Retrospective Analysis of Angiographic Radial Artery Spasm Predictors
Abstract
1. Introduction
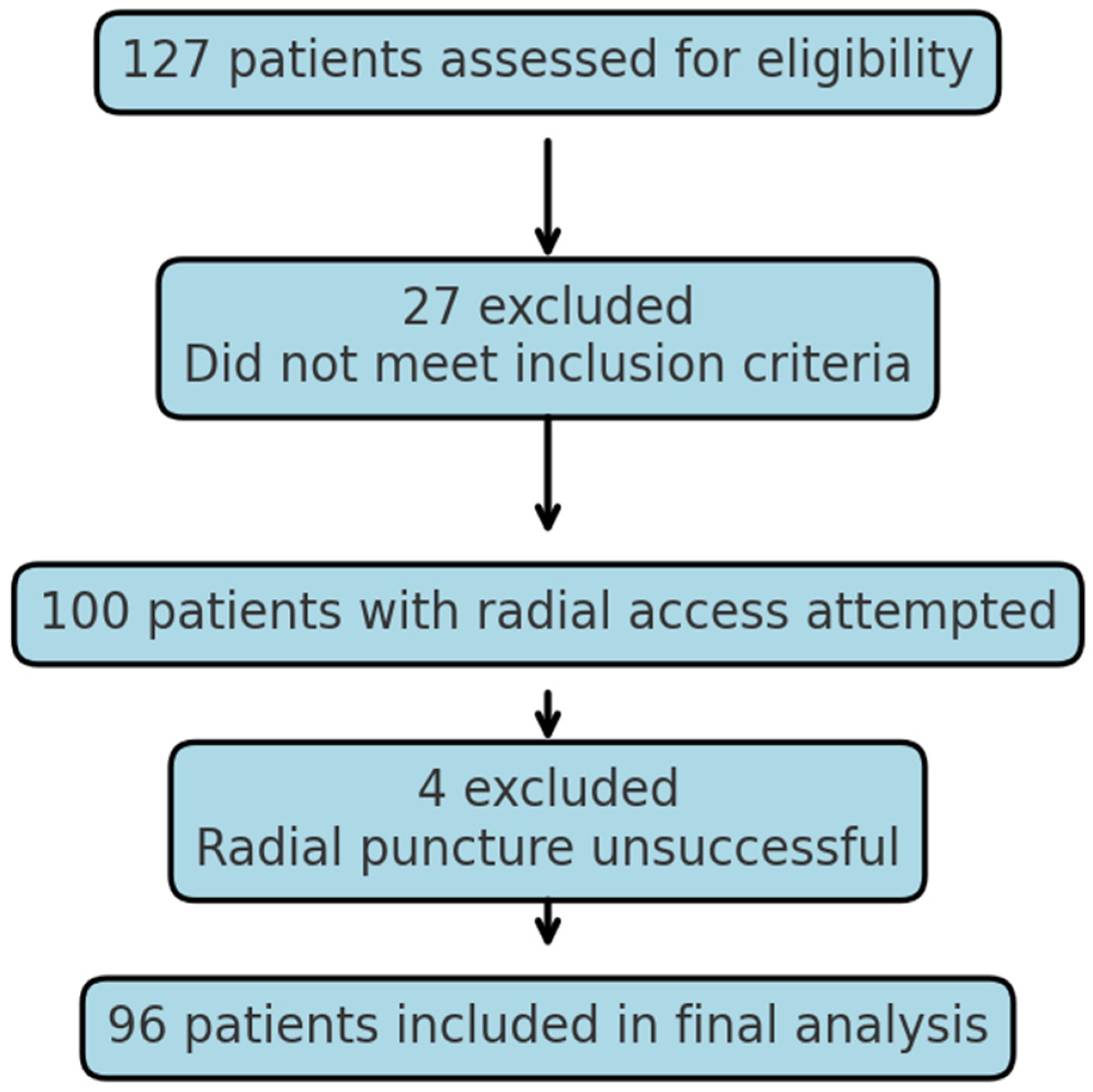
2. Materials and Methods
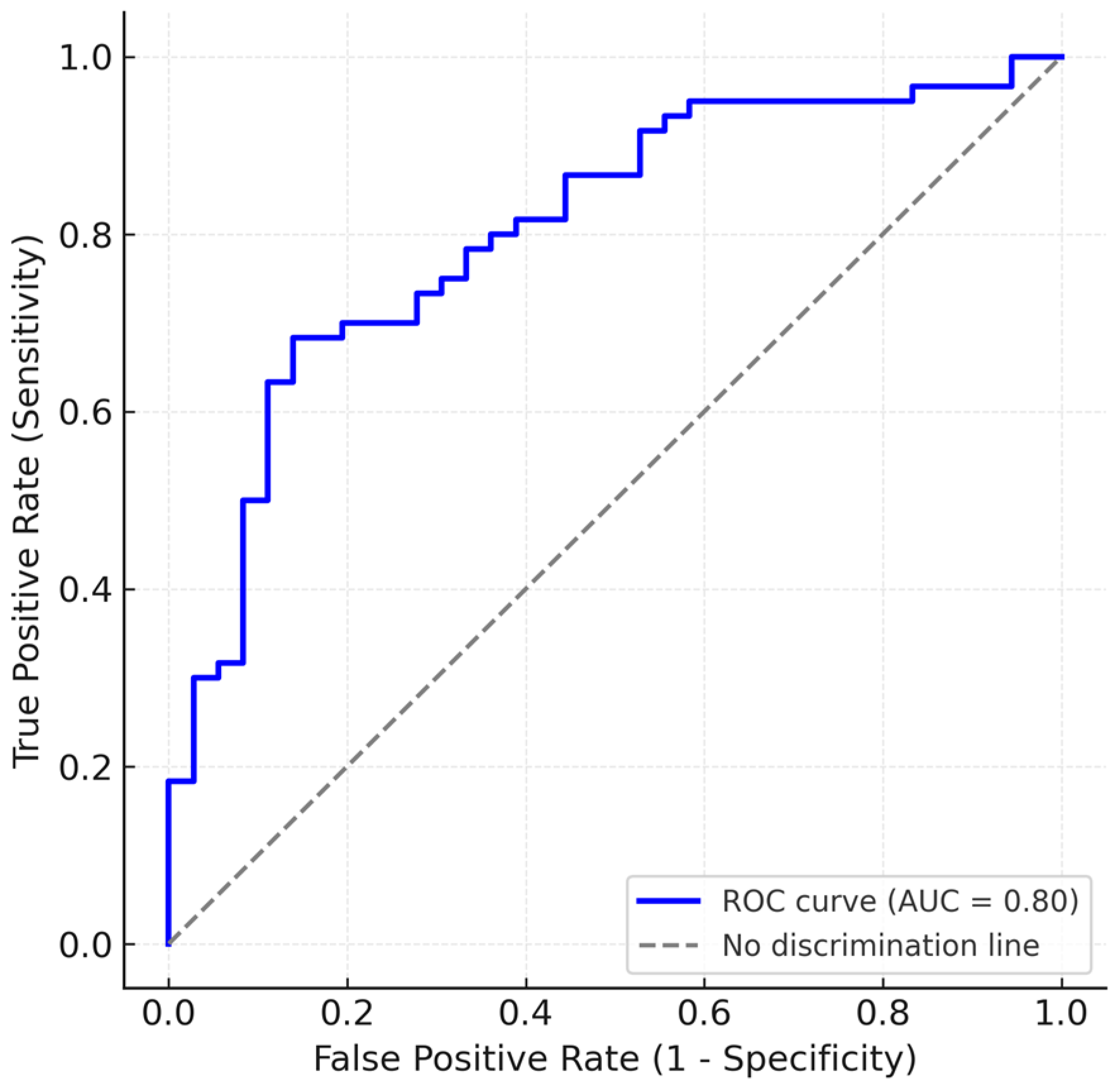
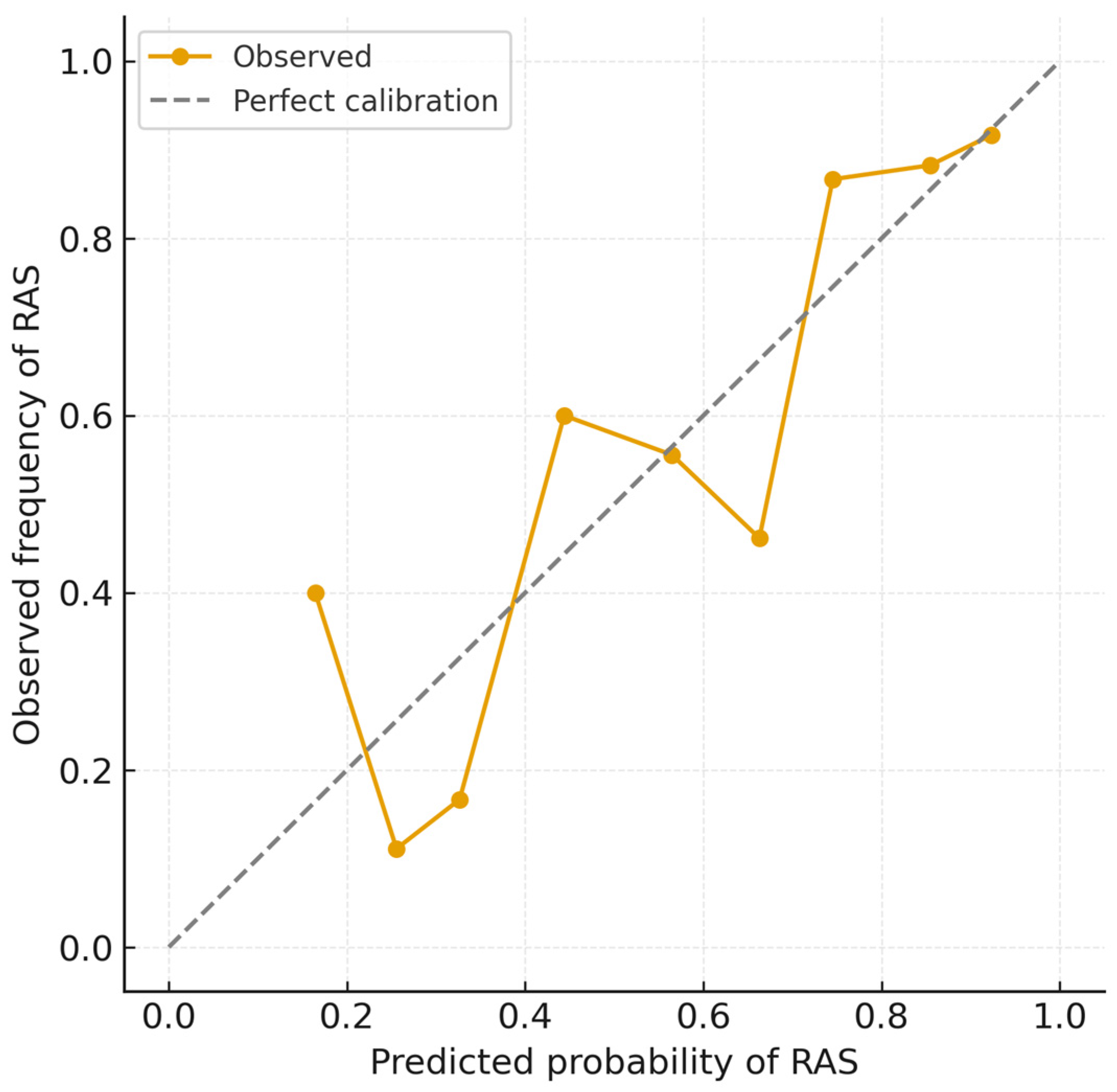
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under curve |
| CI | confidence interval. |
| DBP | diastolic blood pressure. |
| DM | diabetes mellitus. |
| HR | heart rate. |
| IQR | interquartile range. |
| RAS | radial artery spasm. |
| ROC | receiver operating characteristics |
| SBP | systolic blood pressure. |
| SD | standard deviation. |
References
- Chiarito, M.; Cao, D.; Nicolas, J.; Roumeliotis, A.; Power, D.; Chandiramani, R.; Sartori, S.; Camaj, A.; Goel, R.; Claessen, B.E.; et al. Radial versus Femoral Access for Coronary Interventions: An Updated Systematic Review and Meta-analysis of Randomized Trials. Catheter. Cardiovasc. Interv. 2021, 97, 1387–1396. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Hamon, M.; Pristipino, C.; Di Mario, C.; Nolan, J.; Ludwig, J.; Tubaro, M.; Sabate, M.; Mauri-Ferré, J.; Huber, K.; Niemelä, K.; et al. Consensus Document on the Radial Approach in Percutaneous Cardiovascular Interventions: Position Paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention 2013, 8, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, E.J.; Cheon, W.S.; Kim, M.-K.; Park, W.J.; Cho, G.-Y.; Choi, Y.J.; Rhim, C.Y. Comparative Study of Nicorandil and a Spasmolytic Cocktail in Preventing Radial Artery Spasm During Transradial Coronary Angiography. Int. J. Cardiol. 2007, 120, 325–330. [Google Scholar] [CrossRef]
- Ruiz-Salmerón, R.J.; Mora, R.; Vélez-Gimón, M.; Ortiz, J.; Fernández, C.; Vidal, B.; Masotti, M.; Betriu, A. Radial artery spasm in transradial cardiac catheterization. Assessment of factors related to its occurrence, and of its consequences during follow-up. Rev. Esp. Cardiol. 2005, 58, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Dowling, C.; Zaman, S.; Cameron, J.; Kuhn, L. Predictors of Radial to Femoral Artery Crossover During Primary Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis. Aust. Crit. Care 2023, 36, 915–923. [Google Scholar] [CrossRef]
