Multilayered Regulation of Fungal Phosphate Metabolism: From Molecular Mechanisms to Ecological Roles in the Global Phosphorus Cycle
Abstract
1. Introduction
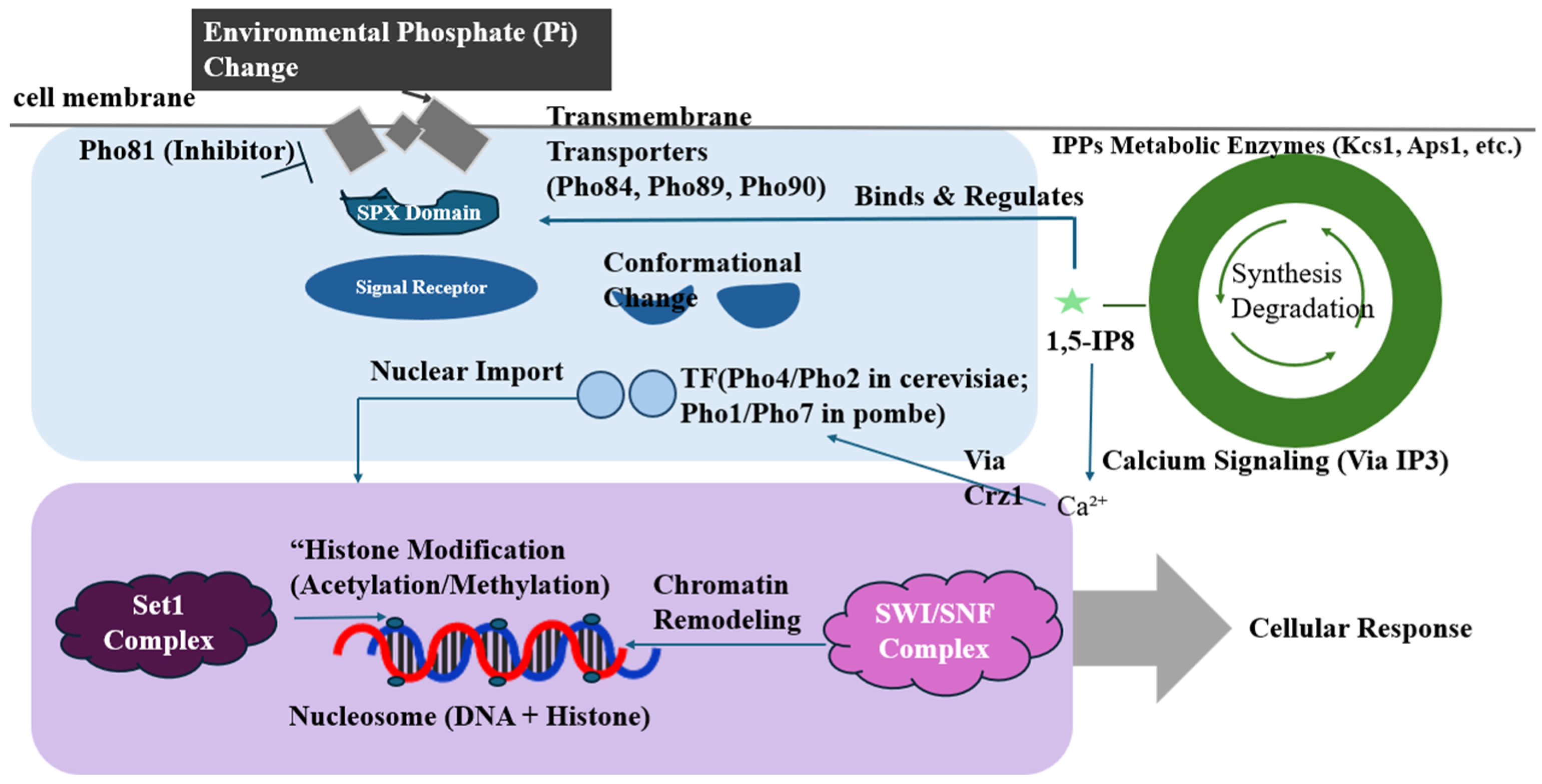
2. Phosphate-Sensing and Transcriptional Regulation Mechanism in Fungi
2.1. Pho Signalling Pathway and Transmembrane Sensing
| Species | Core Transcription Factor | Upstream Regulatory Factors | Molecular Mechanism |
|---|---|---|---|
| Saccharomyces cerevisiae | Pho4 (bHLH transcription factor) | Pho80–Pho85 complex; Pho81 (CDK inhibitor protein); Pho84 (Pi transporter) | Under low-phosphorus conditions, Pho81 inhibits the Pho80–Pho85 kinase complex. Following dephosphorylation, Pho4 enters the nucleus, where it binds to Pho2 to activate PHO gene transcription [31,32,33]. |
| Schizosaccharomyces pombe | Pho7 (Zn2+-Cys6-type transcription factor) | Cdkp1–Pho80–Pho85-like complex; Asp1 (IP8 synthase); SPX protein | Pho7 directly recognizes low-phosphorus response gene promoters; IP8 regulates transcription responses by modulating SPX proteins, employing a non-homologous mechanism distinct from that in S. cerevisiae [27,34,35]. |
| Candida albicans | Pho4 (regulatory factor) | Pho80–Pho85–Pho81 complex; Pho84 | Pho4 nuclear localization is regulated by Pho85 phosphorylation; under low-phosphorus induction, Pho4 activates phosphatase genes and enhances cell wall and oxidative stress tolerance [35]. |
| Cryptococcus neoformans | Pho4 | Pho85–Pho80–Pho81 homologs; SPX–IP7 regulatory axis | Pho4 regulates phosphorus uptake and virulence-related genes; IP7 interacts with the SPX domain to stabilize the Pho pathway, influencing fungal survival within the host. |
| Aspergillus nidulans | PhoP (Zn2+-Cys6 transcription factor) | PhoR–PhoS two-component system; SPX domain protein; VTC complex | PhoP is regulated by PhoR–PhoS signaling, controlling phosphate uptake and polyphosphate metabolism; it cross-regulates with secondary metabolism and developmental pathways. |
2.2. Inositol Pyrophosphate and the Mechanism of SPX Structural Domain Regulation
2.3. Epigenetic Regulation of the PHO Pathway
3. Phosphate Metabolism and the Synergistic Network of Homeostasis in the Body
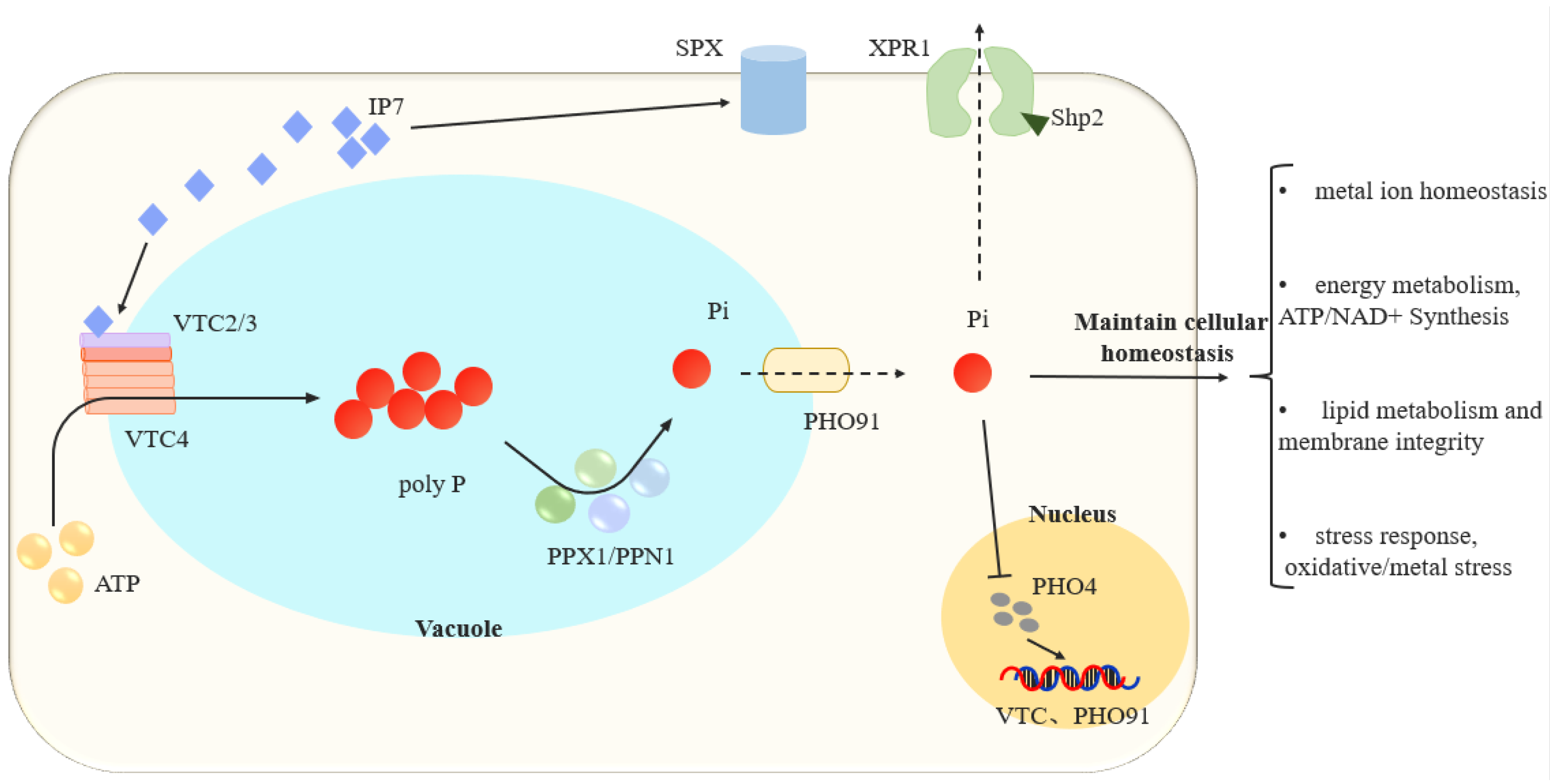
