Analysis of Pharmacological Properties of Nigella sativa L. Bioactive Compounds and Their Therapeutic Relevance in the Management of Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Pharmacological Basis and Experimental Evidence
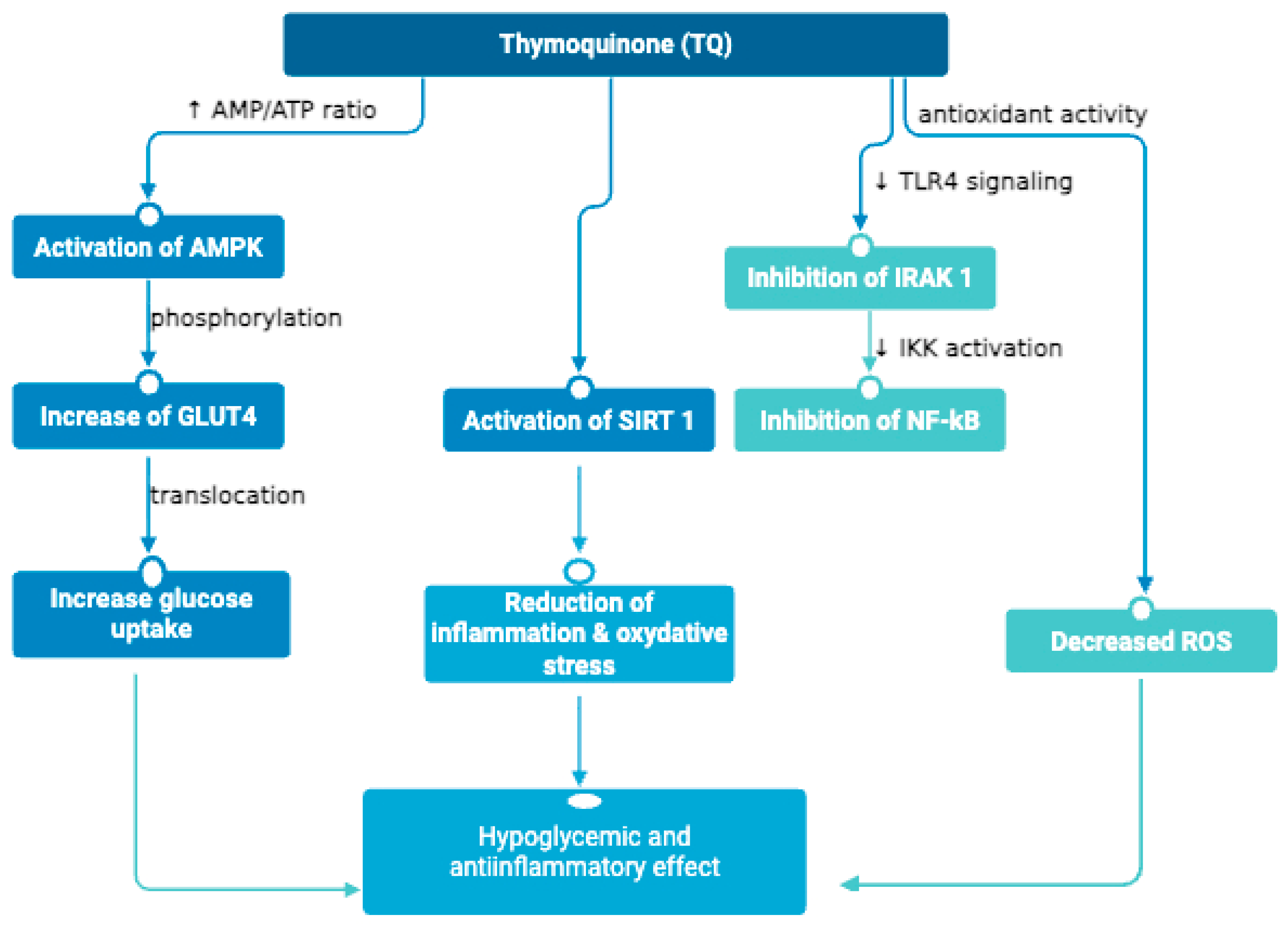
2.1. In vitro Studies on the Antidiabetic Potential of NS
2.2. In vivo Studies on the Antidiabetic Potential of NS
3. Nigella sativa L.’s Evidence of T2DM Complications
4. Evidence of Nigella sativa L. in Commonly Associated T2DM Conditions
5. Innovations in the Ways of NS Administration
6. Safety and Adverse Effects of NS
7. Future Directions of Research as a Supplement in T2DM
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| NS | Nigella sativa |
| TQ | Thymoquinone |
| THQ | Thymohydroquinone |
| DTQ | Dithymoquinone |
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public. Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Tönnies, T.; Rathmann, W.; Hoyer, A.; Brinks, R.; Kuss, O. Quantifying the Underestimation of Projected Global Diabetes Prevalence by the International Diabetes Federation (IDF) Diabetes Atlas. BMJ Open Diabetes Res. Care 2021, 9, e002122. [Google Scholar] [CrossRef]
- Kundnani, N.R.; Lolescu, B.; Dinu, A.-R.; Berceanu-Vaduva, D.M.; Dumitrescu, P.; Tamaș, T.-P.; Sharma, A.; Popa, M.-D. Biotechnology Revolution Shaping the Future of Diabetes Management. Biomolecules 2024, 14, 1563. [Google Scholar] [CrossRef]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef]
- Duarte, A.M.; Guarino, M.P.; Barroso, S.; Gil, M.M. Phytopharmacological Strategies in the Management of Type 2 Diabetes Mellitus. Foods 2020, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Rydén, L.; Lindsten, J. The History of the Nobel Prize for the Discovery of Insulin. Diabetes Res. Clin. Pract. 2021, 175, 108819. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, W.; Liu, J.; Xie, M.; Liu, Q.; Li, S. Vascular Complications of Diabetes: A Narrative Review. Medicine 2023, 102, e35285. [Google Scholar] [CrossRef]
- Balbaa, M.; El-Zeftawy, M.; Abdulmalek, S.A. Therapeutic Screening of Herbal Remedies for the Management of Diabetes. Molecules 2021, 26, 6836. [Google Scholar] [CrossRef]
- Behl, T.; Gupta, A.; Albratty, M.; Najmi, A.; Meraya, A.M.; Alhazmi, H.A.; Anwer, M.K.; Bhatia, S.; Bungau, S.G. Alkaloidal Phytoconstituents for Diabetes Management: Exploring the Unrevealed Potential. Molecules 2022, 27, 5851. [Google Scholar] [CrossRef]
- Adam, S.H.; Abu, I.F.; Kamal, D.A.M.; Febriza, A.; Kashim, M.I.A.M.; Mokhtar, M.H. A Review of the Potential Health Benefits of Nigella sativa on Obesity and Its Associated Complications. Plants 2023, 12, 3210. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P.; Cucinella, L.; Nappi, R.E. The Use of Nigella sativa in Cardiometabolic Diseases. Biomedicines 2024, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Ghods, M.; Karimi, S.; Salari, S.; Alem, E.; Bahmani, P.; Karimi, A.; Saeedpour, A.; Noormohamadi, M.; Jahromi, S.R. Anti-diabetic Effect of a Combination of Black Seed (Nigella sativa) and Cumin (Cuminum cyminum), a Two-step Study from Bench to Bed. Funct. Food Sci. Online 2024, 4, 55–68. [Google Scholar] [CrossRef]
- Singh, T.G.; Sharma, R.; Kaur, A.; Dhiman, S.; Singh, R. Evaluation of renoprotective potential of Ficus religiosa in attenuation of diabetic nephropathy in rats. Obes. Med. 2020, 19, 100268. [Google Scholar] [CrossRef]
- Pishdad, R.; Auwaerter, P.G.; Kalyani, R.R. Diabetes, SGLT-2 Inhibitors, and Urinary Tract Infection: A Review. Curr. Diab. Rep. 2024, 24, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Jangjo-Borazjani, S.; Dastgheib, M.; Kiyamarsi, E.; Jamshidi, R.; Rahmati-Ahmadabad, S.; Helalizadeh, M.; Iraji, R.; Cornish, S.M.; Mohammadi-Darestani, S.; Khojasteh, Z.; et al. Effects of resistance training and Nigella sativa on type 2 diabetes: Implications for metabolic markers, low-grade inflammation and liver enzyme production. Arch. Physiol. Biochem. 2023, 129, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B. Roles of Alkaloids from Medicinal Plants in the Management of Diabetes Mellitus. J. Chem. 2021, 2021, 2691525. [Google Scholar] [CrossRef]
- Maurya, A.; Mohan, S.; Verma, S.C. Antidiabetic Potential of Naturally Occurring Sesquiterpenes: A Review. Curr. Top. Med. Chem. 2021, 21, 851–862. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Ye, X.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol. Res. 2022, 177, 106114. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Y.; Dong, J.; He, K.; Che, H. Insulin Resistance-Nutritional Index: A Simple Index and Potential Predictor of Mortality Risk in Patients with Chronic Heart Failure and Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 2024, 17, 4177–4190. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef]
- Khalivulla, S.I.; Mohammed, A.; Mallikarjuna, K. Novel Phytochemical Constituents and their Potential to Manage Diabetes. Curr. Pharm. Des. 2021, 27, 775–788. [Google Scholar] [CrossRef]
- Kumar, S.; Mittal, A.; Babu, D.; Mittal, A. Herbal Medicines for Diabetes Management and its Secondary Complications. Curr. Diabetes Rev. 2021, 17, 437–456. [Google Scholar] [CrossRef]
- Simon-Szabó, L.; Lizák, B.; Sturm, G.; Somogyi, A.; Takács, I.; Németh, Z. Molecular Aspects in the Development of Type 2 Diabetes and Possible Preventive and Complementary Therapies. Int. J. Mol. Sci. 2024, 25, 9113. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; V. Anil Kumar, N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Rasouli, H.; Yarani, R.; Pociot, F.; Popović-Djordjević, J. Anti-diabetic potential of plant alkaloids: Revisiting current findings and future perspectives. Pharmacol. Res. 2020, 155, 104723. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef] [PubMed]
- Aslani, M.R.; Saadat, S.; Boskabady, M.H. Comprehensive and updated review on anti-oxidant effects of Nigella sativa and its constituent, thymoquinone, in various disorders. Iran. J. Basic Med. Sci. 2024, 27, 923–951. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Imran, M.; Ul-Haq, I.; Živković, J.; Abu-Reidah, I.M.; Sen, S.; Taheri, Y.; Acharya, K.; Azadi, H.; et al. Nigella Plants–Traditional Uses, Bioactive Phytoconstituents, Preclinical and Clinical Studies. Front. Pharmacol. 2021, 12, 625386. [Google Scholar] [CrossRef]
- Ojueromi, O.O.; Oboh, G.; Ademosun, A.O. Black Seed (Nigella sativa): A Favourable Alternative Therapy for Inflammatory and Immune System Disorders. Inflammopharmacology 2022, 30, 1623–1643. [Google Scholar] [CrossRef]
- Adam, S.H.; Mohd Nasri, N.; Kashim, M.I.A.M.; Abd Latib, E.H.; Ahmad Juhari, M.A.A.; Mokhtar, M.H. Potential health benefits of Nigella sativa on diabetes mellitus and its complications: A review from laboratory studies to clinical trials. Front. Nutr. 2022, 9, 1057825. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Nigella sativa: A Comprehensive Review of Its Therapeutic Potential, Pharmacological Properties, and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 13410. [Google Scholar] [CrossRef]
- Sam, J.H.; Chan, Y.S.; Siner, A. Antioxidant and Antimalarial Properties of Nigella sativa. Int. J. Chem. Eng. Appl. 2024, 15, 12–15. [Google Scholar] [CrossRef]
- Koshak, D.A.E. Effects of Nigella sativa as a Treatment of Patients With Upper Respiratory Tract Infection Caused by SARS-coronavirus-2: A Prospective, Randomized, Open-label, Controlled Clinical Study. Clin. Gov. 2021, 61, 102769. [Google Scholar]