- Da Silva, R.L.; De Andrade, P.B.; Dangas, G.; Joaquim, R.M.; Da Silva, T.R.W.; Vieira, R.G.; Pereira, V.C.; Sousa, A.G.M.; Feres, F.; Costa, J.R. Randomized Clinical Trial on Prevention of Radial Occlusion After Transradial Access Using Nitroglycerin. JACC Cardiovasc. Interv. 2022, 15, 1009–1018. [Google Scholar] [CrossRef]
- Sandoval, Y.; Bell, M.R.; Gulati, R. Transradial Artery Access Complications. Circ. Cardiovasc. Interv. 2019, 12, e007386. [Google Scholar] [CrossRef]
- Shroff, A.R.; Gulati, R.; Drachman, D.E.; Feldman, D.N.; Gilchrist, I.C.; Kaul, P.; Lata, K.; Pancholy, S.B.; Panetta, C.J.; Seto, A.H.; et al. SCAI Expert Consensus Statement Update on Best Practices for Transradial Angiography and Intervention. Catheter. Cardiovasc. Interv. 2020, 95, 245–252. [Google Scholar] [CrossRef]
- Roczniak, J.; Tarnawski, A.; Dziewierz, A.; Glanowski, S.; Pawlik, A.; Sabatowski, K.; Januszek, R.; Rzeszutko, Ł.; Surdacki, A.; Bartuś, S.; et al. Radial artery spasms—Angiographic morphology, risk factors and management. Adv. Interv. Cardiol. 2024, 20, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Fernandez, R.; Khoo, J.; Weaver, J.; Lee, A.; Halcomb, E. Clinical Predictors and Management for Radial Artery Spasm: An Australian Cross-Sectional Study. BMC Cardiovasc. Disord. 2023, 23, 33. [Google Scholar] [CrossRef]
- Meng, S.; Guo, Q.; Tong, G.; Shen, Y.; Tong, X.; Gu, J.; Li, X. Development and Validation of a Nomogram for Predicting Radial Artery Spasm During Coronary Angiography. Angiology 2023, 74, 242–251. [Google Scholar] [CrossRef]
