Improvement of Liver Fibrosis in Patients with MASLD Undergoing Pioglitazone Treatment: An Update
Abstract
1. Introduction
2. The Burden of MASLD and MASH
3. Mechanism of Action of Pioglitazone and Its Anti-Fibrotic Effects
4. Clinical Evaluation of Liver Fibrosis Improvement During Pioglitazone Treatment
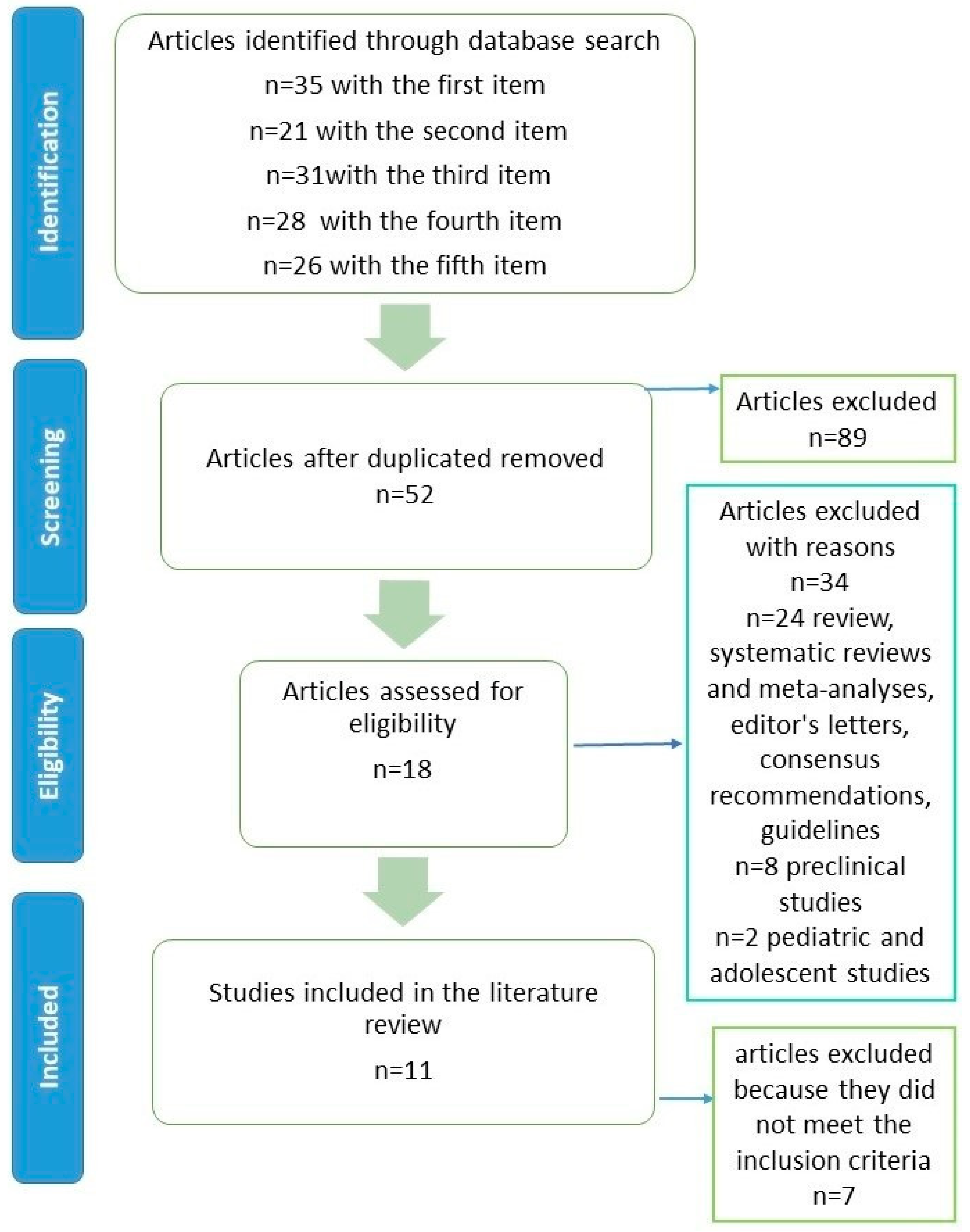
4.1. Methodology of Literature Review
4.2. Literature Review
| Authors | Study Design | Country | Number of Patients | Study Population/Treatment | Follow-Up Time <1 Year | Liver Steatosis/ Methods | Liver Fibrosis/ Non-Invasive Methods | Biopsy |
|---|---|---|---|---|---|---|---|---|
| Abdel Monem et al. [34] | Prospective, randomized, parallel, open-label study | Egypt | 100 | 100 patients with MASH biopsy-confirmed, diabetic and non-diabetic, randomized to pioglitazone 30 mg/day vs. dapagliflozin 10 mg/day | 24 weeks | Dapagliflozin showed a comparable effect on improvement in liver steatosis grade assessed by transient elastography from baseline in diabetics versus a significant superiority in non-diabetics | Dapagliflozin showed a comparable effect on improvement in liver fibrosis stage assessed by transient elastography from baseline in diabetics versus a significant superiority in non-diabetics | Dapagliflozin and pioglitazone had a comparable effect on changes in the MASLD activity score in both the non-diabetic and diabetic groups (p > 0.05), with the exception of Δ hepatocellular ballooning in favor of dapagliflozin in the diabetic group (p = 0.048). The improvement from baseline for non-diabetic patients was not significantly higher in the dapagliflozin group compared to the pioglitazone group. |
| Lee et al. [36] | Randomized, open-label trial | Korea | 50 | 50 patients with TD2M and MASLD randomized to pioglitazone 15 mg/die vs. empaglifozin 10 mg/die vs. pioglitazone plus empaglifozine | 24 weeks | Combination therapy resulted in the largest reduction in liver fat assessed by magnetic resonance imaging-proton density fat fraction | Combination therapy resulted in the largest reduction in liver stiffness assessed by magnetic resonance elastography | |
| Khaliq et al. [37] | prospective, randomized, double-blind, placebo-controlled, interventional study |
Pakistan
Malaysia | 180 completed the trial | 180 MASLD and TD2M patients with fatty liver from grade 1 to grade 3, (ertugliflozin, n = 60; pioglitazone, n = 60; placebo, n = 60) | Final FU at 24 weeks | In the ertugliflozin and pioglitazone groups, 45% to 23.4% and 41.7% to 26.6%, respectively, decreased in the grade 2 group assessed by ultrasonography | FIB-4 index decreased markedly after ertugliflozin treatment. | - |