- Nyulas, K.-I.; Simon-Szabó, Z.; Pál, S.; Fodor, M.-A.; Dénes, L.; Cseh, M.J.; Barabás-Hajdu, E.; Csipor, B.; Szakács, J.; Preg, Z.; et al. Cardiovascular Effects of Herbal Products and Their Interaction with Antihypertensive Drugs—Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 6388. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.; Toaima, W.; Abd El-Aleem, W. Impact of Different Planting Locations on Nigella sativa L. Yield in Egypt. Egypt. J. Desert Res. 2023, 73, 23–38. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Amir, M.; AlJHISI, F.; Alamer, M.H.; Al-Shaban, H.R.; Alsultan, B.M.; Alsadah, Z.A.; Aldawood, N.A.; Chathoth, S.; et al. Variation in Nigella sativa quality and its standardization via instrumental analysis: A study based on geographical origin. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1141–1154. [Google Scholar] [CrossRef]
- Belgaumi, U.I.; Patil, S.; Gandhi, J.M.; Shete, A.S. The Many Therapeutic Applications of Nigella sativa–A Review of Literature. J. Evol. Med. Dent. Sci. 2020, 9, 2151–2157. [Google Scholar] [CrossRef]
- Ferizi, R.; Ramadan, M.F.; Maxhuni, Q. Black Seeds (Nigella sativa) Medical Application and Pharmaceutical Perspectives. J. Pharm. Bioallied Sci. 2023, 15, 63–67. [Google Scholar] [CrossRef]
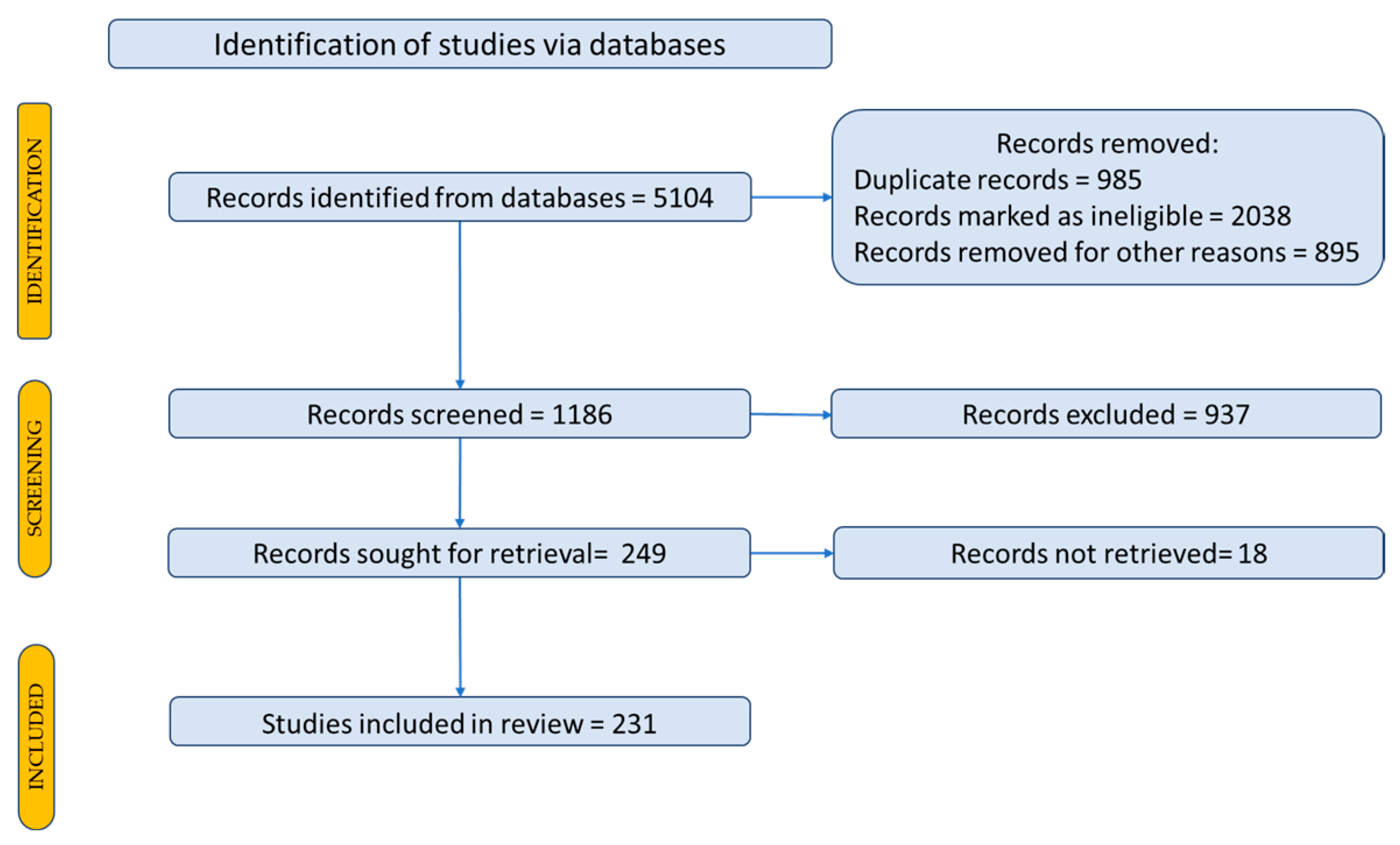
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi-Sardoo, H.; Rezaee, R.; Karimi, G. An overview of in vivo toxicological profile of thymoquinone. Toxin Rev. 2020, 39, 115–122. [Google Scholar] [CrossRef]
- Mohebbati, R.; Abbasnezhad, A. Effects of Nigella sativa on endothelial dysfunction in diabetes mellitus: A review. J. Ethnopharmacol. 2020, 252, 112585. [Google Scholar] [CrossRef] [PubMed]
- Elhariri, S.; Burud, I.; Zulaimy, N.A.; Tong, J.A.; Ahmed, I.; Kar Chun, S.C.; Kumaran, P. Systematic Review of Randomized Controlled Trials in Uses of Nigella sativa (Black Seed) in Metabolic Syndrome. W. Afr. J. Med. 2024, 41, 372–380. [Google Scholar]
- Ahmad, A.; Alqahtani, S.; Jan, B.L.; Raish, M.; Rabba, A.K.; Alkharfy, K.M. Gender effect on the pharmacokinetics of thymoquinone: Preclinical investigation and in silico modeling in male and female rats. Saudi Pharm. J. 2020, 28, 403–408. [Google Scholar] [CrossRef]
- Karimi, Z.; Mirza Alizadeh, A.; Ezzati Nazhad Dolatabadi, J.; Dehghan, P. Nigella sativa and its Derivatives as Food Toxicity Protectant Agents. Adv. Pharm. Bull. 2019, 9, 22–37. [Google Scholar] [CrossRef]
- Kavyani, Z.; Musazadeh, V.; Golpour-hamedani, S.; Moridpour, A.H.; Vajdi, M.; Askari, G. The effect of Nigella sativa (black seed) on biomarkers of inflammation and oxidative stress: An updated systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2023, 31, 1149–1165. [Google Scholar] [CrossRef]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef]
- Shaukat, A.; Zaidi, A.; Anwar, H.; Kizilbash, N. Mechanism of the antidiabetic action of Nigella sativa and Thymoquinone: A review. Front. Nutr. 2023, 10, 1126272. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Legoabe, L.J.; Montesano, D.; Zengin, G. Nigella sativa L. and Its Active Compound Thymoquinone in the Clinical Management of Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 12111. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.G.; Hudu, S.A.; Jega, A.Y.; Taha, A.; Yusuf, A.P.; Usman, D.; Adeshina, K.A.; Umar, Z.U.; Nyakudya, T.T.; Erlwanger, K.H. Thymoquinone: A comprehensive review of its potential role as a monotherapy for metabolic syndrome. Iran. J. Basic. Med. Sci. 2024, 27, 1214–1227. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Kim, J.W.; Kim, J.-K.; Chun, Y.-S.; Choi, J.-S.; Ku, S.-K. Efficacy Confirmation Test of Black Cumin (Nigella sativa L.) Seeds Extract Using a High-Fat Diet Mouse Model. Metabolites 2023, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Maideen, N.M.P. Antidiabetic Activity of Nigella sativa (Black Seeds) and Its Active Constituent (Thymoquinone): A Review of Human and Experimental Animal Studies. Chonnam Med. J. 2021, 57, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef]
- Tiji, S.; Bouhrim, M.; Addi, M.; Drouet, S.; Lorenzo, J.M.; Hano, C.; Bnouham, M.; Mimouni, M. Linking the Phytochemicals and the α-Glucosidase and α-Amylase Enzyme Inhibitory Effects of Nigella sativa Seed Extracts. Foods 2021, 10, 1818. [Google Scholar] [CrossRef]
- Veeramani, S.; Narayanan, A.P.; Yuvaraj, K.; Sivaramakrishnan, R.; Pugazhendhi, A.; Rishivarathan, I.; Jose, S.P.; Ilangovan, R. Nigella sativa flavonoids surface coated gold NPs (Au-NPs) enhancing antioxidant and anti-diabetic activity. Process Biochem. 2022, 114, 193–202. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Divya, M.; Vaseeharan, B.; Chen, J.; Biruntha, M.; Silva, L.P.; Durán-Lara, E.F.; Shreema, K.; Ranjan, S.; Dasgupta, N. Biological Compound Capping of Silver Nanoparticle with the Seed Extracts of Blackcumin (Nigella sativa): A Potential Antibacterial, Antidiabetic, Anti-inflammatory, and Antioxidant. J. Inorg. Organomet. Polym. Mater. 2021, 31, 624–635. [Google Scholar] [CrossRef]
- Dalli, M.; Daoudi, N.E.; Azizi, S.-E.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical Composition Analysis Using HPLC-UV/GC-MS and Inhibitory Activity of Different Nigella sativa Fractions on Pancreatic α-Amylase and Intestinal Glucose Absorption. BioMed Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef] [PubMed]
- Varghese, L.N.; Mehrotra, N. α-Amylase inhibitory activity of microencapsulated Nigella sativa L. and herb- drug interaction: An in vitro analysis. Ann. Phytomed. Int. J. 2020, 9, 107–112. [Google Scholar] [CrossRef]
- Alkis, H.; Demir, E.; Taysi, M.R.; Sagir, S.; Taysi, S. Effects of Nigella sativa oil and thymoquinone on radiation-induced oxidative stress in kidney tissue of rats. Biomed. Pharmacother. 2021, 139, 111540. [Google Scholar] [CrossRef]
- Ali, M.Y.; Akter, Z.; Mei, Z.; Zheng, M.; Tania, M.; Khan, M.A. Thymoquinone in autoimmune diseases: Therapeutic potential and molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111157. [Google Scholar] [CrossRef]
- Mahmud, N.M.; Paraoan, L.; Khaliddin, N.; Kamalden, T.A. Thymoquinone in Ocular Neurodegeneration: Modulation of Pathological Mechanisms via Multiple Pathways. Front. Cell. Neurosci. 2022, 16, 786926. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Kim, M.-Y.; Cho, J.Y. The Role of Thymoquinone in Inflammatory Response in Chronic Diseases. Int. J. Mol. Sci. 2022, 23, 10246. [Google Scholar] [CrossRef]
- Ahmad, A.; Alkharfy, K.M.; Jan, B.L.; Ahad, A.; Ansari, M.A.; Al-Jenoobi, F.I.; Raish, M. Thymoquinone treatment modulates the Nrf2/HO-1 signaling pathway and abrogates the inflammatory response in an animal model of lung fibrosis. Exp. Lung Res. 2020, 46, 53–63. [Google Scholar] [CrossRef]
- Ateş, Ş.; Ülger, H.; Uçar, S.; Okan, A.; Ocak, M.; Güvenilir, E.; Şükranlı, Z.Y.; Kaymak, E.; Doğanyiğit, Z.; Taheri, S.; et al. Evaluation of the Effects of Thymoquinone on RAGE/NOX4 Expressions and Brain Tissue Morphometry in Experimental Alzheimer’s Disease Induced by Amyloid Beta 1-42 Peptide. Biomolecules 2025, 15, 543. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.-H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.; Sleem, R.; Abd-Elaziz, A.; Khalil, H. Regulation of NF-κB Expression by Thymoquinone; A Role in Regulating Pro-Inflammatory Cytokines and Programmed Cell Death in Hepatic Cancer Cells. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 3739–3748. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Biological and therapeutic activities of thymoquinone: Focus on the Nrf2 signaling pathway. Phytother. Res. 2021, 35, 1739–1753. [Google Scholar] [CrossRef]
- Chatterjee, G.; Saha, A.K.; Khurshid, S.; Saha, A. A Comprehensive Review of the Antioxidant, Antimicrobial, and Therapeutic Efficacies of Black Cumin (Nigella sativa L.) Seed Oil and Its Thymoquinone. J. Med. Food 2025, 28, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef]
- Ahmad, A.; Mishra, R.K.; Vyawahare, A.; Kumar, A.; Rehman, M.U.; Qamar, W.; Khan, A.Q.; Khan, R. Thymoquinone (2-Isopropyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019, 27, 1113–1126. [Google Scholar] [CrossRef]
