived stromal/mesenchymal stem cells in xeno-free medium for therapeutic applications. Stem Cell Res. Ther. 2023, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, H.; Chen, L.; Fu, Y. Targeting limbal epithelial stem cells: Master conductors of corneal epithelial regeneration from the bench to multilevel theranostics. J. Transl. Med. 2024, 22, 794. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Tiwari, A.; Kethiri, A.R.; Sangwan, V.S. Current perspectives of limbal-derived stem cells and its application in ocular surface regeneration and limbal stem cell transplantation. Stem Cells Transl. Med. 2021, 10, 1121–1128. [Google Scholar] [CrossRef]
- Jeng, B.H. Preserving the cornea: Corneal storage media. Curr. Opin. Ophthalmol. 2006, 17, 332–337. [Google Scholar] [CrossRef]
- Okada-Tsuchioka, M.; Kajitani, N.; Omori, W.; Kurashige, T.; Boku, S.; Takebayashi, M. Tetraspanin heterogeneity of small extracellular vesicles in human biofluids and brain tissue. Biochem. Biophys. Res. Commun. 2022, 627, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, K.; Koch, L.F.; Tertel, T.; Proestler, E.; Burgers, L.D.; Lipps, C.; Adjaye, J.; Fürst, R.; Giebel, B.; Saul, M.J. Detailed Characterization of Small Extracellular Vesicles from Different Cell Types Based on Tetraspanin Composition by ExoView R100 Platform. Int. J. Mol. Sci. 2022, 23, 8544. [Google Scholar] [CrossRef] [PubMed]
- Chee, K.Y.; Kicic, A.; Wiffen, S.J. Limbal stem cells: The search for a marker. Clin. Exp. Ophthalmol. 2006, 34, 64–73. [Google Scholar] [CrossRef]
- Wang, D.Y.; Hsueh, Y.J.; Yang, V.C.; Chen, J.K. Propagation and phenotypic preservation of rabbit limbal epithelial cells on amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4698–4704. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Barbaro, V.; Ruzza, A.; Ponzin, D.; Pellegrini, G.; De Luca, M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; de Paiva, C.S.; Luo, L.; Kretzer, F.L.; Pflugfelder, S.C.; Li, D.Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 2004, 22, 355–366. [Google Scholar] [CrossRef]