| Khaliq et al. [38] | double-blind, randomized, controlled clinical trial | Pakistan | 173 patients | 173 patients with MASLD and T2DM randomized to receive vitamin E 800 IU daily (n = 42), pioglitazone 30 mg daily (n = 43), ertugliflozin 15 mg daily (n = 44), and vitamin E 800 IU daily + ertugliflozin 15 mg daily (n = 44) combination therapy | 24 weeks | Fatty liver grades markedly decline in the group that received vitamin E combined with ertugliflozin | FIB-4 reduction during treatment with ertugliflozin + vitamin E. Pioglitazone and vitamin E showed no effect on the FIB-4. |
| Authors | Study Design | Country | Number of Patients | Study Population/ Treatment | Follow-Up Time >1 Year | Liver Steatosis/ Methods | Liver Fibrosis/ Methods | Biopsy |
|---|---|---|---|---|---|---|---|---|
| Abdul-Ghani et al. [39] | Clinical Trial (Trial registration number NCT01107717) | USA | 55 | T2DM patients received initial triple therapy with metformin/pioglitazone/exenatide (n = 29) vs. metformin, followed by stepwise addition of glipizide and then insulin glargine (n = 26), achieving glycemic control (HbA1c < 6.5%) | 6 years | Attenuation of progression of liver steatosis assessed by controlled attenuation parameter in patients receiving initial triple therapy | Attenuation of the progression of liver fibrosis assessed by transient elastography in patients receiving initial triple therapy | |
| Albert et al. [40] | Prospective study | USA | 220 | 220 patients with MASLD without diabetes mellitus (pioglitazone vs. vitamin e vs. placebo). | 96 weeks | Pioglitazone and vitamin E both improved histological steatosis | MASLD activity scores improved with pioglitazone by 39%, and with vitamin E by 36%, correlating with changes in FIB-4 | Improvement of steatosis, and inflammation, no change in fibrosis stage |
| Ito et al. [42] | Randomized active-controlled trial | Japan | 61 | MASLD and T2DM patients were randomly assigned to receive either ipragliflozin (n = 31) or pioglitazone (n = 30) | 24 weeks 5 years | Improvement of liver steatosis, as evaluated using the liver-to-spleen attenuation ratio (L/S ratio) on computed tomography for both ipragliflozin and pioglitazone | FIB-4 index significantly improved only in the ipragliflozin group | |
| Pereira et al. [44] | Multi-center retrospective study | Brazil | 65 | MASLD patients treated with pioglitazone (30–45 mg) when MASLD was associated with T2DM or fibrosis stage ≥F2 assessed by liver biopsy | 1–3 years 4–10 years | Significant reduction in CAP levels only in the 4–10 follow-up period, suggesting a reduction in liver steatosis | Significant reduction in FAST™ score between baseline and two follow-up periods | |
| Martínez-Sanchez et al. [46] | Multicentric retrospective observational study | Mexico | 2000 | 2000 patients with T2DM stratified for FIB-4 values (n = 935 in F0, 658 in F1–F2, 407 in F3–F4) | Median duration of T2D = 7 years | Statins and pioglitazone may protect against liver fibrosis | ||
| Shi et al. [47] | Multicenter retrospective cohort study |
China