- Rahman, M.T. Potential benefits of combination of Nigella sativa and Zn supplements to treat COVID-19. J. Herb. Med. 2020, 23, 100382. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.T.; Qadir, R.; Bukhari, I.; Ashraf, R.A.; Malik, Z.; Zahoor, S.; Murtaza, M.A.; Siddique, F.; Shah, S.N.H.; Saadia, M. Antidiabetic potential of Nigella sativa L seed oil in alloxaninduced diabetic rabbits. Trop. J. Pharm. Res. 2020, 19, 283–289. [Google Scholar] [CrossRef]
- Khodaie, S.-A.; Razavi, R.; Nikkhah, H.; Namiranian, N.; Kamalinejad, M. Nigella sativa L. and its bioactive and nutraceutical components in the management of diabetic peripheral neuropathy. Inflammopharmacology 2024, 32, 2897–2920. [Google Scholar] [CrossRef]
- Gomathinayagam, R.; Ha, J.H.; Jayaraman, M.; Song, Y.S.; Isidoro, C.; Dhanasekaran, D.N. Chemopreventive and Anticancer Effects of Thymoquinone: Cellular and Molecular Targets. J. Cancer Prev. 2020, 25, 136–151. [Google Scholar] [CrossRef]
- Akter, Z.; Ahmed, F.R.; Tania, M.; Khan, M.A. Targeting Inflammatory Mediators: An Anticancer Mechanism of Thymoquinone Action. Curr. Med. Chem. 2021, 28, 80–92. [Google Scholar] [CrossRef]
- Pop, R.M.; Sabin, O.; Suciu, Ș.; Vesa, S.C.; Socaci, S.A.; Chedea, V.S.; Bocsan, I.C.; Buzoianu, A.D. Nigella sativa’s Anti-Inflammatory and Antioxidative Effects in Experimental Inflammation. Antioxidants 2020, 9, 921. [Google Scholar] [CrossRef]
- Elsharkawy, E.R.; Abdallah, E.M.; Markb, A.A. Potential Cytotoxic, Antifungal, and Antioxidant Activity of Dithymoquinone and Thymoquinone. J. Hunan Univ. Nat. Sci. 2021, 48, 90–99. [Google Scholar]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 11 July 2025).
- Sakib, R.; Caruso, F.; Aktar, S.; Belli, S.; Kaur, S.; Hernandez, M.; Rossi, M. Antioxidant Properties of Thymoquinone, Thymohydroquinone and Black Cumin (Nigella sativa L.) Seed Oil: Scavenging of Superoxide Radical Studied Using Cyclic Voltammetry, DFT and Single Crystal X-ray Diffraction. Antioxidants 2023, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Esharkawy, E.R.; Almalki, F.; Hadda, T.B. In vitro potential antiviral SARS-CoV-19- activity of natural product thymohydroquinone and dithymoquinone from Nigella sativa. Bioorganic Chem. 2022, 120, 105587. [Google Scholar] [CrossRef]
- Tesarova, H.; Svobodova, B.; Kokoska, L.; Marsik, P.; Pribylova, M.; Landa, P.; Vadlejch, J. Determination of oxygen radical absorbance capacity of black cumin (Nigella sativa) seed quinone compounds. Nat. Prod. Commun. 2011, 6, 213–216. [Google Scholar] [CrossRef]
- Sturabotti, E.; Moldoveanu, V.G.; Camilli, A.; Martinelli, A.; Simonetti, G.; Valletta, A.; Serangeli, I.; Giustini, A.; Miranda, E.; Migneco, L.M.; et al. Thymol-Functionalized Hyaluronic Acid as Promising Preservative Biomaterial for the Inhibition of Candida albicans Biofilm Formation. ACS Macro Lett. 2023, 12, 1079–1084. [Google Scholar] [CrossRef]
- Azizi, Z.; Salimi, M.; Amanzadeh, A.; Majelssi, N.; Naghdi, N. Carvacrol and Thymol Attenuate Cytotoxicity Induced by Amyloid β25-35 Via Activating Protein Kinase C and Inhibiting Oxidative Stress in PC12 Cells. Iran. Biomed. J. 2020, 24, 243–250. [Google Scholar] [CrossRef]
- Agarwal, S.; Tripathi, R.; Mohammed, A.; Rizvi, S.I.; Mishra, N. Effects of thymol supplementation against type 2 diabetes in streptozotocin-induced rat model. Plant Arch. 2020, 20, 863–869. [Google Scholar]
- Bagińska, S.; Golonko, A.; Świsłocka, R.; Lewandowski, W. Monoterpenes as Medicinal Agents: Exploring the Pharmaceutical Potential of p-Cymene, p-Cymenene, and γ-Terpinene. Acta Pol. Pharm. 2024, 80, 879–892. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Krause, S.T.; Liao, P.; Crocoll, C.; Boachon, B.; Förster, C.; Leidecker, F.; Wiese, N.; Zhao, D.; Wood, J.C.; Buell, C.R.; et al. The biosynthesis of thymol, carvacrol, and thymohydroquinone in Lamiaceae proceeds via cytochrome P450s and a short-chain dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Trindade, H. Sequencing and variation of terpene synthase gene (TPS2) as the major gene in biosynthesis of thymol in different Thymus species. Phytochemistry 2020, 169, 112126. [Google Scholar] [CrossRef]
- Jin, H.; Leng, Q.; Zhang, C.; Zhu, Y.; Wang, J. P-cymene prevent high-fat diet-associated colorectal cancer by improving the structure of intestinal flora. J. Cancer 2021, 12, 4355–4361. [Google Scholar] [CrossRef] [PubMed]
- Formiga, R.d.O.; Alves Júnior, E.B.; Vasconcelos, R.C.; Guerra, G.C.B.; Antunes de Araújo, A.; Carvalho, T.G.D.; Garcia, V.B.; de Araújo Junior, R.F.; Gadelha, F.A.A.F.; Vieira, G.C.; et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. [Google Scholar] [CrossRef]
- Pyo, Y.; Jung, Y.J. Microbial Fermentation and Therapeutic Potential of p-Cymene: Insights into Biosynthesis and Antimicrobial Bioactivity. Fermentation 2024, 10, 488. [Google Scholar] [CrossRef]
- Ciesielska-Figlon, K.; Daca, A.; Kokotkiewicz, A.; Łuczkiewicz, M.; Zabiegała, B.; Witkowski, J.M.; Lisowska, K.A. The influence of Nigella sativa essential oil on proliferation, activation, and apoptosis of human T lymphocytes in vitro. Biomed. Pharmacother. 2022, 153, 113349. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Kasprzak-Drozd, K.; Oniszczuk, A.; Borowicz-Reutt, K. Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies-A Non-Systematic Review. Int. J. Mol. Sci. 2021, 22, 7366. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, C.; Yu, J. Natural Monoterpenes as Potential Therapeutic Agents against Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 2429. [Google Scholar] [CrossRef] [PubMed]
- Neopane, D.; Kushwaha, P. Carvacrol in asthma management: A comprehensive review of its therapeutic potential and mechanisms of action. Pharmacol. Rep. 2025, 77, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Della Rocca, Y.; Fonticoli, L.; Guarnieri, S.; Carradori, S.; Rajan, T.S.; Pizzicannella, J.; Diomede, F. The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations. Antioxidants 2022, 11, 386. [Google Scholar] [CrossRef]
- Hoca, M.; Becer, E.; Vatansever, H.S. Carvacrol is potential molecule for diabetes treatment. Arch. Physiol. Biochem. 2024, 130, 823–830. [Google Scholar] [CrossRef]
- Cicalău, G.I.P.; Babes, P.A.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.M.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, X.; Wang, S.; Xu, C.; Gao, M.; Liu, S.; Li, X.; Cheng, N.; Han, Y.; Wang, X.; et al. Thymoquinone Prevents Dopaminergic Neurodegeneration by Attenuating Oxidative Stress Via the Nrf2/ARE Pathway. Front. Pharmacol. 2021, 11, 615598. [Google Scholar] [CrossRef]
- Isaev, N.K.; Chetverikov, N.S.; Stelmashook, E.V.; Genrikhs, E.E.; Khaspekov, L.G.; Illarioshkin, S.N. Thymoquinone as a Potential Neuroprotector in Acute and Chronic Forms of Cerebral Pathology. Biochem. Biokhimiia 2020, 85, 167–176. [Google Scholar] [CrossRef]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 319–329. [Google Scholar] [CrossRef]
- Khan, M.A.; Younus, H. Thymoquinone Shows the Diverse Therapeutic Actions by Modulating Multiple Cell Signaling Pathways: Single Drug for Multiple Targets. Curr. Pharm. Biotechnol. 2018, 19, 934–945. [Google Scholar] [CrossRef]
- Isaev, N.K.; Genrikhs, E.E.; Stelmashook, E.V. Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases. Antioxidants 2023, 12, 433. [Google Scholar] [CrossRef]
- Modarresi Chahardehi, A.; Ojaghi, H.R.; Motedayyen, H.; Arefnezhad, R. Nano-based formulations of thymoquinone are new approaches for psoriasis treatment: A literature review. Front. Immunol. 2024, 15, 1416842. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017, 57, 3911–3928. [Google Scholar] [CrossRef]