- Gragnano, F.; Jolly, S.S.; Mehta, S.R.; Branca, M.; Van Klaveren, D.; Frigoli, E.; Gargiulo, G.; Leonardi, S.; Vranckx, P.; Di Maio, D.; et al. Prediction of Radial Crossover in Acute Coronary Syndromes: Derivation and Validation of the MATRIX Score. EuroIntervention 2021, 17, e971–e980. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Raisakis, K.; Synetos, A.; Davlouros, P.; Hahalis, G.; Alexopoulos, D.; Tousoulis, D.; Lekakis, J.; Stefanadis, C.; Cleman, M.W.; et al. A Predictive Score of Radial Artery Spasm in Patients Undergoing Transradial Percutaneous Coronary Intervention. Int. J. Cardiol. 2015, 188, 76–80. [Google Scholar] [CrossRef]
- Gorgulu, S.; Norgaz, T.; Karaahmet, T.; Dagdelen, S. Incidence and Predictors of Radial Artery Spasm at the Beginning of A Transradial Coronary Procedure. J. Interv. Cardiol. 2013, 26, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Numasawa, Y.; Kawamura, A.; Kohsaka, S.; Takahashi, M.; Endo, A.; Arai, T.; Ohno, Y.; Yuasa, S.; Maekawa, Y.; Fukuda, K. Anatomical variations affect radial artery spasm and procedural achievement of transradial cardiac catheterization. Heart Vessel. 2014, 29, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pishgahi, M.; Mehrabi, M.A.; Adeli, M. Incidence Rate and Risk Factors of Radial Artery Spasm During Transradial Coronary Angiography. Arch. Men’s Health 2021, 5, e31. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Raisakis, K.; Hahalis, G.; Kaoukis, A.; Kossyvakis, C.; Avramides, D.; Pappas, L.; Panagopoulou, V.; Pyrgakis, V.; et al. Moderate Procedural Sedation and Opioid Analgesia During Transradial Coronary Interventions to Prevent Spasm. JACC Cardiovasc. Interv. 2013, 6, 267–273. [Google Scholar] [CrossRef]
- Ercan, S.; Unal, A.; Altunbas, G.; Kaya, H.; Davutoglu, V.; Yuce, M.; Ozer, O. Anxiety Score as a Risk Factor for Radial Artery Vasospasm During Radial Interventions: A Pilot Study. Angiology 2014, 65, 67–70. [Google Scholar] [CrossRef]