Korea United Kingdom Italy Japan Sweden Malaysia France Singapore Virginia Spain Massachusetts | 7867 | 7867 patients with T2D and MASLD Treated with pioglitazone, GLP-1RAs, and SGLT-2 inhibitors | 5.1 years | SGLT-2 inhibitor had a significantly lower risk of liver stiffness progression | At baseline, 1599 patients underwent liver biopsy, of whom 42.4% had advanced fibrosis and 71.3% had MASH | |
| Lee et al. [48] | Real-world prospective cohort study | China | 888 | 888 patients with T2D and MASLD Treated with pioglitazone, GLP-1RAs, and SGLT-2 inhibitors | 3.9 years | * | Participants had received a median of three reassessment VCTEs and their FAST * scores overall improved significantly from baseline. Dual or triple combination treatment was significantly associated with a greater annual reduction in FAST scores from baseline |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolically-dysfunction-associated steatotic liver disease |
| MAFLD | Metabolically-dysfunctional-associated fatty liver disease |
| T2DM | type 2 diabetes |
| MASL | Metabolically-dysfunction-associated steatotic liver |
| MASH | Metabolically-dysfunction-associated steatohepatitis |
| HCC | Hepatocellular carcinoma |
| AASLD | American Association for the Study of Liver Diseases |
| EASL-EASD-EASO | European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity |
| LSM | Liver stiffness measurement |
| FIB-4 | Fibrosis-4 |
| MetALD | Metabolic and alcohol-associated liver disease |
| AGA | American Gastroenterological Association |
| AACE | American Association of Clinical Endocrinology |
| ADA | American Diabetes Association |
| VCTE | Vibration-controlled transient elastography |
| HCSs | Hepatic stellate cells |
| PPARs | Peroxisome proliferator-activated receptors |
| TNF-α | Tumor necrosis factor-α |
| ROS | Reactive oxygen species |
| NLRP3 | Inflammasome 3 |
| ASC | Apoptosis-associated speck-like protein containing a caspase recruitment domain |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme oxygenase-1 |
| HGF | Hepatocyte growth factor |
| PDGF | Platelet-derived growth factor |
| HbA1c | Hemoglobin A1c |
| FAST™ | FibroScan-AST |
| NAFIC | Type IV collagen 7S values |
| CAP | Controlled Attenuation Parameter |
| AST | Aspartate Aminotransferase |
References
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- En Li Cho, E.; Ang, C.Z.; Quek, J.; Fu, C.E.; Lim, L.K.E.; Heng, Z.E.Q.; Tan, D.J.H.; Lim, W.H.; Yong, J.N.; Zeng, R.; et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: An updated systematic review and meta-analysis. Gut 2023, 72, 2138–2148. [Google Scholar] [CrossRef]
- Stasi, C. Post-COVID-19 Pandemic Sequelae in Liver Diseases. Life 2025, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Nour, T.Y.; Altintaş, K.H. Effect of the COVID-19 pandemic on obesity and it is risk factors: A systematic review. BMC Public Health 2023, 23, 1018. [Google Scholar] [CrossRef]
- World Heart Federation. World Heart Report 2025. Obesity & Cardiovascular Disease. 2025. Available online: https://world-heart-federation.org/resource/world-heart-report-2025/ (accessed on 16 May 2025).