- Negi, P.; Rathore, C.; Sharma, G.; Singh, B.; Katare, O.P. Thymoquinone a Potential Therapeutic Molecule from the Plant Nigella sativa: Role of Colloidal Carriers in its Effective Delivery. Recent. Pat. Drug Deliv. Formul. 2018, 12, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.M.; El-Kholy, W.M.; El-Sawi, M.R.; El-Shafai, N.M.; Alotaibi, B.S.; Ghamry, H.I.; Shukry, M. Ameliorative Effect of Thymoquinone and Thymoquinone Nanoparticles against Diazinon-Induced Hepatic Injury in Rats: A Possible Protection Mechanism. Toxics 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, E.; Imenshahidi, M.; Hosseinzadeh, H. Molecular mechanisms and signaling pathways of black cumin (Nigella sativa) and its active constituent, thymoquinone: A review. Mol. Biol. Rep. 2023, 50, 5439–5454. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Manzoor, N.; Muneer, Z.; Bhatti, S.A.; Imran, I. Reserpine-induced altered neuro-behavioral, biochemical and histopathological assessments prevent by enhanced antioxidant defence system of thymoquinone in mice. Metab. Brain Dis. 2021, 36, 2535–2552. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. A Review on Possible Therapeutic Effect of Nigella sativa and Thymoquinone in Neurodegenerative Diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Jamaddar, S.; Islam, T.; Mondal, M.; Islam, M.T.; Mubarak, M.S. Therapeutic perspectives of the black cumin component thymoquinone: A review. Food Funct. 2021, 12, 6167–6213. [Google Scholar] [CrossRef] [PubMed]
- Imam, A.L.; Okesina, A.A.; Sulaimon, F.A.; Imam, A.; Ibiyeye, R.Y.; Oyewole, L.A.; Biliaminu, S.A.; Shehu, M.; Alli, A.O.; Omoola, O.O.; et al. Thymoquinone ameliorate oxidative stress, GABAergic neuronal depletion and memory impairment through Nrf2/ARE signaling pathway in the dentate gyrus following cypermethrin administration. BMC Neurosci. 2024, 25, 45. [Google Scholar] [CrossRef]
- Kantar, D.; Acun, A.D.; Danışman, B. Effects of thymoquinone on scopolamine-induced spatial and echoic memory changes through regulation of lipid peroxidation and cholinergic impairment. Behav. Brain Res. 2022, 431, 113972. [Google Scholar] [CrossRef]
- Tiwari, G.; Gupta, M.; Devhare, L.D.; Tiwari, R. Therapeutic and Phytochemical Properties of Thymoquinone Derived from Nigella sativa. Curr. Drug Res. Rev. 2024, 16, 145–156. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Neuroprotective Effects of Thymoquinone in Acrylamide-Induced Peripheral Nervous System Toxicity Through MAPKinase and Apoptosis Pathways in Rat. Neurochem. Res. 2019, 44, 1101–1112. [Google Scholar] [CrossRef]
- Zahra, N.; Fatima, S.; Nazir, A.; Farrukh, S.Y.; Anwer, A.; Sarwar, A.; Aziz, T.; Al Asmari, F.; Nahari, A.M.; Jalal, R.S.; et al. In vivo and in silico analysis of anti inflammatory, antipyretic and analgesic activity of methanolic extract of Nigella sativa. J. Mol. Histol. 2025, 56, 118. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, U.; Padhye, S.; Špirtović-Halilović, S.; Osmanović, A.; Veljović, E.; Roca, S.; Novaković, I.; Mandić, B.; Turel, I.; Kljun, J.; et al. Synthesis, Biological Evaluation and Docking Studies of Benzoxazoles Derived from Thymoquinone. Molecules 2018, 23, 3297. [Google Scholar] [CrossRef]
- Fatima Shad, K.; Soubra, W.; Cordato, D.J. The role of thymoquinone, a major constituent of Nigella sativa, in the treatment of inflammatory and infectious diseases. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1445–1453. [Google Scholar] [CrossRef]
- Eissa, N.; Alwattar, J.K.; Jayaprakash, P.; Chkier, D.; Ahmed, A.O.; Ahmed, A.; Rizwan, R.; Mujeeb, S.; Rahal, M.; Sadek, B. The Effects of Novel Thymoquinone-Loaded Nanovesicles as a Promising Avenue to Modulate Autism Associated Dysregulation by Restoring Oxidative Stress in Autism in Mice. Int. J. Nanomedicine 2025, 20, 8041–8061. [Google Scholar] [CrossRef] [PubMed]
- Dahmash, E.Z.; Ali, D.K.; Alyami, H.S.; AbdulKarim, H.; Alyami, M.H.; Aodah, A.H. Novel Thymoquinone Nanoparticles Using Poly(ester amide) Based on L-Arginine-Targeting Pulmonary Drug Delivery. Polymers 2022, 14, 1082. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, T.; Akin, A.T.; Karabulut, D.; Tan, F.C.; Taşkiran, M.; Yakan, B. Therapeutic effect of thymoquinone on brain damage caused by nonylphenol exposure in rats. J. Biochem. Mol. Toxicol. 2023, 37, e23471. [Google Scholar] [CrossRef]
- Bin Abdulrahman, K.; Bamosa, A.; Bukhari, A.; Siddiqui, I.; Arafa, M.; Mohsin, A.; Althageel, M.; Aljuaeed, M.; Aldeailej, I.; Alrajeh, A.; et al. The Effect of Short Treatment with Nigella sativa on Symptoms, the Cluster of Differentiation (CD) Profile, and Inflammatory Markers in Mild COVID-19 Patients: A Randomized, Double-Blind Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 11798. [Google Scholar] [CrossRef]
- Badary, O.A.; Hamza, M.S.; Tikamdas, R. Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure. Drug Des. Devel. Ther. 2021, 15, 1819–1833. [Google Scholar] [CrossRef]
- Shoaib, A.; Javed, S.; Wahab, S.; Azmi, L.; Tabish, M.; Sultan, M.H.; Abdelsalam, K.; Alqahtani, S.S.; Ahmad, M.F. Cellular, Molecular, Pharmacological, and Nano-Formulation Aspects of Thymoquinone-A Potent Natural Antiviral Agent. Molecules 2023, 28, 5435. [Google Scholar] [CrossRef]
- Wahab, S.; Alsayari, A. Potential Pharmacological Applications of Nigella Seeds with a Focus on Nigella sativa and Its Constituents against Chronic Inflammatory Diseases: Progress and Future Opportunities. Plants 2023, 12, 3829. [Google Scholar] [CrossRef]
- Tiwari, P.; Jena, S.; Satpathy, S.; Sahu, P.K. Nigella sativa: Phytochemistry, Pharmacology and its Therapeutic Potential. Res. J. Pharm. Technol. 2019, 12, 3111. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sharfaraz, A.; Dutta, A.; Ahsan, A.; Masud, M.A.; Ahmed, I.A.; Goh, B.H.; Urbi, Z.; Sarker, M.M.R.; Ming, L.C. A review of ethnobotany, phytochemistry, antimicrobial pharmacology and toxicology of Nigella sativa L. Biomed. Pharmacother. Biomedecine Pharmacother. 2021, 143, 112182. [Google Scholar] [CrossRef]
- Abbas, M.; Gururani, M.A.; Ali, A.; Bajwa, S.; Hassan, R.; Batool, S.W.; Imam, M.; Wei, D. Antimicrobial Properties and Therapeutic Potential of Bioactive Compounds in Nigella sativa: A Review. Molecules 2024, 29, 4914. [Google Scholar] [CrossRef]
- Ouattar, H.; Zouirech, O.; Kara, M.; Assouguem, A.; Almutairi, S.M.; Al-Hemaid, F.M.; Rasheed, R.A.; Ullah, R.; Abbasi, A.M.; Aouane, M.; et al. In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds. Molecules 2022, 27, 5946. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.D.S.; Guimarães, A.G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Gheorghita, D.; Robu, A.; Antoniac, A.; Antoniac, I.; Ditu, L.M.; Raiciu, A.-D.; Tomescu, J.; Grosu, E.; Saceleanu, A. In Vitro Antibacterial Activity of Some Plant Essential Oils against Four Different Microbial Strains. Appl. Sci. 2022, 12, 9482. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Shafodino, F.S.; Lusilao, J.M.; Mwapagha, L.M. Phytochemical characterization and antimicrobial activity of Nigella sativa seeds. PLoS ONE 2022, 17, e0272457. [Google Scholar] [CrossRef]
- Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Nazzaro, F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 6073. [Google Scholar] [CrossRef]
- Santos, W.B.R.; Melo, M.A.O.; Alves, R.S.; De Brito, R.G.; Rabelo, T.K.; Prado, L.D.S.; Silva, V.K.d.S.; Bezerra, D.P.; De Menezes-Filho, J.E.R.; Souza, D.S.; et al. p-Cymene attenuates cancer pain via inhibitory pathways and modulation of calcium currents. Phytomedicine 2019, 61, 152836. [Google Scholar] [CrossRef] [PubMed]
- Satira, A.; Espro, C.; Paone, E.; Calabrò, P.S.; Pagliaro, M.; Ciriminna, R.; Mauriello, F. The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene. Catalysts 2021, 11, 387. [Google Scholar] [CrossRef]