- Da Silva, R.L.; Moreira, D.M.; Fattah, T.; da Conceição, R.S.; Trombetta, A.P.; Panata, L.; Thiago, L.E.K.S.; Giuliano, L.C. Pain Assessment During Transradial Catheterization Using the Visual Analogue Scale. Rev. Bras. De Cardiol. Invasiva (Engl. Ed.) 2015, 23, 207–210. [Google Scholar] [CrossRef]
- Dehghani, P.; Mohammad, A.; Bajaj, R.; Hong, T.; Suen, C.M.; Sharieff, W.; Chisholm, R.J.; Kutryk, M.J.B.; Fam, N.P.; Cheema, A.N. Mechanism and Predictors of Failed Transradial Approach for Percutaneous Coronary Interventions. JACC Cardiovasc. Interv. 2009, 2, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Trilla, M.; Freixa, X.; Regueiro, A.; Fernández-Rodriguez, D.; Brugaletta, S.; Martin-Yuste, V.; Jiménez, M.; Betriu, A.; Sabaté, M.; Masotti, M. Impact of Aging on Radial Spasm During Coronary Catheterization. J. Invasive Cardiol. 2015, 27, E303–E307. [Google Scholar] [PubMed]
- Bertrand, O.F.; Rao, S.V.; Pancholy, S.; Jolly, S.S.; Rodés-Cabau, J.; Larose, É.; Costerousse, O.; Hamon, M.; Mann, T. Transradial Approach for Coronary Angiography and Interventions. JACC Cardiovasc. Interv. 2010, 3, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Stables, R.H.; Pauriah, M.; Hakeem, A.; Mills, J.D.; Palmer, N.D.; Perry, R.A.; Morris, J.L. Impact of Length and Hydrophilic Coating of the Introducer Sheath on Radial Artery Spasm During Transradial Coronary Intervention. JACC Cardiovasc. Interv. 2010, 3, 475–483. [Google Scholar] [CrossRef]
- Goldsmit, A.; Kiemeneij, F.; Gilchrist, I.C.; Kantor, P.; Kedev, S.; Kwan, T.; Dharma, S.; Valdivieso, L.; Wenstemberg, B.; Patel, T. Radial Artery Spasm Associated with Transradial Cardiovascular Procedures: Results from the RAS Registry. Catheter. Cardiovasc. Interv. 2014, 83, E32–E36. [Google Scholar] [CrossRef] [PubMed]