3.1. Synthesis and Regulatory Mechanisms of Polyphosphate
3.2. Intracellular Transport of Phosphate
3.3. Coupling of Phosphate Homeostasis and Cellular Homeostasis
4. Fungal Environmental Interactions and Ecological Functions Regulated by the Phosphate Signalling Pathway
4.1. Ecological Status of Fungi in the Global Phosphorus Cycle
4.2. Promotion of Fungal Phosphorus Response in Plant Symbiotic Interactions
4.3. Biological Significance of Phosphate Metabolism of Pathogenic Fungi
4.4. Fungal-Based Biostimulants Effectively Enhance Phosphorus Availability
5. Conclusions and Perspectives
6. Materials and Methods
6.1. Literature Search and Selection
6.2. Data Organization and Integration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kritmetapak, K.; Kumar, R. Phosphate as a Signaling Molecule. Calcif. Tissue Int. 2021, 108, 16–31. [Google Scholar] [CrossRef]
- Bhadada, S.K.; Rao, S.D. Role of Phosphate in Biomineralization. Calcif. Tissue Int. 2021, 108, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.I.; Poza-Carrión, C.; Martinez-Hevia, I.; Perez-Liens, L.; Paz-Ares, J. Recent Advances in Research on Phosphate Starvation Signaling in Plants. J. Plant Res. 2024, 137, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Chen, H.; Shi, Y.; Xiao, X.; Xu, L.; Qin, C.; Zhu, Y.; Yi, K.; Lei, M.; Zeng, H. PHOSPHATE1-Mediated Phosphate Translocation from Roots to Shoots Regulates Floral Transition in Plants. J. Exp. Bot. 2024, 75, 5054–5075. [Google Scholar] [CrossRef] [PubMed]
- Ikeh, M.A.C.; Kastora, S.L.; Day, A.M.; Herrero-de-Dios, C.M.; Tarrant, E.; Waldron, K.J.; Banks, A.P.; Bain, J.M.; Lydall, D.; Veal, E.A.; et al. Pho4 Mediates Phosphate Acquisition in Candida albicans and Is Vital for Stress Resistance and Metal Homeostasis. Mol. Biol. Cell 2016, 27, 2784–2801. [Google Scholar] [CrossRef]
- Ahmed, Y.; Ikeh, M.A.C.; MacCallum, D.M.; Day, A.M.; Waldron, K.; Quinn, J. Blocking Polyphosphate Mobilization Inhibits Pho4 Activation and Virulence in the Pathogen Candida albicans. mBio 2022, 13, e00342-22. [Google Scholar] [CrossRef]
- King, W.R.; Acosta-Zaldívar, M.; Qi, W.; Cherico, N.; Cooke, L.; Köhler, J.R.; Patton-Vogt, J. Glycerophosphocholine Provision Rescues Candida albicans Growth and Signaling Phenotypes Associated with Phosphate Limitation. mSphere 2023, 8, e00231-23. [Google Scholar] [CrossRef]