- Seifi-Nahavandi, B.; Yaghmaei, P.; Ahmadian, S.; Ghobeh, M.; Ebrahim-Habibi, A. Cymene consumption and physical activity effect in Alzheimer’s disease model: An in vivo and in vitro study. J. Diabetes Metab. Disord. 2020, 19, 1381–1389. [Google Scholar] [CrossRef]
- Shareef, S.H.; Al-Medhtiy, M.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Jabbar, A.A.; Galali, Y.; Agha, N.F.S.; Aziz, P.Y.; Thabit, M.A.; Agha, D.N.F.; et al. Gastroprophylactic Effects of p-Cymene in Ethanol-Induced Gastric Ulcer in Rats. Processes 2022, 10, 1314. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Wang, Y.; Leng, Q.; Sun, Y.; Hoffman, R.M.; Jin, H. The Anti-oxidant Monoterpene p-Cymene Reduced the Occurrence of Colorectal Cancer in a Hyperlipidemia Rat Model by Reducing Oxidative Stress and Expression of Inflammatory Cytokines. Anticancer Res. 2021, 41, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bahuguna, A.; Shukla, S.; Aziz, F.; Chauhan, A.K.; Ansari, M.B.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Antimicrobial potential of the food-grade additive carvacrol against uropathogenic E. coli based on membrane depolarization, reactive oxygen species generation, and molecular docking analysis. Microb. Pathog. 2020, 142, 104046. [Google Scholar] [CrossRef]
- Anaeigoudari, A. Hepato- and reno-protective effects of thymoquinone, crocin, and carvacrol: A comprehensive review. Asian Pac. J. Trop. Biomed. 2022, 12, 185. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Hajiaghaalizadeh, M.; Sheikharabi, M.; Jazi, M.S.; Alhashem, R.; Hosseini, S.S. Anti-biofilm activity of carvacrol-thymoquinone nanocarriers on vulvovaginal candidiasis isolates. Diagn. Microbiol. Infect. Dis. 2025, 111, 116606. [Google Scholar] [CrossRef]
- Addo, K.A.; Li, H.; Yu, Y.; Xiao, X. Unraveling the mechanism of the synergistic antimicrobial effect of cineole and carvacrol on Escherichia coli O157:H7 inhibition and its application on fresh-cut cucumbers. Food Control 2023, 144, 109339. [Google Scholar] [CrossRef]
- Azizi, Z.; Majlessi, N.; Choopani, S.; Naghdi, N. Neuroprotective effects of carvacrol against Alzheimer’s disease and other neurodegenerative diseases: A review. Avicenna J. Phytomedicine 2022, 12, 371–387. [Google Scholar] [CrossRef]
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and anti-biofilm properties of carvacrol alone and in combination with cefixime against Escherichia coli. BMC Microbiol. 2023, 23, 55. [Google Scholar] [CrossRef]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Mohammadpour, A.H.; Boskabady, M.H. Safety and tolerability of carvacrol in healthy subjects: A phase I clinical study. Drug Chem. Toxicol. 2021, 44, 177–189. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Ghorani, V.; Boskabady, M.H. Experimental and clinical evidence on the effect of carvacrol on respiratory, allergic, and immunologic disorders: A comprehensive review. BioFactors 2022, 48, 779–794. [Google Scholar] [CrossRef]
- Qu, C.; Li, Z.; Wang, X. UHPLC-HRMS-Based Untargeted Lipidomics Reveal Mechanism of Antifungal Activity of Carvacrol against Aspergillus flavus. Foods 2021, 11, 93. [Google Scholar] [CrossRef]
- Souza, G.H.d.A.d.; Radai, J.A.d.S.; Vaz, M.S.M.; Silva, K.E.d.; Fraga, T.L.; Barbosa, L.S.; Simionatto, S. In vitro and in vivo antibacterial activity assays of carvacrol: A candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae. PLoS ONE 2021, 16, e0246003. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Moshtagh, M.; Anaeigoudari, A.; Jafari, S.; Kazemi, T. Protective effects of carvacrol on lipid profiles, oxidative stress, hypertension, and cardiac dysfunction–A comprehensive review. Food Sci. Nutr. 2024, 12, 3137–3149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, T.; Chen, K.; Pan, L.; Xie, J.; Xi, B. Antimicrobial and Antivirulence Activities of Carvacrol against Pathogenic Aeromonas hydrophila. Microorganisms 2022, 10, 2170. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Chen, P.; Sheikh, S.; Ahmad, A.; Ahmad, M.; Paithankar, M.; Desai, B.; Patel, P.; Khan, M.; Chaturvedi, A.; et al. Thymoquinone with Metformin Decreases Fasting, Post Prandial Glucose, and HbA1c in Type 2 Diabetic Patients. Drug Res. 2021, 71, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Zaidi, K.U. Protective Effect of Nigella sativa Seed Extract and its BioactiveCompound Thymoquinone on Streptozotocin-induced Diabetic Rats. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 51–59. [Google Scholar] [CrossRef]
- Fadishei, M.; Ghasemzadeh Rahbardar, M.; Imenshahidi, M.; Mohajeri, A.; Razavi, B.M.; Hosseinzadeh, H. Effects of Nigella sativa oil and thymoquinone against bisphenol A-induced metabolic disorder in rats. Phytother. Res. 2021, 35, 2005–2024. [Google Scholar] [CrossRef]
- Sadiq, N.; Subhani, G.; Fatima, S.A.; Nadeem, M.; Zafer, S.; Mohsin, M. Antidiabetic effect of Nigella sativa compared with metformin on blood glucose levels in streptozotocin induced diabetic albino wistar rats. Int. J. Basic Clin. Pharmacol. 2021, 10, 361–367. [Google Scholar] [CrossRef]
- Faisal Lutfi, M.; Abdel-Moneim, A.-M.H.; Alsharidah, A.S.; Mobark, M.A.; Abdellatif, A.A.H.; Saleem, I.Y.; Al Rugaie, O.; Mohany, K.M.; Alsharidah, M. Thymoquinone Lowers Blood Glucose and Reduces Oxidative Stress in a Rat Model of Diabetes. Molecules 2021, 26, 2348. [Google Scholar] [CrossRef]
- Dong, J.; Liang, Q.; Niu, Y.; Jiang, S.; Zhou, L.; Wang, J.; Ma, C.; Kang, W. Effects of Nigella sativa seed polysaccharides on type 2 diabetic mice and gut microbiota. Int. J. Biol. Macromol. 2020, 159, 725–738. [Google Scholar] [CrossRef]
- Mohebbati, R.; Abbasnezhad, A.; Havakhah, S.; Mousavi, M. The Effect of Nigella sativa on Renal Oxidative Injury in Diabetic Rats. Saudi J. Kidney Dis. Transplant. 2020, 31, 775. [Google Scholar] [CrossRef]
- Mostafa, M.D.; Amer, M.E.; ElKomy, M.A.; Othman, A.I.; El--Missiry, M.A. Thymoquinone controlled obesity and invigorated cognitive and memory performance in rats consuming a high-fat diet via modulating oxidative stress, inflammation and apoptosis. Sci. Rep. 2025, 15, 20171. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, H.; Kaya, S.; Seker, U.; Nergiz, Y. Comparison of the anti-diabetic and nephroprotective activities of vitamin E, metformin, and Nigella sativa oil on kidney in experimental diabetic rats. Iran. J. Basic Med. Sci. 2023, 26, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Alsahli, M.A.; Khan, A.A.; Rahmani, A.H. Thymoquinone, the Most Prominent Constituent of Nigella sativa, Attenuates Liver Damage in Streptozotocin-Induced Diabetic Rats via Regulation of Oxidative Stress, Inflammation and Cyclooxygenase-2 Protein Expression. Appl. Sci. 2021, 11, 3223. [Google Scholar] [CrossRef]
- Rahmani, A.; Niknafs, B.; Naseri, M.; Nouri, M.; Tarighat-Esfanjani, A. Effect of Nigella sativa Oil on Oxidative Stress, Inflammatory, and Glycemic Control Indices in Diabetic Hemodialysis Patients: A Randomized Double-Blind, Controlled Trial. Evid. Based Complement. Alternat. Med. 2022, 2022, 2753294. [Google Scholar] [CrossRef]
- Kooshki, A.; Tofighiyan, T.; Rastgoo, N.; Rakhshani, M.H.; Miri, M. Effect of Nigella sativa oil supplement on risk factors for cardiovascular diseases in patients with type 2 diabetes mellitus. Phytother. Res. 2020, 34, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.; Daryabeygi-Khotbehsara, R.; Mirmiran, P.; McVicar, J.; Hadi, V.; Soleimani, D.; Askari, G. Effect of Nigella sativa oil extract on cardiometabolic risk factors in type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2021, 35, 3747–3755. [Google Scholar] [CrossRef]
- El-Afify, D.; El Amrousy, D. Cardioprotective Effect of Nigella sativa in Pediatric Patients with Type 1 Diabetes Mellitus: A Randomized Controlled Study. Pediatr. Drugs 2025, 27, 481–489. [Google Scholar] [CrossRef]