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Canivet, C.M.; Ongaro, M.; Conquet, N.; Spahr, L.; Goossens, N. Validation of the American Association for the Study of Liver Disease/European Association for the Study of the Liver Multistep Screening Strategies for Metabolic Dysfunction-associated Steatotic Liver disease. Gastro Hep Adv. 2025, 4, 100747. [Google Scholar] [CrossRef]
- Vidal-Trécan, T.; Julla, J.B.; El Khoury, T.; Venteclef, N.; Riveline, J.P.; Paradis, V.; Valla, D.; Gautier, J.F.; Castera, L. Effectiveness of Six International Guidelines Using Fibrosis-4 and FibroScan for Risk Stratification of Metabolic Dysfunction-associated Steatotic Liver Disease in Type 2 Diabetes. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2025; advance online publication. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Udompap, P.; Therneau, T.M.; Canning, R.E.; Benson, J.T.; Allen, A.M. Performance of American Gastroenterological Association Clinical Care Pathway for the risk stratification of patients with nonalcoholic fatty liver disease in the US population. Hepatology 2023, 77, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2022, 28, 528–562. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S59–S85. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, D.; Mao, R.; Yao, Z.; Wu, Q.; Lv, W. Global burden of MAFLD, MAFLD related cirrhosis and MASH related liver cancer from 1990 to 2021. Sci. Rep. 2025, 15, 7083. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Danpanichkul, P.; Pang, Y.; Suparan, K.; Auttapracha, T.; Sirimangklanurak, S.; Attia, A.M.; Thimphitthaya, C.; Ni Law, M.S.; Yu, Z.; Soliman, M.A.; et al. Increased MASH-associated liver cancer in younger demographics. Hepatol. Commun. 2025, 9, e0629. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients with Type 2 Diabetes. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2024, 22, 1999–2010.e8. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakul, W.; Charatcharoenwitthaya, N.; Charatcharoenwitthaya, P. Metabolic dysfunction-associated steatotic liver disease and the risk of mortality in individuals with type 2 diabetes: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2024, 36, 351–358. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Paik, J.M.; Lazarus, J.V.; Burra, P.; Eguchi, Y.; Tacke, F.; Crespo, J.; Villela-Nogueira, C.A.; Brennan, P.N.; Al-Omar, H.A.; et al. Projected Global Clinical, Humanistic, and Economic Impact of Metabolic Dysfunction-Associated Steatohepatitis (MASH): The Cost of Inaction Based on Data from Nine Countries. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2025; advance online publication. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Alswat, K.; Aljumah, A.A.; Sanai, F.M.; Abaalkhail, F.; Alghamdi, M.; Al Hamoudi, W.K.; Al Khathlan, A.; Al Quraishi, H.; Al Rifai, A.; Al Zaabi, M.; et al. Nonalcoholic fatty liver disease burden–Saudi Arabia and United Arab Emirates, 2017–2030. Saudi J. Gastroenterol. 2018, 24, 211–219. [Google Scholar] [CrossRef]
- Stefan, N.; Yki-Järvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatotic liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef]
- Hashim, M.M.A.; Khan, M.A.M.; Ashraf, M.U.; Mohsin, S.; Zahoor, K.; Niazi, J.; Khan, A.; Muzaffar, S.; Makhdumi, M.; Ibad, O.A.; et al. Pathological Evolution and Internal Medicine Management of Nonalcoholic Fatty Liver Disease (NAFLD) in the Era of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Cureus 2025, 17, e86963. [Google Scholar] [CrossRef]