- Qu, X.; Bhalla, K.; Horianopoulos, L.C.; Hu, G.; Alcázar Magaña, A.; Foster, L.J.; Roque Da Silva, L.B.; Kretschmer, M.; Kronstad, J.W. Phosphate Availability Conditions Caspofungin Tolerance, Capsule Attachment and Titan Cell Formation in Cryptococcus neoformans. Front. Fungal Biol. 2024, 5, 1447588. [Google Scholar] [CrossRef]
- Suraby, E.J.; Agisha, V.N.; Dhandapani, S.; Sng, Y.H.; Lim, S.H.; Naqvi, N.I.; Sarojam, R.; Yin, Z.; Park, B.S. Plant Growth Promotion under Phosphate Deficiency and Improved Phosphate Acquisition by New Fungal Strain, Penicillium Olsonii TLL1. Front. Microbiol. 2023, 14, 1285574. [Google Scholar] [CrossRef]
- Xie, X.; Lai, W.; Che, X.; Wang, S.; Ren, Y.; Hu, W.; Chen, H.; Tang, M. A SPX Domain-containing Phosphate Transporter from Rhizophagus irregularis Handles Phosphate Homeostasis at Symbiotic Interface of Arbuscular Mycorrhizas. New Phytol. 2022, 234, 650–671. [Google Scholar] [CrossRef]
- Bhalla, K.; Qu, X.; Kretschmer, M.; Kronstad, J.W. The Phosphate Language of Fungi. Trends Microbiol. 2022, 30, 338–349. [Google Scholar] [CrossRef]
- Li, H.; Smith, S.E.; Holloway, R.E.; Zhu, Y.; Smith, F.A. Arbuscular Mycorrhizal Fungi Contribute to Phosphorus Uptake by Wheat Grown in a Phosphorus-fixing Soil Even in the Absence of Positive Growth Responses. New Phytol. 2006, 172, 536–543. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; George, T.S.; Feng, G.; Zhang, L. Arbuscular Mycorrhizal Fungi Enhance Plant Phosphorus Uptake through Stimulating Hyphosphere Soil Microbiome Functional Profiles for Phosphorus Turnover. New Phytol. 2023, 238, 2578–2593. [Google Scholar] [CrossRef] [PubMed]
- Van’T Padje, A.; Werner, G.D.A.; Kiers, E.T. Mycorrhizal Fungi Control Phosphorus Value in Trade Symbiosis with Host Roots When Exposed to Abrupt ‘Crashes’ and ‘Booms’ of Resource Availability. New Phytol. 2021, 229, 2933–2944. [Google Scholar] [CrossRef]
- Köhler, J.; Yang, N.; Pena, R.; Raghavan, V.; Polle, A.; Meier, I.C. Ectomycorrhizal Fungal Diversity Increases Phosphorus Uptake Efficiency of European Beech. New Phytol. 2018, 220, 1200–1210. [Google Scholar] [CrossRef]