- Rao, A.S.; Hegde, S.; Pacioretty, L.M.; DeBenedetto, J.; Babish, J.G. Nigella sativa and Trigonella foenum-graecum Supplemented Chapatis Safely Improve HbA1c, Body Weight, Waist Circumference, Blood Lipids, and Fatty Liver in Overweight and Diabetic Subjects: A Twelve-Week Safety and Efficacy Study. J. Med. Food 2020, 23, 905–919. [Google Scholar] [CrossRef]
- Mahmoudian, A.; Ashouri, A.; Mohammadzadeh, F.; Rahmani Bilandi, R.; Dashti, S.; Bahri, N. Effect of Nigella sativa-L supplementation on glycemia in adolescent polycystic ovarian syndrome: Secondary analysis of a randomized controlled trial study. J. Ovarian Res. 2025, 18, 46. [Google Scholar] [CrossRef]
- Ammar, I.M.M.; Salem, M.A.A. Amelioration of polycystic ovary syndrome-related disorders by supplementation of thymoquinone and metformin. Middle East. Fertil. Soc. J. 2021, 26, 29. [Google Scholar] [CrossRef]
- Mostafa, T.M.; Hegazy, S.K.; Elnaidany, S.S.; Shehabeldin, W.A.; Sawan, E.S. Nigella sativa as a promising intervention for metabolic and inflammatory disorders in obese prediabetic subjects: A comparative study of Nigella sativa versus both lifestyle modification and metformin. J. Diabetes Complicat. 2021, 35, 107947. [Google Scholar] [CrossRef]
- Syuhada, S.; Anggadiredja, K.; Kurniati, N.F.; Akrom, A. The Potential of Nigella sativa oil on Clinical output improvement of diabetic neuropathy. J. Appl. Pharm. Sci. 2023, 13, 9–17. [Google Scholar] [CrossRef]
- Khodaie, S.-A.; Nikkhah, H.; Namiranian, N.; Abotorabi, M.; Askari, M.; Khalilzadeh, S.H.; Khatibi Aghda, A.; Kamalinejad, M. Topical Nigella sativa L. product: A new candidate for the management of diabetic peripheral neuropathy. Inflammopharmacology 2024, 32, 551–559. [Google Scholar] [CrossRef]
- Alkhalaf, M.I.; Hussein, R.H.; Hamza, A. Sinteza verde a nanoparticulelor de argint cu extract de Nigella sativa atenuează neuropatia diabetică prin efecte antiinflamatorii și antioxidante. Saudi J. Biol. Sci. 2020, 27, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Alrafiah, A. Thymoquinone Protects Neurons in the Cerebellum of Rats through Mitigating Oxidative Stress and Inflammation Following High-Fat Diet Supplementation. Biomolecules 2021, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Elsawy, H.; Sedky, A. Thymoquinone Abrogates Acrylamide-Induced Cerebellar Toxicity via Modulation of Nuclear Factor Erythroid 2-Related Factor 2/Nuclear Factor Kappa B Signaling, Oxidative Neuroinflammation, and Neuroapoptosis in Rats. J. Med. Food 2024, 27, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Okoh, O.S.; Akintunde, J.K.; Akamo, A.J.; Akpan, U. Thymoquinone inhibits Neuroinflammatory mediators and vasoconstriction injury via NF-κB dependent NeuN/GFAP/Ki-67 in hypertensive Dams and F1 male pups on exposure to a mixture of Bisphenol-A analogues. Toxicol. Appl. Pharmacol. 2025, 494, 117162. [Google Scholar] [CrossRef]
- Abo Mansour, H.E.; Elberri, A.I.; Ghoneim, M.E.-S.; Samman, W.A.; Alhaddad, A.A.; Abdallah, M.S.; El-Berri, E.I.; Salem, M.A.; Mosalam, E.M. The Potential Neuroprotective Effect of Thymoquinone on Scopolamine-Induced In Vivo Alzheimer’s Disease-like Condition: Mechanistic Insights. Molecules 2023, 28, 6566. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kasikci, M. Low-dose rosmarinic acid and thymoquinone accelerate wound healing in retinal pigment epithelial cells. Int. Ophthalmol. 2023, 43, 3811–3821. [Google Scholar] [CrossRef]
- Nehar, S.; Rani, P.; Kumar, C. Evaluation of genoprotective and antioxidative potentiality of ethanolic extract of N. sativa seed in streptozotocin induced diabetic albino rats. Vegetos 2021, 34, 453–459. [Google Scholar] [CrossRef]
- Shoaei-Hagh, P.; Kamelan Kafi, F.; Najafi, S.; Zamanzadeh, M.; Heidari Bakavoli, A.; Ramezani, J.; Soltanian, S.; Asili, J.; Hosseinzadeh, H.; Eslami, S.; et al. A randomized, double-blind, placebo-controlled, clinical trial to evaluate the benefits of Nigella sativa seeds oil in reducing cardiovascular risks in hypertensive patients. Phytother. Res. 2021, 35, 4388–4400. [Google Scholar] [CrossRef]
- Shirazi, M.; Khodakarami, F.; Feizabad, E.; Ghaemi, M. The effects of Nigella sativa on anthropometric and biochemical indices in postmenopausal women with metabolic syndrome. Endocrine 2020, 69, 49–52. [Google Scholar] [CrossRef]
- Pop, R.M.; Vassilopoulou, E.; Jianu, M.-E.; Roșian, Ș.H.; Taulescu, M.; Negru, M.; Bercian, C.; Boarescu, P.-M.; Bocsan, I.C.; Feketea, G.; et al. Nigella sativa oil attenuates inflammation and oxidative stress in experimental myocardial infarction. BMC Complement Med. Ther. 2024, 24, 362. [Google Scholar] [CrossRef]
- Medhet, M.; El-Bakly, W.M.; Badr, A.M.; Awad, A.; El-Demerdash, E. Thymoquinone attenuates isoproterenol-induced myocardial infarction by inhibiting cytochrome C and matrix metalloproteinase-9 expression. Clin. Exp. Pharmacol. Physiol. 2022, 49, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Bocsan, I.C.; Pop, R.M.; Sabin, O.; Sarkandy, E.; Boarescu, P.-M.; Roşian, Ş.H.; Leru, P.M.; Chedea, V.S.; Socaci, S.A.; Buzoianu, A.D. Comparative Protective Effect of Nigella sativa Oil and Vitis vinifera Seed Oil in an Experimental Model of Isoproterenol-Induced Acute Myocardial Ischemia in Rats. Molecules 2021, 26, 3221. [Google Scholar] [CrossRef]
- Rathod, S.; Agrawal, Y.; Sherikar, A.; Nakhate, K.T.; Patil, C.R.; Nagoor Meeran, M.F.; Ojha, S.; Goyal, S.N. Thymoquinone Produces Cardioprotective Effect in β-Receptor Stimulated Myocardial Infarcted Rats via Subsiding Oxidative Stress and Inflammation. Nutrients 2022, 14, 2742. [Google Scholar] [CrossRef]
- Adıyaman, M.Ş.; Adıyaman, Ö.A.; Dağlı, A.F.; Karahan, M.Z.; Dağlı, M.N. Prevention of doxorubicin-induced experimental cardiotoxicity by Nigella sativa in rats. Rev. Port. Cardiol. 2022, 41, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.H.; Ez Elarab, S.M.; Tohamy, H.G.; El-Far, A.H. Thymoquinone attenuates diabetes-induced hepatic damage in rat via regulation of oxidative/nitrosative stress, apoptosis, and inflammatory cascade with molecular docking approach. Sci. Rep. 2024, 14, 13016. [Google Scholar] [CrossRef]
- Owumi, S.; Otunla, M.; Akindipe, P.; Oluwawibe, B.; Babalola, J.O.; Chimezie, J.; Arunsi, U.; Owoeye, O.; Oyelere, A.K. Thymoquinone modulates oxidative stress and inflammation, correcting mercury-induced HO-1/NRF/Trx pathway disruption in experimental rat hepatorenal system: An in vivo and in silico study. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2025, 38, 1179–1202. [Google Scholar] [CrossRef] [PubMed]
- Esmail, M.; Anwar, S.; Kandeil, M.; El-Zanaty, A.M.; Abdel-Gabbar, M. Effect of Nigella sativa, atorvastatin, or L-Carnitine on high fat diet-induced obesity in adult male Albino rats. Biomed. Pharmacother. 2021, 141, 111818. [Google Scholar] [CrossRef]
- Ramineedu, K.; Sankaran, K.R.; Mallepogu, V.; Rendedula, D.P.; Gunturu, R.; Gandham, S.; Md, S.I.; Meriga, B. Thymoquinone mitigates obesity and diabetic parameters through regulation of major adipokines, key lipid metabolizing enzymes and AMPK/p-AMPK in diet-induced obese rats. 3 Biotech 2024, 14, 16. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Safi, S.; Mazaheri, M.; Khalesi, S.; Nazari, M.; Mirmiran, P.; Nadjarzadeh, A. A crossover randomized controlled trial examining the effects of black seed (Nigella sativa) supplementation on IL-1β, IL-6 and leptin, and insulin parameters in overweight and obese women. BMC Complement. Med. Ther. 2024, 24, 22. [Google Scholar] [CrossRef]