- Dahm, J.B.; Wolpers, H.G.; Becker, J.; Hansen, C.; Felix, S.B. Transradial Access in Percutaneous Coronary Interventions: Technique and Procedure. Herz 2010, 35, 482–487. [Google Scholar] [CrossRef]
- Kotowycz, M.A.; Johnston, K.W.; Ivanov, J.; Asif, N.; Almoghairi, A.M.; Choudhury, A.; Nagy, C.D.; Sibbald, M.; Chan, W.; Seidelin, P.H.; et al. Predictors of Radial Artery Size in Patients Undergoing Cardiac Catheterization: Insights from the Good Radial Artery Size Prediction (GRASP) Study. Can. J. Cardiol. 2014, 30, 211–216. [Google Scholar] [CrossRef]
- Zencirci, E.; Değirmencioğlu, A. Can Radial Artery Pulse Grading Predict Radial Artery Spasm During Transradial Approach? Kardiol. Pol. 2017, 75, 360–367. [Google Scholar] [CrossRef]
- Ruiz-Salmerón, R.J.; Mora, R.; Masotti, M.; Betriu, A. Assessment of the Efficacy of Phentolamine to Prevent Radial Artery Spasm During Cardiac Catheterization Procedures: A Randomized Study Comparing Phentolamine vs. Verapamil. Cathet. Cardiovasc. Intervent. 2005, 66, 192–198. [Google Scholar] [CrossRef]
- Mercado, N.; Rubio, M.; Cisneros, M.; Trejo, S.; Giraudo, M. Smoking as an Independent Predictor of Radial Spasm. Rev. Argent. de Cardioangiol. Interv. 2020, 11, 194–201. [Google Scholar] [CrossRef]
- Horie, K.; Tada, N.; Isawa, T.; Matsumoto, T.; Taguri, M.; Kato, S.; Honda, T.; Ootomo, T.; Inoue, N. A Randomised Comparison of Incidence of Radial Artery Occlusion and Symptomatic Radial Artery Spasm Associated with Elective Transradial Coronary Intervention Using 6.5 Fr SheathLess Eaucath Guiding Catheter vs. 6.0 Fr Glidesheath Slender. EuroIntervention 2018, 13, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.H.; Roberts, J.S.; Abu-Fadel, M.S.; Czak, S.J.; Latif, F.; Jain, S.P.; Raza, J.A.; Mangla, A.; Panagopoulos, G.; Patel, P.M.; et al. Real-Time Ultrasound Guidance Facilitates Transradial Access. JACC Cardiovasc. Interv. 2015, 8, 283–291. [Google Scholar] [CrossRef] [PubMed]
| All Patients = 96 | Median | IQR | |