- Ji, B.; Bever, J.D. Plant Preferential Allocation and Fungal Reward Decline with Soil Phosphorus: Implications for Mycorrhizal Mutualism. Ecosphere 2016, 7, e01256. [Google Scholar] [CrossRef]
- Yang, G.; Liu, N.; Lu, W.; Wang, S.; Kan, H.; Zhang, Y.; Xu, L.; Chen, Y. The Interaction between Arbuscular Mycorrhizal Fungi and Soil Phosphorus Availability Influences Plant Community Productivity and Ecosystem Stability. J. Ecol. 2014, 102, 1072–1082. [Google Scholar] [CrossRef]
- Chen, E.; Liao, H.; Chen, B.; Peng, S. Arbuscular Mycorrhizal Fungi Are a Double-edged Sword in Plant Invasion Controlled by Phosphorus Concentration. New Phytol. 2020, 226, 295–300. [Google Scholar] [CrossRef]
- Pánek, M.; Vlková, T.; Michalová, T.; Borovička, J.; Tedersoo, L.; Adamczyk, B.; Baldrian, P.; Lopéz-Mondéjar, R. Variation of Carbon, Nitrogen and Phosphorus Content in Fungi Reflects Their Ecology and Phylogeny. Front. Microbiol. 2024, 15, 1379825. [Google Scholar] [CrossRef] [PubMed]
- Takado, M.; Komamura, T.; Nishimura, T.; Ohkubo, I.; Ohuchi, K.; Matsumoto, T.; Takeda, K. Phosphate Uptake Restriction, Phosphate Export, and Polyphosphate Synthesis Contribute Synergistically to Cellular Proliferation and Survival. J. Biol. Chem. 2023, 299, 105454. [Google Scholar] [CrossRef] [PubMed]
- Groth, B.; Lee, Y.-C.; Huang, C.-C.; McDaniel, M.; Huang, K.; Lee, L.-H.; Lin, S.-J. The Histone Deacetylases Hst1 and Rpd3 Integrate De Novo NAD+ Metabolism with Phosphate Sensing in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2023, 24, 8047. [Google Scholar] [CrossRef]
- Chen, Y.; Farooq, A.; Wei, X.; Qin, L.; Wang, Y.; Zhang, L.; Xiang, Q.; Zhao, K.; Yu, X.; Chen, Q.; et al. Transcriptomic and Metabolomic Analysis of Recalcitrant Phosphorus Solubilization Mechanisms in Trametes gibbosa. Front. Microbiol. 2025, 16, 1520459. [Google Scholar] [CrossRef]
- Ming Yip, H.; Cheng, S.; Olson, E.J.; Crone, M.; Maerkl, S.J. Perfect Adaptation Achieved by Transport Limitations Governs the Inorganic Phosphate Response in S. Cerevisiae. Proc. Natl. Acad. Sci. USA 2023, 120, e2212151120. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; Mayer, A. Phosphate Homeostasis—A Vital Metabolic Equilibrium Maintained Through the INPHORS Signaling Pathway. Front. Microbiol. 2020, 11, 1367. [Google Scholar] [CrossRef]