- Bittencourt, A.; Brum, P.O.; Ribeiro, C.T.; Gasparotto, J.; Bortolin, R.C.; De Vargas, A.R.; Heimfarth, L.; De Almeida, R.F.; Moreira, J.C.F.; De Oliveira, J.; et al. High fat diet-induced obesity causes a reduction in brain tyrosine hydroxylase levels and non-motor features in rats through metabolic dysfunction, neuroinflammation and oxidative stress. Nutr. Neurosci. 2022, 25, 1026–1040. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Wei, W.-Y.; Tsai, K.-J.; Wang, L.-C. High Fat Diet Suppresses Peroxisome Proliferator-Activated Receptors and Reduces Dopaminergic Neurons in the Substantia Nigra. Int. J. Mol. Sci. 2019, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.O.; Gaspar, J.M. Obesity-Induced Brain Neuroinflammatory and Mitochondrial Changes. Metabolites 2023, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.; Fernandes, A.; Barateiro, A. The complex relationship between obesity and neurodegenerative diseases: An updated review. Front. Cell. Neurosci. 2023, 17, 1294420. [Google Scholar] [CrossRef]
- Harborg, S.; Kjærgaard, K.A.; Thomsen, R.W.; Borgquist, S.; Cronin-Fenton, D.; Hjorth, C.F. New Horizons: Epidemiology of Obesity, Diabetes Mellitus, and Cancer Prognosis. J. Clin. Endocrinol. Metab. 2024, 109, 924–935. [Google Scholar] [CrossRef]
- Behrooz, A.B.; Cordani, M.; Fiore, A.; Donadelli, M.; Gordon, J.W.; Klionsky, D.J.; Ghavami, S. The obesity-autophagy-cancer axis: Mechanistic insights and therapeutic perspectives. Semin. Cancer Biol. 2024, 99, 24–44. [Google Scholar] [CrossRef]
- Ravi, Y.; Vethamoni, P.I.; Saxena, S.N.; Kaviyapriya, M.; Santhanakrishnan, V.P.; Raveendran, M.; Ashoka, N.N.; Choudhary, S.; Verma, A.K.; Harisha, C.B.; et al. Anticancer potential of Thymoquinone from Nigella sativa L.: An in-silico and cytotoxicity study. PLoS ONE 2025, 20, e0323804. [Google Scholar] [CrossRef]
- Gnanasekaran, P.; Roy, A.; Sirpu Natesh, N.; Raman, V.; Ganapathy, P.; Arumugam, M.K. Removal of microbial pathogens and anticancer activity of synthesized nano-thymoquinone from Nigella sativa seeds. Environ. Technol. Innov. 2021, 24, 102068. [Google Scholar] [CrossRef]
- Alsanosi, S.; Sheikh, R.A.; Sonbul, S.; Altayb, H.N.; Batubara, A.S.; Hosawi, S.; Al-Sakkaf, K.; Abdullah, O.; Omran, Z.; Alhosin, M. The Potential Role of Nigella sativa Seed Oil as Epigenetic Therapy of Cancer. Molecules 2022, 27, 2779. [Google Scholar] [CrossRef]
- Karimi, M.; Pirzad, S.; Pourfaraji, S.M.A.; Sedgi, F.M.; Darouei, B.; Amani-Beni, R.; Kazemi, K.; Rabiee, R. Effects of black seed (Nigella sativa L.) on cardiometabolic indices in type 2 diabetic patients: A systematic review and meta-analysis of RCTs. Complement. Ther. Med. 2025, 90, 103174. [Google Scholar] [CrossRef]
- Saadati, S.; Naseri, K.; Asbaghi, O.; Abhari, K.; Zhang, P.; Li, H.-B.; Gan, R.-Y. Nigella sativa supplementation improves cardiometabolic indicators in population with prediabetes and type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 977756. [Google Scholar] [CrossRef]
- Ke, J.; Pan, J.; Lin, H.; Gu, J. Diabetic cardiomyopathy: A brief summary on lipid toxicity. ESC Heart Fail. 2023, 10, 776–790. [Google Scholar] [CrossRef]
- Tekbaş, A.; Bremer-Streck, S.; Wissenbach, D.K.; Peters, F.T.; von Lilienfeld-Toal, M.; Soonawalla, Z.; Rauchfuß, F.; Settmacher, U.; Dahmen, U. Gas Chromatography–Mass Spectrometry Detection of Thymoquinone in Oil and Serum for Clinical Pharmacokinetic Studies. Int. J. Mol. Sci. 2023, 24, 16431. [Google Scholar] [CrossRef]
- Shehata, T.M.; Almostafa, M.M.; Elsewedy, H.S. Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect. Polymers 2022, 14, 3021. [Google Scholar] [CrossRef] [PubMed]
- Zakarial Ansar, F.H.; Latifah, S.Y.; Wan Kamal, W.H.B.; Khong, K.C.; Ng, Y.; Foong, J.N.; Gopalsamy, B.; Ng, W.K.; How, C.W.; Ong, Y.S.; et al. Pharmacokinetics and Biodistribution of Thymoquinone-loaded Nanostructured Lipid Carrier After Oral and Intravenous Administration into Rats. Int. J. Nanomedicine 2020, 15, 7703–7717. [Google Scholar] [CrossRef] [PubMed]
- Behzadifar, S.; Barras, A.; Plaisance, V.; Pawlowski, V.; Szunerits, S.; Abderrahmani, A.; Boukherroub, R. Polymer-Based Nanostructures for Pancreatic Beta-Cell Imaging and Non-Invasive Treatment of Diabetes. Pharmaceutics 2023, 15, 1215. [Google Scholar] [CrossRef] [PubMed]
- Rathore, C.; Rathbone, M.J.; Chellappan, D.K.; Tambuwala, M.M.; Pinto, T.D.J.A.; Dureja, H.; Hemrajani, C.; Gupta, G.; Dua, K.; Negi, P. Nanocarriers: More than tour de force for thymoquinone. Expert. Opin. Drug Deliv. 2020, 17, 479–494. [Google Scholar] [CrossRef]
- Shariare, M.H.; Khan, M.A.; Al-Masum, A.; Khan, J.H.; Uddin, J.; Kazi, M. Development of Stable Liposomal Drug Delivery System of Thymoquinone and Its In Vitro Anticancer Studies Using Breast Cancer and Cervical Cancer Cell Lines. Molecules 2022, 27, 6744. [Google Scholar] [CrossRef]
- Rathore, C.; Hemrajani, C.; Sharma, A.K.; Gupta, P.K.; Jha, N.K.; Aljabali, A.A.A.; Gupta, G.; Singh, S.K.; Yang, J.-C.; Dwivedi, R.P.; et al. Self-nanoemulsifying drug delivery system (SNEDDS) mediated improved oral bioavailability of thymoquinone: Optimization, characterization, pharmacokinetic, and hepatotoxicity studies. Drug Deliv. Transl. Res. 2023, 13, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S.; Almatroudi, A.; Alrumaihi, F.; Aljaghwani, A.; Alnuqaydan, A.M.; Khalilullah, H.; Younus, H.; El-Kady, A.M.; Aldakheel, F.M.; Khan, A.A.; et al. Antimicrobial, Immunomodulatory and Anti-Inflammatory Potential of Liposomal Thymoquinone: Implications in the Treatment of Bacterial Pneumonia in Immunocompromised Mice. Biomedicines 2021, 9, 1673. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Dalamaga, M.; Pavlou, A.; Rebelos, E.; Karamanolis, N.N.; Papachristoforou, E.; Mavrothalassitis, E.; Eleftheriadou, I.; Tentolouris, N.; Kounatidis, D. The Transformative Role of Nanotechnology in the Management of Diabetes Mellitus: Insights from Current Research. Biomolecules 2025, 15, 653. [Google Scholar] [CrossRef] [PubMed]
- Manral, K.; Singh, A.; Singh, Y. Nanotechnology as a potential treatment for diabetes and its complications: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2024, 18, 103159. [Google Scholar] [CrossRef]
- Shan, X.; Cai, Y.; Zhu, B.; Zhou, L.; Sun, X.; Xu, X.; Yin, Q.; Wang, D.; Li, Y. Rational strategies for improving the efficiency of design and discovery of nanomedicines. Nat. Commun. 2024, 15, 9990. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Adamus, G.; Heaselgrave, W.; Radecka, I. Thymoquinone: Hydroxypropyl-β-cyclodextrin Loaded Bacterial Cellulose for the Management of Wounds. Pharmaceutics 2022, 14, 2816. [Google Scholar] [CrossRef]
- Parveen, R.; Ali, F.; Singh, S.D. Innovative Nanocomposites for Drug Delivery: A Novel Approach for Diabetic Foot Ulcer. Curr. Drug Deliv. 2024, 22, 1–21. [Google Scholar] [CrossRef]
- Xue, Q.; Lin, Y. In vitro and functional investigation reveals the curative effect of thymoquinone from black cumin-loaded chitosan nanoparticles on streptozotocin induced paediatric diabetes. Regen. Ther. 2024, 25, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Shoukat, A.; Khalid, W.; Ejaz, A.; Itrat, N.; Majeed, I.; Koraqi, H.; Imran, M.; Nisa, M.U.; Nazir, A.; et al. A Narrative Review on Various Oil Extraction Methods, Encapsulation Processes, Fatty Acid Profiles, Oxidative Stability, and Medicinal Properties of Black Seed (Nigella sativa). Foods 2022, 11, 2826. [Google Scholar] [CrossRef]
- Hannan, M.A.; Zahan, M.S.; Sarker, P.P.; Moni, A.; Ha, H.; Uddin, M.J. Protective Effects of Black Cumin (Nigella sativa) and Its Bioactive Constituent, Thymoquinone against Kidney Injury: An Aspect on Pharmacological Insights. Int. J. Mol. Sci. 2021, 22, 9078. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Novatek Pharmaceuticals A Randomized, Double-Blind, Placebo-Controlled Study. To Evaluate the Safety and Efficacy of TQ Formula in Treating Participants Who Have Tested Positive for Novel Coronavirus 2019 (BOSS-Covid-19). Clin. Gov. 2024. Available online: https://clinicaltrials.gov/study/NCT04914377 (accessed on 11 July 2025).