|---|---|---|---|
| Height (centimeters) | 169.5 | 163–175 | |
| HR (beats per minute) | 70 | 60–80 | |
| Pain score (0–10 scale) | 3 | 2–6 | |
| mean | SD | CI | |
| Age (years) | 63.1 | 11.3 | 60.8 to 65.3 |
| Weight (kg) | 81.7 | 15.2 | 78.6 to 84.8 |
| SBP (mmHg) | 158.3 | 27.3 | 152.8 to 163 |
| DBP (mmHg) | 72.4 | 10.7 | 70.3 to 74.6 |
| Radial artery diameter (mm) | 2.45 | 0.49 | 2.36 to 2.55 |
| Sheath-to-artery diameter ratio | 0.85 | 0.17 | 0.81 to 0.88 |
| All Patients = 96 | ||
|---|---|---|
| Acute presentation Male sex | 48/96 (50%) 67/96 (69.8%) | |
| PAD | 23/96 (24%) | |
| CKD | 18/96 (18.8%) | |
| Dyslipidemia | 92/96 (95.8%) | |
| DM | 31/96 (32.3%) | |
| Smoking | 35/96 (36.5%) | |
| Pulse grade | 1 = poor | 32/96 (33.3%) |
| 2 = adequate | 48/96 (50%) | |
| 3 = good | 16/96 (16.7%) | |
| RAS | 60/96 (62.5%) |
| All Patients N = 96 | RAS (60/96) Median | RAS (60/96) IQR | No RAS (36/96) Median | No RAS (36/96) IQR | p | ||
|---|---|---|---|---|---|---|---|
| Height (centimeters) | 166 | 160–175 | 171 | 165–175.25 | 0.0233 | ||
| HR (beats per minute) | 65.5 | 60–75 | 72 | 60–86.25 | 0.3972 | ||
| Pain score (0–10 scale) | 4 | 3–7 | 3 | 2–4 | 0.0022 | ||
| RAS (60/96) mean | RAS (60/96) SD | RAS (60/96) CI | No RAS (36/96) mean | No RAS (36/96) SD | No RAS (36/96) CI | p | |
| Age (years) | 62.9 | 11.5 | 59.95–65.89 | 63.3 | 11 | 59.6–67 | 0.8232 |
| Weight (kg) | 77.4 | 14 | 73.78–81.02 | 88.9 | 14.5 | 84–93.8 | 0.0002 |
| SBP (mmHg) | 157.5 | 25.6 | 150.9–164.1 | 159.6 | 30.2 | 149.4–169.8 | 0.6959 |
| DBP (mmHg) | 71 | 9.7 | 68.5–73.5 | 74.9 | 11.8 | 70.9–78.9 | 0.0429 |
| Radial artery diameter (mm) | 2.32 | 0.47 | 2.2–2.45 | 2.67 | 0.44 | 2.52–2.82 | 0.0014 |
| Sheath-to-artery diameter ratio | 0.89 | 0.18 | 0.85–0.95 | 0.76 | 0.11 | 0.73–0.80 | 0.00002 |
| All Patients N = 96 | RAS (60 Patients) | No RAS (36 Patients) | p |
|---|---|---|---|