- Pipercevic, J.; Kohl, B.; Gerasimaite, R.; Comte-Miserez, V.; Hostachy, S.; Müntener, T.; Agustoni, E.; Jessen, H.J.; Fiedler, D.; Mayer, A.; et al. Inositol Pyrophosphates Activate the Vacuolar Transport Chaperone Complex in Yeast by Disrupting a Homotypic SPX Domain Interaction. Nat. Commun. 2023, 14, 2645. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, S.; Chen, R.; Zhu, Q.; Shi, P.; Shen, Y. The Transporter PHO84/NtPT1 Is a Target of Aluminum to Affect Phosphorus Absorption in Saccharomyces cerevisiae and Nicotiana tabacum L. Metallomics 2023, 15, mfad069. [Google Scholar] [CrossRef]
- Henry, T.C.; Power, J.E.; Kerwin, C.L.; Mohammed, A.; Weissman, J.S.; Cameron, D.M.; Wykoff, D.D. Systematic Screen of Schizosaccharomyces pombe Deletion Collection Uncovers Parallel Evolution of the Phosphate Signal Transduction Pathway in Yeasts. Eukaryot. Cell 2011, 10, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.F.; O’Brien, E.M.; Zhao, J.; Liang, J.; Bruce, B.J.; Zhang, Y.; Zhu, W.; Cassier, T.; Schnicker, N.J.; Zhou, X.; et al. Divergence in a Eukaryotic Transcription Factor’s Co-TF Dependence Involves Multiple Intrinsically Disordered Regions 2024. Nat. Commun. 2025, 16, 5340. [Google Scholar] [CrossRef]
- Komamura, T.; Nishimura, T.; Ohta, N.; Takado, M.; Matsumoto, T.; Takeda, K. The Putative Polyamine Transporter Shp2 Facilitates Phosphate Export in an Xpr1-Independent Manner and Contributes to High Phosphate Tolerance. J. Biol. Chem. 2025, 301, 108056. [Google Scholar] [CrossRef]
- Speedman, D.; Sauer, D.B. Pho Pictures Provide Powerful Perspectives of Phosphate Importing Proteins. Structure 2024, 32, 849–850. [Google Scholar] [CrossRef]
- Choi, J.; Rajagopal, A.; Xu, Y.-F.; Rabinowitz, J.D.; O’Shea, E.K. A Systematic Genetic Screen for Genes Involved in Sensing Inorganic Phosphate Availability in Saccharomyces cerevisiae. PLoS ONE 2017, 12, e0176085. [Google Scholar] [CrossRef]
- Ogawa, N.; DeRisi, J.; Brown, P.O. New Components of a System for Phosphate Accumulation and Polyphosphate Metabolism in Saccharomyces cerevisiae Revealed by Genomic Expression Analysis. Mol. Biol. Cell 2000, 11, 4309–4321. [Google Scholar] [CrossRef]
