Association of Chromosome 3p21.32 Haplotype Blocks Introgressed from Neanderthals with Critical COVID-19 in a Spanish Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Genotyping
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, R.E.; Krause, J.; Briggs, A.W.; Maricic, T.; Stenzel, U.; Kircher, M.; Patterson, N.; Li, H.; Zhai, W.; Fritz, M.H.-Y.; et al. A draft sequence of the Neandertal genome. Science 2010, 328, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, K.; Racimo, F.; Patterson, N.; Jay, F.; Sankararaman, S.; Sawyer, S.; Heinze, A.; Renaud, G.; Sudmant, P.H.; De Filippo, C.; et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 2014, 505, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Kircher, M.; Gansauge, M.-T.; Li, H.; Racimo, F.; Mallick, S.; Schraiber, J.G.; Jay, F.; Prüfer, K.; De Filippo, C.; et al. A high-coverage genome sequence from an archaic Denisovan individual. Science 2012, 338, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Vernot, B.; Akey, J.M. Resurrecting Surviving Neandertal lineages from modern human genomes. Science 2014, 343, 1017–1021. [Google Scholar] [CrossRef]
- Racimo, F.; Sankararaman, S.; Nielsen, R.; Huerta-Sánchez, E. Evidence for archaic adaptive introgression in humans. Nat. Rev. Genet. 2015, 16, 359–371. [Google Scholar] [CrossRef]
- Liang, S.-A.; Ren, T.; Zhang, J.; He, J.; Wang, X.; Jiang, X.; He, Y.; McCoy, R.C.; Fu, Q.; Akey, J.M.; et al. A refined analysis of Neanderthal-introgressed sequences in modern humans with a complete reference genome. Genome Biol. 2025, 26, 1–20. [Google Scholar] [CrossRef]
- Ongaro, L.; Huerta-Sanchez, E. A history of multiple Denisovan introgression events in modern humans. Nat. Genet. 2024, 56, 2612–2622. [Google Scholar] [CrossRef]
- Li, L.; Comi, T.J.; Bierman, R.F.; Akey, J.M. Recurrent gene flow between Neanderthals and modern humans over the past 200,000 years. Science 2024, 385, eadi1768. [Google Scholar] [CrossRef]
- Iasi, L.N.M.; Chintalapati, M.; Skov, L.; Mesa, A.B.; Hajdinjak, M.; Peter, B.M.; Moorjani, P. Neanderthal ancestry through time: Insights from genomes of ancient and present-day humans. Science 2024, 386, eadq3010. [Google Scholar] [CrossRef]
- Sümer, A.P.; Rougier, H.; Villalba-Mouco, V.; Huang, Y.; Iasi, L.N.M.; Essel, E.; Mesa, A.B.; Furtwaengler, A.; Peyrégne, S.; de Filippo, C.; et al. Earliest modern human genomes constrain timing of Neanderthal admixture. Nature 2024, 638, 711–717. [Google Scholar] [CrossRef]