- Thomas, P.B.; Liu, Y.H.; Zhuang, F.F.; Selvam, S.; Song, S.W.; Smith, R.E.; Trousdale, M.D.; Yiu, S.C. Identification of Notch-1 expression in the limbal basal epithelium. Mol. Vis. 2007, 13, 337–344. [Google Scholar] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Li, N.; Zhao, L.; Wei, Y.; Ea, V.L.; Nian, H.; Wei, R. Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res. Ther. 2019, 10, 278. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Saini, J.S.; Azuara-Blanco, A.; Gupta, P. Limbal stem cell deficiency: Concept, aetiology, clinical presentation, diagnosis and management. Indian J. Ophthalmol. 2000, 48, 83–92. [Google Scholar] [PubMed]
- Knickelbein, J.E.; Liu, B.; Arakelyan, A.; Zicari, S.; Hannes, S.; Chen, P.; Li, Z.; Grivel, J.C.; Chaigne-Delalande, B.; Sen, H.N.; et al. Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4101–4107. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef] [PubMed]
- Zabeo, D.; Cvjetkovic, A.; Lässer, C.; Schorb, M.; Lötvall, J.; Höög, J.L. Exosomes purified from a single cell type have diverse morphology. J. Extracell. Vesicles 2017, 6, 1329476. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, C.M.; Zheng, R.; Kenney, J.; Van De Water, L. Integrin-mediated regulation of epidermal wound functions. Cell Tissue Res. 2016, 365, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, T.; Cao, Z.; Zhong, W.; Zhang, C.; Li, H.; Song, J. Integrin α9β1 as a Novel Therapeutic Target for Refractory Diseases: Recent Progress and Insights. Front. Immunol. 2021, 12, 638400. [Google Scholar] [CrossRef]
- Hight-Warburton, W.; Felix, R.; Burton, A.; Maple, H.; Chegkazi, M.S.; Steiner, R.A.; McGrath, J.A.; Parsons, M. α4/α9 Integrins Coordinate Epithelial Cell Migration Through Local Suppression of MAP Kinase Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 750771. [Google Scholar] [CrossRef]
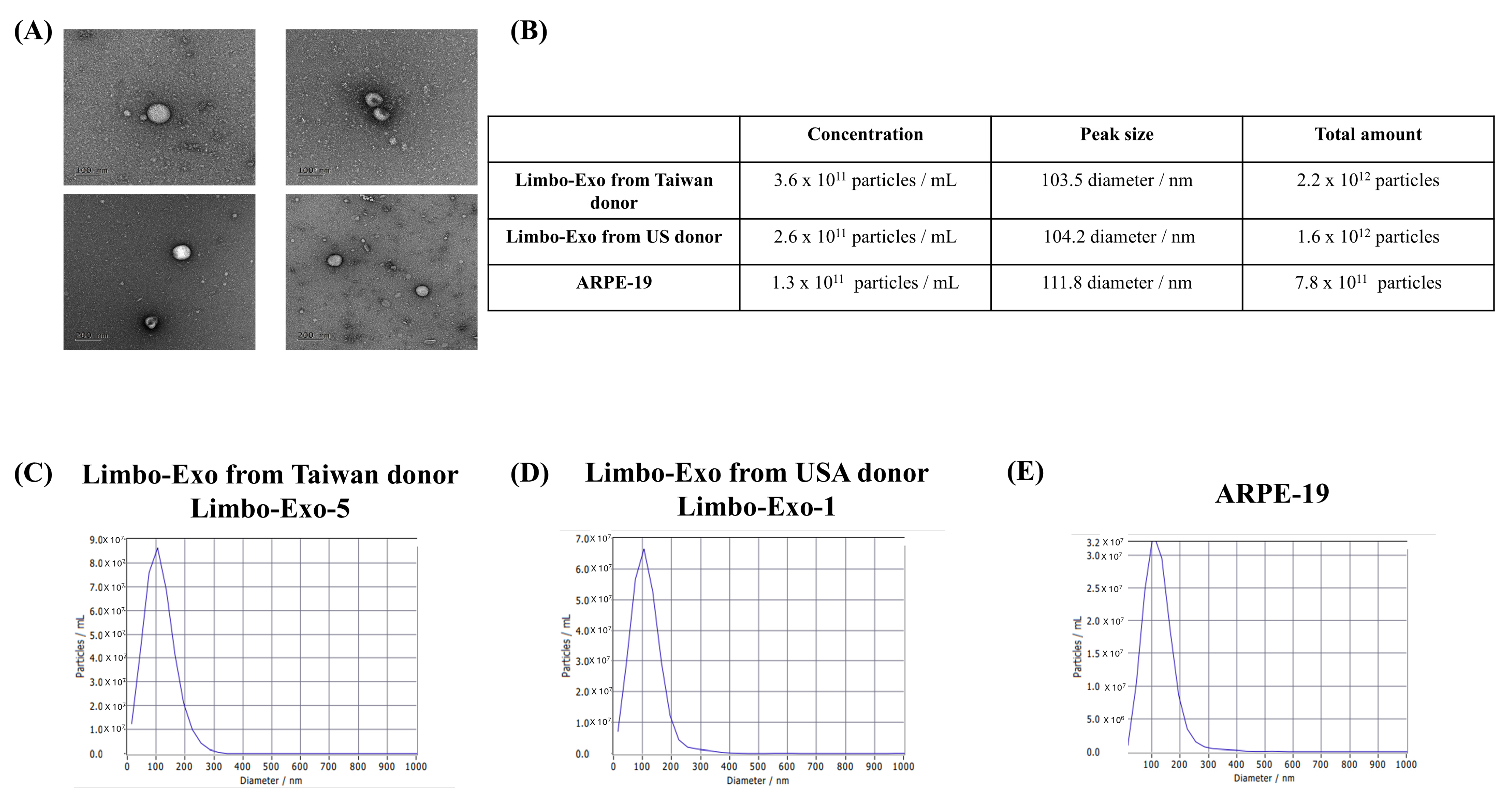
| Limbo-Exo No. | Origin | Age | Sex | Primary Cause of Death | Corneal Endothelial Density (Cells/mm2) | Duration a | Extracellular Vesicles Concentration (Particles/mL) | Extracellular Vesicles Peak Size (Diameter/nm) | PDI | Span |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | US | 64 | unknown | liver CA | 2801 | 14 | 113 | 2 × 1011 | 0.37 | 1.40 |
| 2 | US | 65 | F | CHF | 3006 | 18 | 101 | 3× 1011 | 0.35 | 1.60 |
| 3 | US | 61 | M | CHF | 2942 | 19 | 103.1 | 4 × 1011 | 0.34 | 1.47 |
| 4 | US | 68 | unknown | SAH | 2890 | 18 | 104.1 | 4 × 1011 | 0.24 | 1.37 |
| 5 | Taiwan | 44 | M | SAH | 2710 | 17 | 95.5 | 6.1 × 1011 | 0.19 | 1.37 |