- Secco, D.; Wang, C.; Shou, H.; Whelan, J. Phosphate Homeostasis in the Yeast Saccharomyces cerevisiae, the Key Role of the SPX Domain-containing Proteins. FEBS Lett. 2012, 586, 289–295. [Google Scholar] [CrossRef]
- Carter-O’Connell, I.; Peel, M.T.; Wykoff, D.D.; O’Shea, E.K. Genome-Wide Characterization of the Phosphate Starvation Response in Schizosaccharomyces pombe. BMC Genom. 2012, 13, 697. [Google Scholar] [CrossRef]
- Estill, M.; Kerwin-Iosue, C.L.; Wykoff, D.D. Dissection of the PHO Pathway in Schizosaccharomyces pombe Using Epistasis and the Alternate Repressor Adenine. Curr. Genet. 2015, 61, 175–183. [Google Scholar] [CrossRef]
- Chabert, V.; Kim, G.-D.; Qiu, D.; Liu, G.; Michaillat Mayer, L.; Jamsheer K, M.; Jessen, H.J.; Mayer, A. Inositol Pyrophosphate Dynamics Reveals Control of the Yeast Phosphate Starvation Program through 1,5-IP8 and the SPX Domain of Pho81. eLife 2023, 12, RP87956. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sanchez, A.M.; Schwer, B.; Prucker, I.; Jork, N.; Jessen, H.J.; Shuman, S. Activities and Genetic Interactions of Fission Yeast Aps1, a Nudix-Type Inositol Pyrophosphatase and Inorganic Polyphosphatase. mBio 2024, 15, e01084-24. [Google Scholar] [CrossRef]
- Bednor, L.; Sanchez, A.M.; Garg, A.; Shuman, S.; Schwer, B. Genetic Suppressor Screen Identifies Tgp1 (Glycerophosphocholine Transporter), Kcs1 (IP6 Kinase), and Plc1 (Phospholipase C) as Determinants of Inositol Pyrophosphate Toxicosis in Fission Yeast. mBio 2024, 15, e03062-23. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Ortiz, M.; Walla, E.; Fleig, U.; Saiardi, A. The PPIP5K Family Member Asp1 Controls Inorganic Polyphosphate Metabolism in S. Pombe. J. Fungi 2021, 7, 626. [Google Scholar] [CrossRef]
- Schwer, B.; Prucker, I.; Sanchez, A.M.; Babor, J.; Jessen, H.J.; Shuman, S. Tandem Inactivation of Inositol Pyrophosphatases Asp1, Siw14, and Aps1 Illuminates Functional Redundancies in Inositol Pyrophosphate Catabolism in Fission Yeast. mBio 2025, 16, e00389-25. [Google Scholar] [CrossRef] [PubMed]
- Sunder, S.; Bauman, J.S.; Decker, S.J.; Lifton, A.R.; Kumar, A. The Yeast AMP-Activated Protein Kinase Snf1 Phosphorylates the Inositol Polyphosphate Kinase Kcs1. J. Biol. Chem. 2024, 300, 105657. [Google Scholar] [CrossRef]