- Iturbe-Rey, S.; Maccali, C.; Arrese, M.; Aspichueta, P.; Oliveira, C.P.; Castro, R.E.; Lapitz, A.; Izquierdo-Sanchez, L.; Bujanda, L.; Perugorria, M.J.; et al. Lipotoxicity-driven metabolic dysfunction-associated steatotic liver disease (MASLD). Atherosclerosis 2025, 400, 119053. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Butruille, L.; Francque, S. Treating NASH by targeting peroxisome proliferator-activated receptors. J. Hepatol. 2023, 79, 1302–1316. [Google Scholar] [CrossRef]
- Chiarelli, F.; Di Marzio, D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: Current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [CrossRef]
- Ju, Z.; Su, M.; Hong, J.; Kim, E.; Jung, J.H. Anti-inflammatory effects of an optimized PPAR-γ agonist via NF-κB pathway inhibition. Bioorganic Chem. 2020, 96, 103611. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, R.; Gu, Y.; Ji, Y.; Lou, Y.; Wu, J. Therapeutic Targeting of PPARγ in Nonalcoholic Fatty Liver Disease: Efficacy, Safety, and Drug Development. Drug Des. Dev. Ther. 2025, 19, 7293–7319. [Google Scholar] [CrossRef]
- Stasi, C.; Milani, S.; Galli, A. Gut-liver the role of serotonin and its pathways in hepatic fibrogenesis. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes, 1st ed.; Stasi, C., Ed.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Kawaguchi, K.; Sakaida, I.; Tsuchiya, M.; Omori, K.; Takami, T.; Okita, K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem. Biophys. Res. Commun. 2004, 315, 187–195. [Google Scholar] [CrossRef]
- Deng, W.; Meng, Z.; Sun, A.; Yang, Z. Pioglitazone suppresses inflammation and fibrosis in nonalcoholic fatty liver disease by down-regulating PDGF and TIMP-2: Evidence from in vitro study. Cancer Biomark. Sect. A Dis. Markers 2017, 20, 411–415. [Google Scholar] [CrossRef]
- Miyahara, T.; Schrum, L.; Rippe, R.; Xiong, S.; Yee, H.F.; Motomura, K., Jr.; Anania, F.A.; Willson, T.M.; Tsukamoto, H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J. Biol. Chem. 2000, 275, 35715–35722. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Wilson, L.A.; Yates, K.P.; Cummings, O.W.; Vilar-Gomez, E.; Ajmera, V.; Kowdley, K.V.; Rosenberg, W.M.; Tonascia, J.; Chalasani, N. Relationship of ELF and PIIINP with Liver Histology and Response to Vitamin E or Pioglitazone in the PIVENS Trial. Hepatol. Commun. 2021, 5, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Abdel Monem, M.S.; Adel, A.; Abbassi, M.M.; Abdelaziz, D.H.; Hassany, M.; Raziky, M.E.; Sabry, N.A. Efficacy and safety of dapagliflozin compared to pioglitazone in diabetic and non-diabetic patients with non-alcoholic steatohepatitis: A randomized clinical trial. Clin. Res. Hepatol. Gastroenterol. 2025, 49, 102543. [Google Scholar] [CrossRef]
- Sayadishahraki, M.; Mirfendereski, S.; Kachuei, A.; Rafiee Zadeh, A.; Mirghaderi, A. Effect of Pioglitazone on Nonalcoholic Fatty Liver Disease in Morbid Obese Patients; a Randomized Controlled Trial. Adv. Biomed. Res. 2023, 12, 27. [Google Scholar] [CrossRef]
- Lee, M.; Hong, S.; Cho, Y.; Rhee, H.; Yu, M.H.; Bae, J.; Lee, Y.H.; Lee, B.W.; Kang, E.S.; Cha, B.S. Synergistic benefit of thiazolidinedione and sodium-glucose cotransporter 2 inhibitor for metabolic dysfunction-associated steatotic liver disease in type 2 diabetes: A 24-week, open-label, randomized controlled trial. BMC Med. 2025, 23, 266. [Google Scholar] [CrossRef]
- Khaliq, A.; Badshah, H.; Shah, Y.; Rehman, I.U.; Khan, K.U.; Ming, L.C.; Cheng, M.H. The effect of ertugliflozin in patients with nonalcoholic fatty liver disease associated with type 2 diabetes mellitus: A randomized controlled trial. Medicine 2024, 103, e40356. [Google Scholar] [CrossRef]