- Li, T.; Tan, Q.; Wei, C.; Zou, H.; Liu, X.; Mei, Z.; Zhang, P.; Cheng, J.; Fu, J. Design, Synthesis, and Acute Toxicity Assays for Novel Thymoquinone Derivative TQFL12 in Mice and the Mechanism of Resistance to Toxicity. Molecules 2023, 28, 5149. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Alnuqaydan, A.M.; Almatroudi, A.; Alrumaihi, F.; Aljaghwani, A.; Khalilullah, H.; Younus, H.; Khan, A.; Khan, M.A. Safety and Therapeutic Efficacy of Thymoquinone-Loaded Liposomes against Drug-Sensitive and Drug-Resistant Acinetobacter baumannii. Pharmaceutics 2021, 13, 677. [Google Scholar] [CrossRef]
- Sanpinit, S.; Wetchakul, P.; Chonsut, P.; Ngamdokmai, N.; Ahmad, A.R.; Warinhomhoun, S. Repeated 28-Day Oral Toxicological Study and Gastroprotective Effects of Nigella sativa L. Oil (Shuhada) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients 2023, 15, 1532. [Google Scholar] [CrossRef] [PubMed]
| Bioactive Compounds | Therapeutic Properties | References |
|---|---|---|
| Thymoquinone | Anti-inflammatory Antioxidant Anti-nociceptive Hypoglycemic Hepatoprotective Neuroprotective Antitumoral Antimicrobial Immunomodulatory Antihistamine | [102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127] |
| Thymohidroquinone | Antibacterial Antioxidant Anti-inflammatory activity | [81,89,128,129] |
| Dithymoquinone | Antioxidant Antifungals Antitumoral Antiviral | [78,81,130] |
| Thymol | Anti-inflammatory Antifungal Antimicrobial activity Antioxidant Neuroprotective Antidiabetic | [90,131,132,133,134,135] |
| γ-terpinen | Antioxidant Anti-inflammatory Anti-nociceptive Analgesic | [86,136,137] |
| p-Cymene | Antioxidant Antitumoral Antimicrobial Anti-inflammatory Neuroprotective Anti-atherosclerotic | [132,133,138,139,140,141,142,143,144] |
| Carvacrol | Anti-inflammatory Antioxidant Antidiabetic Analgesic Cardioprotective Reno-protective Antimicrobial Neuroprotective | [131,145,146,147,148,149,150,151,152,153,154,155,156,157] |
| Author of the Study | Study | Dose Administered | Period | Observed Effects |
|---|---|---|---|---|
| Khan and Zaidi (2024) [159] | Rats | 100 mg/kg body weight NS extract 10 mg/kg body weight thymoquinone | 28 days | Significant decrease in blood glucose, total cholesterol and low-density lipoprotein; Protective effect of NS seed extract and TQ on diabetic rats |
| Fadishei (2021) [160] | 2 mg/kg body weight TQ | |||
| Rats with metabolic disorder | Daily peritoneal injection | 54 days | Decreased lipid profile, hepatic enzymes, insulin and the blood pressure | |
| Akhtar (2020) [73] | Rabbits | 2.5 mL/kg body weight NS seed oil | 24 days | NS oil treatment lowered serum blood glucose levels and lipid contents, total cholesterol, triglycerides and low density lipoprotein cholesterol |
| Oral administration | ||||
| Sadiq (2021) [161] | 0.5 mL NS | Significant decrease in blood glucose and partial regeneration | ||
| Rats | 1 mL NS 1.5 mL NS Oral administration | 40 days of beta islet cells of the pancreas | ||
| Faisal Lutfi (2021) [162] | Rats | 50 mg/kg body weight TQ Daily | 4 weeks | Decreased glycated hemoglobin (HbA1c) level, lipid peroxidase and nitric oxide (NO); higher TAC in diabetic rats treated with TQ and attenuated of diabetic nephropathy |
| Dong (2020) [163] | Mice | 140 mg/kg 70 mg/kg 35 mg/kg, (0.1 mL/10 g) NS | 4 weeks | NS reduced blood glucose levels, triglycerides, total cholesterol and LDL-C; antihyperglycemic and antihyperlipidemic action after the NS treatment |
| Mohebbati (2020) [164] | Rats | 200 mg/kg NS extract 400 mg/kg NS extract Orally | 6 weeks | Decreased serum glucose level and improved the lipid level in rats treated with NS; Increased antioxidant status (CAT) after NS administration |
| Mostafa (2025) [165] | Rats | 40 mg/kg body weight TQ, orally via stomach tube | 4 weeks | TQ balanced HbA1c levels and insulin resistance, controlled inflammatory cytokines IL-1β, IL-6, TNF-α and CRP |
| Ayaz (2023) [166] | Wistar Albino rats | 2.5 mL/kg body weight NS oil | 56 days | Amelioration of hyperglycemia and pathological renal changes caused by diabetes; NS can favorably regulate oxidative stress; NS proves nephroprotective effect and anti-apoptotic potential |
| Almatroodi (2021) [167] | Diabetic rats | 150 mg/kg body weight TQ | 8 weeks | Improvement of serum glucose levels and insulin, improving lipid metabolism (TC, TG, LDL-C); |
| Orally | TQ confirms the essential role in the antidiabetic activity | |||
| Author of the Study | Subjects | Dose Administered | Period | Observed Effects |
|---|---|---|---|---|
| Rahmani (2022) [168] | 46 diabetic patients | 2 g/day NS oil | 12 weeks | Notable improvement in serum HbA1c and fasting blood sugar levels (FBS); NS significantly increased antioxidant levels (SOD, TAC) |
| Kooshki (2020) [169] | 50 patients | 1000 mg NS oil as two capsules | 8 weeks | Significant decrease in fasting blood glucose and improved lipid panel (TC, TG, LDL-C) |
| Hadi (2021) [170] | 43 patients | 500 mg NS oil twice daily | 8 weeks | Favorable action on glycemic control body weight in diabetic patients and improvement of lipid parameters |
| El-Afify (2025) [171] | 60 pediatric diabetic patients | 450 mg NS oil, Twice daily | 3 months | Notable decrease in cholesterol and LDL-C levels in pediatric patients with type 1 diabetes; Representative reduction in MDA and nitric oxide levels, highlighting the ability of NS to eliminate free radicals |
| Jangjo- Borazjani (2023) [17] | 40 patients with type 2 diabetes | 2 g NS capsules (crushed seeds) | 8 weeks | Significant decrease in insulin level, LDL-C and C-reactive protein; Improvement of diabetic biomarkers |
| Rao (2020) [172] | 40 subjects | 4.7 g NS + 0.75 g fenugreek Twice daily | 12 weeks | Pronounced decrease in postprandial blood glucose, FBG and HbA1c after twelve weeks of treatment |
| Mahmoudian (2025) [173] | 103 adolescent girls | 1000 mg NS/day | 16 weeks | Improving the glycemic profile of adolescent girls (FBG, plasma glucose 2-h postprandial) |
| Ghods (2024) [14] | 80 patients | 2 g NS-Cuminum cyminum Soft gel | 12 weeks | Pronounced decrease in FBG and HbA1c levels compared to placebo; NS-CC oil may decrease blood glucose levels and insulin resistance |
| Ammar (2021) [174] | 127 prediabetic patients | 500 mg TQ + 500 mg Metformin Three times daily | 6 months | Significant weight loss, improving glycemic control, better body fat distribution (waist circumference, body mass index), achieving normal oxidative balance; Increased serum superoxide dismutase activity |
| Mostafa (2021) [175] | 117 obese prediabetic subjects | 450 mg 2x/day NS oil soft gelatin capsules | 6 months | Favorable effects on glycemic parameters; Improving lipid panel |
| Suppression of inflammation (TNF-α) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morar, M.T.; Pallag, A.; Burlou-Nagy, C.; Vicaș, L.G.; Dejeu, I.L.; Horvath, T.; Bei, D.; Vesa, C. Analysis of Pharmacological Properties of Nigella sativa L. Bioactive Compounds and Their Therapeutic Relevance in the Management of Type 2 Diabetes Mellitus. Life 2025, 15, 1681. https://doi.org/10.3390/life15111681
Morar MT, Pallag A, Burlou-Nagy C, Vicaș LG, Dejeu IL, Horvath T, Bei D, Vesa C. Analysis of Pharmacological Properties of Nigella sativa L. Bioactive Compounds and Their Therapeutic Relevance in the Management of Type 2 Diabetes Mellitus. Life. 2025; 15(11):1681. https://doi.org/10.3390/life15111681
Chicago/Turabian StyleMorar (Romocea), Monica Tabita, Annamaria Pallag, Cristina Burlou-Nagy (Fati), Laura Grațiela Vicaș, Ioana Lavinia Dejeu, Tünde Horvath, Diana Bei, and Cosmin Vesa. 2025. "Analysis of Pharmacological Properties of Nigella sativa L. Bioactive Compounds and Their Therapeutic Relevance in the Management of Type 2 Diabetes Mellitus" Life 15, no. 11: 1681. https://doi.org/10.3390/life15111681
APA StyleMorar, M. T., Pallag, A., Burlou-Nagy, C., Vicaș, L. G., Dejeu, I. L., Horvath, T., Bei, D., & Vesa, C. (2025). Analysis of Pharmacological Properties of Nigella sativa L. Bioactive Compounds and Their Therapeutic Relevance in the Management of Type 2 Diabetes Mellitus. Life, 15(11), 1681. https://doi.org/10.3390/life15111681