- Massilani, D.; Skov, L.; Hajdinjak, M.; Gunchinsuren, B.; Tseveendorj, D.; Yi, S.; Lee, J.; Nagel, S.; Nickel, B.; Devièse, T.; et al. Denisovan ancestry and population history of early East Asians. Science 2020, 370, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Vespasiani, D.M.; Jacobs, G.S.; Cook, L.E.; Brucato, N.; Leavesley, M.; Kinipi, C.; Ricaut, F.-X.; Cox, M.P.; Romero, I.G. Denisovan introgression has shaped the immune system of present-day Papuans. PLoS Genet. 2022, 18, e1010470. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Mallick, S.; Dannemann, M.; Prüfer, K.; Kelso, J.; Pääbo, S.; Patterson, N.; Reich, D. The genomic landscape of Neanderthal ancestry in present-day humans. Nature 2014, 507, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Juric, I.; Aeschbacher, S.; Coop, G. The strength of selection against Neanderthal introgression. PLoS Genet. 2016, 12, e1006340. [Google Scholar] [CrossRef]
- Reilly, P.F.; Tjahjadi, A.; Miller, S.L.; Akey, J.M.; Tucci, S. The contribution of Neanderthal introgression to modern human traits. Curr. Biol. 2022, 32, R970–R983. [Google Scholar] [CrossRef]
- Abood, S.; Oota, H. Human dispersal into East Eurasia: Ancient genome insights and the need for research on physiological adaptations. J. Physiol. Anthr. 2025, 44, 5. [Google Scholar] [CrossRef]
- Dannemann, M.; Kelso, J. The Contribution of Neanderthals to Phenotypic Variation in Modern Humans. Am. J. Hum. Genet. 2017, 101, 578–589. [Google Scholar] [CrossRef]
- Dannemann, M.; Andrés, A.M.; Kelso, J. Introgression of Neandertal- and Denisovan-like Haplotypes Contributes to Adaptive Variation in Human Toll-like Receptors. Am. J. Hum. Genet. 2016, 98, 22–33. [Google Scholar] [CrossRef]
- McArthur, E.; Rinker, D.C.; Capra, J.A. Quantifying the contribution of Neanderthal introgression to the heritability of complex traits. Nat. Commun. 2021, 12, 4481. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, Y.; Xu, S.; Wang, J.; Jin, L. Neanderthal introgression at chromosome 3p21.31 was under positive natural selection in East Asians. Mol. Biol. Evol. 2013, 31, 683–695. [Google Scholar] [CrossRef]