- Khaliq, A.; Badshah, H.; Shah, Y. Combination therapy with vitamin E and ertugliflozin in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: A randomized clinical trial. Ir. J. Med. Sci. 2025, 194, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; Puckett, C.; Abdelgani, S.; Merovci, A.; Lavrynenko, O.; Adams, J.; Triplitt, C.; DeFronzo, R.A. Glycemic and non-glycemic benefits of initial triple therapy versus sequential add-on therapy in patients with new-onset diabetes: Results from the EDICT study. BMJ Open Diabetes Res. Care 2025, 13, e004981. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.G.; Wood, E.M. FIB-4 as a screening and disease monitoring method in pre-fibrotic stages of metabolic dysfunction-associated fatty liver disease (MASLD). J. Diabetes Its Complicat. 2024, 38, 108777. [Google Scholar] [CrossRef]
- Jalali, M.; Rahimlou, M.; Mahmoodi, M.; Moosavian, S.P.; Symonds, M.E.; Jalali, R.; Zare, M.; Imanieh, M.H.; Stasi, C. The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: An up-to date systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 159, 104799. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Shimizu, S.; Haisa, A.; Yanagisawa, S.; Inoue, K.; Saito, D.; Sumita, T.; Yanagisawa, M.; Uchida, Y.; Inukai, K.; et al. Long-term effects of ipragliflozin and pioglitazone on metabolic dysfunction-associated steatotic liver disease in patients with type 2 diabetes: 5 year observational follow-up of a randomized, 24 week, active-controlled trial: Effect of ipragliflozin in MASLD. J. Diabetes Investig. 2024, 15, 1220–1230. [Google Scholar] [CrossRef]
- Hooshmand Gharabagh, L.; Shargh, A.; Mohammad Hosseini Azar, M.R.; Esmaeili, A. Comparison between the effect of Empagliflozin and Pioglitazone added to metformin in patients with type 2 diabetes and nonalcoholic fatty liver disease. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102279. [Google Scholar] [CrossRef]
- Pereira, I.V.A.; de Oliveira, A.B.S.; Zitelli, P.M.Y.; Barbieri, L.A.; Cardoso, A.C.; Monteiro, M.J.S.D.; de Oliveira, J.S.; Stefano, J.T.; Altikes, R.; Reis, A.L.G.; et al. Long-term pioglitazone use in MASLD patients: Insights from a multicentric preliminary study. Clinics 2025, 80, 100737. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef]
- Martínez-Sánchez, F.D.; Corredor-Nassar, M.J.; Feria-Agudelo, S.M.; Paz-Zarza, V.M.; Martinez-Perez, C.; Diaz-Jarquin, A.; Manzo-Santana, F.; Sánchez-Gómez, V.A.; Rosales-Padron, A.; Baca-García, M.; et al. Factors Associated with Advanced Liver Fibrosis in a Population with Type 2 Diabetes: A Multicentric Study in Mexico City. J. Clin. Exp. Hepatol. 2025, 15, 102536. [Google Scholar] [CrossRef]
- Shi, Y.; Kim, S.U.; Yip, T.C.; Tsochatzis, E.; Petta, S.; Nakajima, A.; Hagström, H.; Bugianesi, E.; Chan, W.K.; Boursier, J.; et al. Effect of Antidiabetic Drug Classes on the Risk of Liver-Related Events in Individuals with T2D and MASLD. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2025; advance online publication. [Google Scholar] [CrossRef]
- Lee, C.H.; Lui, D.T.; Mak, L.Y.; Fong, C.H.; Chan, K.S.; Mak, J.H.; Cheung, C.Y.; Chow, W.S.; Woo, Y.C.; Yuen, M.F.; et al. Benefits of combining SGLT2 inhibitors and pioglitazone on risk of MASH in type 2 diabetes-A real-world study. Diabetes Obes. Metab. 2025, 27, 574–582. [Google Scholar] [CrossRef]
- ClinicalTrials.gov ID NCT04501406 Low-Dose Pioglitazone in Patients with NASH (AIM 2). Last Update Posted 4 April 2025. Available online: https://clinicaltrials.gov/study/NCT04501406?cond=NASH%20-%20Nonalcoholic%20Steatohepatitis&intr=pioglitazone&rank=2 (accessed on 4 April 2025).