| 6 | Taiwan | 7 | F | Thrombocytopenia | 3226 | 13 | 114.8 | 4.8 × 1010 | 0.18 | 1.16 |
| 7 | Taiwan | 40 | M | EDH | 2747 | 16 | 100.5 | 3.2 × 1010 | 0.22 | 1.23 |
| 8 | Taiwan | 59 | F | cancer | 2169 | 15 | 90.7 | 9.0 × 1010 | 0.27 | 1.87 |
| 9 | US | 61 | M | CHF | 2855 | 17 | 103.2 | 3.8 × 1011 | 0.27 | 1.46 |
| 10 | US | 68 | unknown | SAH | 2825 | 18 | 106 | 4.6 × 1011 | 0.27 | 1.41 |
| 11 | US | 73 | unknown | lung CA | 2358 | 14 | 92.9 | 2.4 × 1011 | 0.25 | 1.44 |
| 12 | US | 78 | F | dyspnea | 2841 | 15 | 98.6 | 6.4 × 1010 | 0.30 | 1.53 |
| 13 | US | 78 | F | dyspnea | 2865 | 19 | 101.1 | 7 × 1010 | 0.59 | 1.76 |
| 14 | Taiwan | 44 | M | SAH | 2688 | 17 | 109.0 | 1.1 × 1011 | 0.23 | 1.33 |
| 15 | Taiwan | 40 | M | cancer | 2833 | 17 | 100.9 | 1.20 × 1011 | 0.21 | 1.53 |
| 16 | Taiwan | 59 | F | cancer | 2632 | 15 | 126.9 | 1.0 × 1010 | 0.41 | 2.06 |
| 17 | Taiwan | 18 | M | SAH | 3448 | 18 | 110.2 | 2.80 × 1011 | 0.25 | 1.29 |
| 18 | Taiwan | 18 | M | SAH | 3425 | 18 | 105.8 | 3.10 × 1011 | 0.31 | 1.42 |
| 19 | Taiwan | 39 | M | spine tumor | 2841 | 18 | 102.5 | 2.70 × 1011 | 0.20 | 1.30 |
| 20 | Taiwan | 39 | M | spine tumor | 2941 | 18 | 101.5 | 2.90 × 1011 | 0.23 | 1.29 |
| 21 | Taiwan | 49 | M | amyotrophic lateral sclerosis | 2639 | 13 | 94 | 2.60 × 1011 | 0.21 | 1.35 |
| 22 | Taiwan | 44 | M | respiratory failure | 2740 | 12 | 71.9 | 3.00 × 1011 | 0.43 | 1.43 |
| 23 | Taiwan | 41 | M | respiratory failure | 2809 | 17 | 87.7 | 3.00 × 1011 | 0.27 | 1.56 |
| 24 | Taiwan | 52 | M | respiratory failure | 2646 | 20 | 74.2 | 4.00 × 1011 | 0.29 | 1.74 |
| 25 | Taiwan | 52 | M | respiratory failure | 2646 | 20 | 81.6 | 4.40 × 1011 | 0.25 | 1.19 |
| 26 | Taiwan | 55 | M | cancer | 2801 | 14 | 99.1 | 3.00 × 1011 | 0.26 | 1.39 |
| 27 | Taiwan | 36 | M | brain tumor | 3484 | 15 | 98.6 | 3.40 × 1011 | 0.17 | 1.28 |
| 28 | Taiwan | 49 | F | breast cancer | 2639 | 15 | 113.1 | 8.10 × 1011 | 0.23 | 1.25 |
| 29 | Taiwan | 43 | M | hypoxia | 2882 | 20 | 97.1 | 2.20 × 1011 | 0.32 | 0.97 |
| 30 | Taiwan | 43 | M | hypoxia | 3012 | 20 | 107.9 | 2.30 × 1011 | 0.32 | 1.13 |
| 31 | US | 65 | unknown | NSTEMI | 2347 | 13 | 101.3 | 2 × 1011 | 0.13 | 0.89 |
| 32 | US | 57 | unknown | COPD | 2513 | 19 | 100.3 | 1.2 × 1011 | 0.14 | 1.08 |