- Deschamps, M.; Laval, G.; Fagny, M.; Itan, Y.; Abel, L.; Casanova, J.-L.; Patin, E.; Quintana-Murci, L. Genomic Signatures of Selective Pressures and Introgression from Archaic Hominins at Human Innate Immunity Genes. Am. J. Hum. Genet. 2016, 98, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Sams, A.J.; Dumaine, A.; Nédélec, Y.; Yotova, V.; Alfieri, C.; Tanner, J.E.; Messer, P.W.; Barreiro, L.B. Adaptively introgressed Neandertal haplotype at the OAS locus functionally impacts innate immune responses in humans. Genome Biol. 2016, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lu, Y.; Xu, S. Adaptive Evolution of Two Distinct Adaptive Haplotypes of Neanderthal Origin at the Immunoglobulin Heavy-chain Locus in East Asian and European Populations. Mol. Biol. Evol. 2024, 41, msae147. [Google Scholar] [CrossRef] [PubMed]
- Mendez, F.L.; Watkins, J.C.; Hammer, M.F. Neandertal origin of genetic variation at the cluster of OAS immunity genes. Mol. Biol. Evol. 2013, 30, 798–801. [Google Scholar] [CrossRef]
- Jagoda, E.; Lawson, D.J.; Wall, J.D.; Lambert, D.; Muller, C.; Westaway, M.; Leavesley, M.; Capellini, T.D.; Lahr, M.M.; Gerbault, P.; et al. Disentangling Immediate Adaptive Introgression from Selection on Standing Introgressed Variation in Humans. Mol. Biol. Evol. 2017, 35, 623–630. [Google Scholar] [CrossRef]
- Quach, H.; Rotival, M.; Pothlichet, J.; Loh, Y.-H.E.; Dannemann, M.; Zidane, N.; Laval, G.; Patin, E.; Harmant, C.; Lopez, M.; et al. Genetic Adaptation and Neandertal Admixture Shaped the Immune System of Human Populations. Cell 2016, 167, 643–656.e17. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; Asselta, R.; et al. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature 2021, 600, 472–477. [Google Scholar] [CrossRef]
- Zeberg, H.; Pääbo, S. A genomic region associated with protection against severe COVID-19 is inherited from Neandertals. Proc. Natl. Acad. Sci. USA 2021, 118, e2026309118. [Google Scholar] [CrossRef]
- Jagoda, E.; Marnetto, D.; Senevirathne, G.; Gonzalez, V.; Baid, K.; Montinaro, F.; Richard, D.; Falzarano, D.; LeBlanc, E.V.; Colpitts, C.C.; et al. Regulatory dissection of the severe COVID-19 risk locus introgressed by Neanderthals. eLife 2023, 12, e71235. [Google Scholar] [CrossRef]
- Downes, D.J.; Cross, A.R.; Hua, P.; Roberts, N.; Schwessinger, R.; Cutler, A.J.; Munis, A.M.; Brown, J.; Mielczarek, O.; de Andrea, C.E.; et al. Identification of LZTFL1 as a candidate effector gene at a COVID-19 risk locus. Nat. Genet. 2021, 53, 1606–1615. [Google Scholar] [CrossRef]