- Markowska, J.; Kasprzak-Drozd, K.; Niziński, P.; Dragan, M.; Kondracka, A.; Gondek, E.; Oniszczuk, T.; Oniszczuk, A. Quercetin: A Promising Candidate for the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Molecules 2024, 29, 5245. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, T.; Mustafa, M.R.; Achike, F.I.; Murugan, D.D. Quercetin and pioglitazone synergistically reverse endothelial dysfunction in isolated aorta from fructose-streptozotocin (F-STZ)-induced diabetic rats. Eur. J. Pharmacol. 2017, 799, 160–170. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Y.; Yang, L.; Wang, M.; Lai, Y. Comparison of efficacy and safety of pioglitazone and SGLT2 inhibitors in treating Asian patients in MASLD associated with type 2 diabetes: A meta-analysis. J. Diabetes Its Complicat. 2025, 39, 108998. [Google Scholar] [CrossRef]
- Liao, H.W.; Saver, J.L.; Wu, Y.L.; Chen, T.H.; Lee, M.; Ovbiagele, B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: A systematic review and meta-analysis. BMJ Open 2017, 7, e013927. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Shi, W.; Fu, S.; Wang, T.; Zhai, S.; Song, Y.; Han, J. Pioglitazone and bladder cancer risk: A systematic review and meta-analysis. Cancer Med. 2018, 7, 1070–1080. [Google Scholar] [CrossRef]
- Nogueira, A.C.C.; Barreto, J.; Moura, F.A.; Luchiari, B.; Abuhab, A.; Bonilha, I.; Nadruz, W.; Gaziano, J.M.; Gaziano, T.; de Carvalho, L.S.F.; et al. Comparative effectiveness and cost-effectiveness of cardioprotective glucose-lowering therapies for type 2 diabetes in Brazil: A Bayesian network model. Health Econ. Rev. 2023, 13, 50. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasi, C.; Mega, A. Improvement of Liver Fibrosis in Patients with MASLD Undergoing Pioglitazone Treatment: An Update. Life 2025, 15, 1682. https://doi.org/10.3390/life15111682
Stasi C, Mega A. Improvement of Liver Fibrosis in Patients with MASLD Undergoing Pioglitazone Treatment: An Update. Life. 2025; 15(11):1682. https://doi.org/10.3390/life15111682
Chicago/Turabian StyleStasi, Cristina, and Andrea Mega. 2025. "Improvement of Liver Fibrosis in Patients with MASLD Undergoing Pioglitazone Treatment: An Update" Life 15, no. 11: 1682. https://doi.org/10.3390/life15111682
APA StyleStasi, C., & Mega, A. (2025). Improvement of Liver Fibrosis in Patients with MASLD Undergoing Pioglitazone Treatment: An Update. Life, 15(11), 1682. https://doi.org/10.3390/life15111682