| Acute presentation Male sex | 27/60 (45%) 42/60 (70%) | 21/36 (58.3%) 25/36 (69.4%) | 0.2918 1 |
| PAD | 11/60 (18.3%) | 12/36 (33.3%) | 0.1556 |
| CKD | 10/60 (16.7%) | 8/36 (22.2%) | 0.6854 |
| Dyslipidemia | 58/60 (96.7%) | 34/36 (94.4%) | 1 |
| Diabetes mellitus | 19/60 (31.7%) | 12/36 (33.3%) | 1 |
| Smoking | 26/60 (43.3%) | 9/36 (25%) | 0.1123 |
| Pulse grade | 1–21/60 (35%) | 1–11/36 (30.6%) | 0.8948 |
| 2–29 (48.3%) | 2–19/36 (52.8%) | ||
| 3–10 (16.7%) | 3–6/36 (16.7%) |
| Variable | β Coefficient | p-Value |
|---|---|---|
| Weight (kg) | −0.043 | 0.0307 |
| Radial artery diameter (mm) | −1.352 | 0.0200 |
| Height (cm) | −0.015 | 0.6188 |
| DBP (mmHg) | −0.004 | 0.8811 |
| Pain score (0–10) | 0.191 | 0.1272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zus, A.S.; Crișan, S.; Luca, S.; Nișulescu, D.; Valcovici, M.-D.; Pătru, O.; Lazăr, M.-A.; Văcărescu, C.; Gaiță, D.; Luca, C.-T. Retrospective Analysis of Angiographic Radial Artery Spasm Predictors. Life 2025, 15, 1759. https://doi.org/10.3390/life15111759
Zus AS, Crișan S, Luca S, Nișulescu D, Valcovici M-D, Pătru O, Lazăr M-A, Văcărescu C, Gaiță D, Luca C-T. Retrospective Analysis of Angiographic Radial Artery Spasm Predictors. Life. 2025; 15(11):1759. https://doi.org/10.3390/life15111759
Chicago/Turabian StyleZus, Adrian Sebastian, Simina Crișan, Silvia Luca, Daniel Nișulescu, Mihaela-Daniela Valcovici, Oana Pătru, Mihai-Andrei Lazăr, Cristina Văcărescu, Dan Gaiță, and Constantin-Tudor Luca. 2025. "Retrospective Analysis of Angiographic Radial Artery Spasm Predictors" Life 15, no. 11: 1759. https://doi.org/10.3390/life15111759
APA StyleZus, A. S., Crișan, S., Luca, S., Nișulescu, D., Valcovici, M.-D., Pătru, O., Lazăr, M.-A., Văcărescu, C., Gaiță, D., & Luca, C.-T. (2025). Retrospective Analysis of Angiographic Radial Artery Spasm Predictors. Life, 15(11), 1759. https://doi.org/10.3390/life15111759