| 33 | US | 75 | unknown | CHF | 2404 | 16 | 75.3 | 1.5 × 1011 | 0.15 | 1.11 |
| 34 | US | 48 | unknown | pneumonia | 2611 | 17 | 106.1 | 1.8 × 1011 | 0.22 | 1.21 |
| 35 | US | 46 | unknown | pulmonary embolism | 2506 | 17 | 104.2 | 2.6 × 1011 | 0.23 | 1.31 |
| 36 | US | 65 | unknown | ESRD | 2584 | 17 | 101.8 | 1.7 × 1011 | 0.19 | 1.34 |
| 37 | US | 46 | unknown | pulmonary embolism | 2404 | 17 | 104.8 | 2.5 × 1011 | 0.24 | 1.40 |
| 38 | US | 66 | unknown | CHF | 2833 | 19 | 71.3 | 2.4 × 1011 | 0.25 | 1.11 |
| 39 | US | 43 | M | ICH | 2747 | 19 | 126 | 1.9 × 1011 | 0.17 | 1.09 |
| 40 | US | 58 | M | pulmonary fibrosis | 3125 | 18 | 70.4 | 2.2 × 1011 | 0.21 | 1.00 |
| 41 | US | 58 | M | pulmonary fibrosis | 3514 | 18 | 105.8 | 2.8 × 1011 | 0.31 | 1.64 |
| 42 | US | 54 | unknown | trauma | 3012 | 19 | 106.2 | 3.1 × 1011 | 0.30 | 1.43 |
| Mean | 51.62 ± 15.56 | 2808.12 ± 304.20 | 16.88 ± 2.15 | 99.52 ± 13.00 | 2.58 × 1011 ± 1.53 × 1011 | 0.26 ± 0.08 | 1.36 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.-Y.; Hsieh, M.-C.; Wu, H.-H.; Lee, C.-W.; Liu, S.-H.; Lin, H.-Y.; Chen, Y.-W.; Chiang, C.-C.; Hsieh, Y.-C.; Wu, Y.-H.; et al. Isolation and Characterization of Integrin α9 Positive Extracellular Vesicles Derived from Human Corneoscleral Rings. Life 2025, 15, 1780. https://doi.org/10.3390/life15111780
Lai H-Y, Hsieh M-C, Wu H-H, Lee C-W, Liu S-H, Lin H-Y, Chen Y-W, Chiang C-C, Hsieh Y-C, Wu Y-H, et al. Isolation and Characterization of Integrin α9 Positive Extracellular Vesicles Derived from Human Corneoscleral Rings. Life. 2025; 15(11):1780. https://doi.org/10.3390/life15111780
Chicago/Turabian StyleLai, Hung-Yin, Ming-Chieh Hsieh, Hao-Hsiang Wu, Chien-Wei Lee, Shih-Hua Liu, Hsing-Yu Lin, Yi-Wen Chen, Chun-Chi Chiang, Yi-Ching Hsieh, Ying-Hsuen Wu, and et al. 2025. "Isolation and Characterization of Integrin α9 Positive Extracellular Vesicles Derived from Human Corneoscleral Rings" Life 15, no. 11: 1780. https://doi.org/10.3390/life15111780
APA StyleLai, H.-Y., Hsieh, M.-C., Wu, H.-H., Lee, C.-W., Liu, S.-H., Lin, H.-Y., Chen, Y.-W., Chiang, C.-C., Hsieh, Y.-C., Wu, Y.-H., Li, Y.-L., Tung, H.-F., Ho, J. H.-C., & Tsai, Y.-Y. (2025). Isolation and Characterization of Integrin α9 Positive Extracellular Vesicles Derived from Human Corneoscleral Rings. Life, 15(11), 1780. https://doi.org/10.3390/life15111780