- Yan, S.M.; Sherman, R.M.; Taylor, D.J.; Nair, D.R.; Bortvin, A.N.; Schatz, M.C.; McCoy, R.C. Local adaptation and archaic introgression shape global diversity at human structural variant loci. eLife 2021, 10, e67615. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Ricci, G.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: Further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev. 2021, 58, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Bowlin, T.L.; Lukacs, N.W. Respiratory syncytial virus–induced chemokine production: Linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 2004, 189, 1419–1430. [Google Scholar] [CrossRef]
- John, A.E.; Gerard, C.J.; Schaller, M.; Miller, A.L.; Berlin, A.A.; Humbles, A.A.; Lukacs, N.W. Respiratory syncytial virus-induced exag-geration of allergic airway disease is dependent upon CCR1-associated immune responses. Eur. J. Immunol. 2005, 35, 108–116. [Google Scholar] [CrossRef]
- Sørensen, L.N.; Paludan, S.R. Blocking CC chemokine receptor (CCR) 1 and CCR5 during herpes simplex virus type 2 infection in vivo impairs host defence and perturbs the cytokine response. Scand. J. Immunol. 2004, 59, 321–333. [Google Scholar] [CrossRef]
- Trump, S.; Lukassen, S.; Anker, M.S.; Chua, R.L.; Liebig, J.; Thürmann, L.; Corman, V.M.; Binder, M.; Loske, J.; Klasa, C.; et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat. Biotechnol. 2020, 39, 705–716. [Google Scholar] [CrossRef]
- Enard, D.; Petrov, D.A. Evidence that RNA Viruses Drove Adaptive Introgression between Neanderthals and Modern Humans. Cell 2018, 175, 360–371.e13. [Google Scholar] [CrossRef]
- Barrie, W.; Yang, Y.; Irving-Pease, E.K.; Attfield, K.E.; Scorrano, G.; Jensen, L.T.; Armen, A.P.; Dimopoulos, E.A.; Stern, A.; Refoyo-Martinez, A.; et al. Elevated genetic risk for multiple sclerosis emerged in steppe pastoralist populations. Nature 2024, 625, 321–328. [Google Scholar] [CrossRef]
- Allentoft, M.E.; Sikora, M.; Refoyo-Martínez, A.; Irving-Pease, E.K.; Fischer, A.; Barrie, W.; Ingason, A.; Stenderup, J.; Sjögren, K.-G.; Pearson, A.; et al. Population genomics of post-glacial western Eurasia. Nature 2024, 625, 301–311. [Google Scholar] [CrossRef]
- Green, M.H. The Four Black Deaths. Am. Hist. Rev. 2020, 125, 1601–1631. [Google Scholar] [CrossRef]
- Klunk, J.; Vilgalys, T.P.; Demeure, C.E.; Cheng, X.; Shiratori, M.; Madej, J.; Beau, R.; Elli, D.; Patino, M.I.; Redfern, R.; et al. Evolution of immune genes is associated with the Black Death. Nature 2022, 611, 312–319. [Google Scholar] [CrossRef]
- Hamilton, F.; Mentzer, A.J.; Parks, T.; Baillie, J.K.; Smith, G.D.; Ghazal, P.; Timpson, N.J. Variation in ERAP2 has opposing effects on severe respiratory infection and autoimmune disease. Am. J. Hum. Genet. 2023, 110, 691–702. [Google Scholar] [CrossRef]
- Ye, C.J.; Chen, J.; Villani, A.-C.; Gate, R.E.; Subramaniam, M.; Bhangale, T.; Lee, M.N.; Raj, T.; Raychowdhury, R.; Li, W.; et al. Genetic analysis of isoform usage in the human anti-viral response reveals influenza-specific regulation of ERAP2 transcripts under balancing selection. Genome Res. 2018, 28, 1812–1825. [Google Scholar] [CrossRef]
- Raja, A.; Kuiper, J.J.W. Evolutionary immuno-genetics of endoplasmic reticulum aminopeptidase II (ERAP2). Genes Immun. 2023, 24, 295–302. [Google Scholar] [CrossRef]
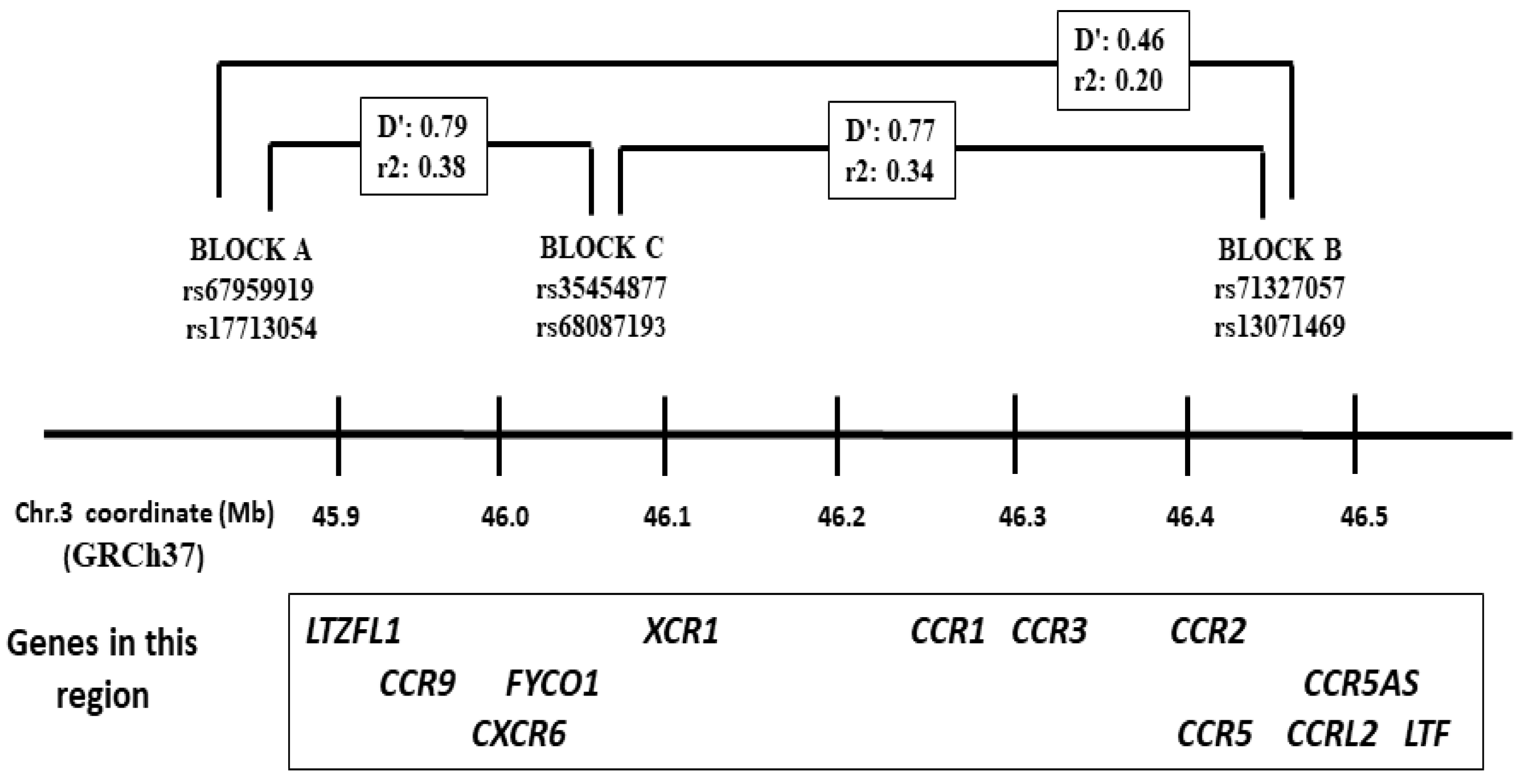
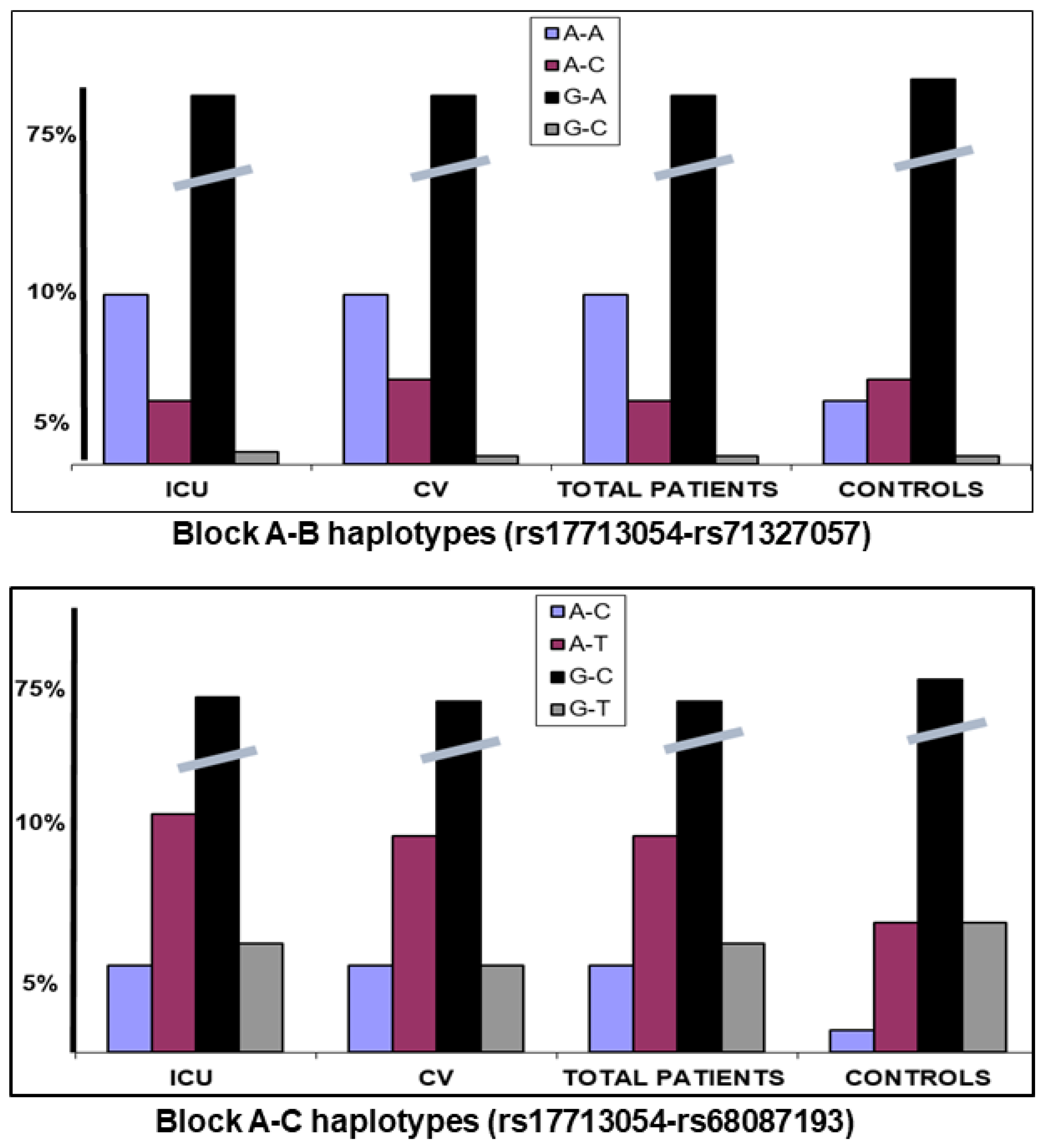

| ICU N = 446 | NO ICU N = 532 | p-Value | OR (95% CI) | DEATH N = 85 | |
|---|---|---|---|---|---|
| Male Female | 320 (72%) 126 (28%) | 306 (58%) 226 (42%) | <0.001 | 1.88 (1.44–2.46) | 60 (71%) 25 (29%) |
| Hypertensives | 243 (54%) | 172 (32%) | <0.001 | 2.51 (1.93–3.25) | 58 (68%) |
| Hypercholesterol | 207 (46%) | 154 (29%) | <0.001 | 2.13 (1.63–2.77) | 45 (53%) |
| Median age (range) years | 65 (18–89) | 59 (18–91) | <0.001 | 1.02 (1.01–1.03) | 73 (32–95) |
| <65 years ≥65 years | 232 (52%) 214 (48%) | 339 (64%) 193 (36%) | <0.001 | 1.62 (1.25–2.10) | 65 (77%) 20 (23%) |
| Block A rs17713054 G > A | |||||
| 22 GG | 322 (72%) | 423 (80%) | 62 (73%) | ||
| 12 AG | 117 (26%) | 104 (19%) | 0.007 | 1.49 (1.11–2.01) | 21 (25%) |
| 11 AA | 7 (2%) | 5 (1%) | 2 (2%) | ||
| MAF A | 0.15 | 0.11 | 0.15 | ||
| Block B rs71327057 A > C | |||||
| 11 AA | 376 (84%) | 466 (88%) | 72 (85%) | ||
| 12 AC | 65 (15%) | 64 (12%) | 0.14 | 1.13 (0.91–1.89) | 12 (14%) |
| 22 CC | 5 (1%) | 2 (<1%) | 1 (1%) | ||
| MAF C | 0.08 | 0.06 | |||
| BLOCK C rs34454877 T > C | |||||
| TT | 320 (71%) | 408 (77%) | 65 (71%) | ||
| CT | 110 (25%) | 115 (21%) | 0.08 | 1.29 (0.97–1.73) | 17 (26%) |
| CC | 16 (4%) | 9 (2%) | 3 (3%) | ||
| MAF C | 0.16 | 0.13 | 0.14 |
| ICU < 65 N = 232 | NO ICU < 65 N = 339 | ICU ≥ 65 N = 232 | NO ICU ≥ 65 N = 193 | Total N = 978 | Controls N = 500 | |
|---|---|---|---|---|---|---|
| Block A rs17713054 G > A | ||||||
| GG | 159 (68%) | 267 (79%) | 163 (76%) | 156 (81%) | 745 (76%) | 415 (83%) |
| AG | 67 (29%) | 67 (20%) | 50 (23%) | 37 (19%) | 221 (23%) | 81 (16%) |
| AA | 6 (3%) | 5 (1%) | 1 (1%) | 0 | 12 (1%) | 4 (1%) |
| A | 0.17 | 0.11 | 0.12 | 0.10 | 0.13 | 0.09 |
| Eurx A = 0.08 | p = 0.006 | p = 0.24 | p = 0.003, OR = 1.53 (1.16–2.01) | |||
| Block B rs71327057 A > C | ||||||
| AA | 191 (82%) | 294 (87%) | 185 (86%) | 172 (89%) | 842 (86%) | 449 (89%) |
| AC | 38 (16%) | 43 (13%) | 27 (13%) | 21 (11%) | 129 (13%) | 45 (9%) |
| CC | 3 (2%) | 2 (1%) | 2 (2%) | 0 | 7 (1%) | 6 (2%) |
| C | 0.08 | 0.07 | 0.07 | 0.04 | 0.07 | 0.06 |
| Eurx C = 0.07 | p = 0.12 | p = 0.29 | p = 0.04, OR = 1.42 (1.01–2.00) | |||
| Block C rs68087193 C > T | ||||||
| CC | 160 (69%) | 258 (76%) | 160 (75%) | 150 (78%) | 728 (74%) | 405 (81%) |
| CT | 59 (25%) | 77 (23%) | 51 (22%) | 38 (20%) | 225 (23%) | 90 (18%) |
| TT | 13 (6%) | 4 (1%) | 3 (1%) | 5 (2%) | 25 (3%) | 5 (1%) |
| T | 0.18 | 0.13 | 0.13 | 0.12 | 0.14 | 0.10 |
| Eurx T = 0.11 | p = 0.06 | p = 0.49 | p = 0.005, OR = 1.46 (1.12–1.91) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Coto, D.; García-Clemente, M.; Hermida-Valverde, T.; Albaiceta, G.M.; Amado, L.; Vega-Prado, L.M.; García-Lago, C.; Herrero-Puente, P.; Martínez-Borra, J.; Lorca, R.; et al. Association of Chromosome 3p21.32 Haplotype Blocks Introgressed from Neanderthals with Critical COVID-19 in a Spanish Cohort. Life 2025, 15, 1733. https://doi.org/10.3390/life15111733
Vázquez-Coto D, García-Clemente M, Hermida-Valverde T, Albaiceta GM, Amado L, Vega-Prado LM, García-Lago C, Herrero-Puente P, Martínez-Borra J, Lorca R, et al. Association of Chromosome 3p21.32 Haplotype Blocks Introgressed from Neanderthals with Critical COVID-19 in a Spanish Cohort. Life. 2025; 15(11):1733. https://doi.org/10.3390/life15111733
Chicago/Turabian StyleVázquez-Coto, Daniel, Marta García-Clemente, Tamara Hermida-Valverde, Guillermo M. Albaiceta, Laura Amado, Lorena M. Vega-Prado, Claudia García-Lago, Pablo Herrero-Puente, Jesús Martínez-Borra, Rebeca Lorca, and et al. 2025. "Association of Chromosome 3p21.32 Haplotype Blocks Introgressed from Neanderthals with Critical COVID-19 in a Spanish Cohort" Life 15, no. 11: 1733. https://doi.org/10.3390/life15111733
APA StyleVázquez-Coto, D., García-Clemente, M., Hermida-Valverde, T., Albaiceta, G. M., Amado, L., Vega-Prado, L. M., García-Lago, C., Herrero-Puente, P., Martínez-Borra, J., Lorca, R., Gómez, J., & Coto, E. (2025). Association of Chromosome 3p21.32 Haplotype Blocks Introgressed from Neanderthals with Critical COVID-19 in a Spanish Cohort. Life, 15(11), 1733. https://doi.org/10.3390/life15111733