- Gogianu, L.I.; Ruta, L.L.; Farcasanu, I.C. Kcs1 and Vip1: The Key Enzymes behind Inositol Pyrophosphate Signaling in Saccharomyces cerevisiae. Biomolecules 2024, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Chen, J.; Liu, R.; Chen, Y.; Xing, Q.; Du, Z.; Cheng, M.; Hu, J.; Zhang, W.; Mei, W.; et al. The Cytoplasmic Synthesis and Coupled Membrane Translocation of Eukaryotic Polyphosphate by Signal-Activated VTC Complex. Nat. Commun. 2023, 14, 718. [Google Scholar] [CrossRef]
- Martín, J.F. Interaction of Calcium Responsive Proteins and Transcriptional Factors with the PHO Regulon in Yeasts and Fungi. Front. Cell Dev. Biol. 2023, 11, 1225774. [Google Scholar] [CrossRef]
- Schwer, B.; Garg, A.; Sanchez, A.M.; Bernstein, M.A.; Benjamin, B.; Shuman, S. Cleavage-Polyadenylation Factor Cft1 and SPX Domain Proteins Are Agents of Inositol Pyrophosphate Toxicosis in Fission Yeast. mBio 2022, 13, e03476-21. [Google Scholar] [CrossRef]
- Benjamin, B.; Garg, A.; Jork, N.; Jessen, H.J.; Schwer, B.; Shuman, S. Activities and Structure-Function Analysis of Fission Yeast Inositol Pyrophosphate (IPP) Kinase-Pyrophosphatase Asp1 and Its Impact on Regulation of Pho1 Gene Expression. mBio 2022, 13, e01034-22. [Google Scholar] [CrossRef] [PubMed]
- Schwer, B.; Innokentev, A.; Sanchez, A.M.; Garg, A.; Shuman, S. Suppression of Inositol Pyrophosphate Toxicosis and Hyper-Repression of the Fission Yeast PHO Regulon by Loss-of-Function Mutations in Chromatin Remodelers Snf22 and Sol1. mBio 2024, 15, e01252-24. [Google Scholar] [CrossRef]
- Lieleg, C.; Novacic, A.; Musladin, S.; Schmid, A.; Akpinar, G.G.; Barbaric, S.; Korber, P. Nucleosome Remodeling at the Yeast PHO8 and PHO84 Promoters without the Putatively Essential SWI/SNF Remodeler. Int. J. Mol. Sci. 2023, 24, 4949. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sanchez, A.M.; Schwer, B.; Shuman, S. Factors Governing the Transcriptome Changes and Chronological Lifespan of Fission Yeast during Phosphate Starvation. J. Biol. Chem. 2024, 300, 105718. [Google Scholar] [CrossRef]
- Camblong, J.; Iglesias, N.; Fickentscher, C.; Dieppois, G.; Stutz, F. Antisense RNA Stabilization Induces Transcriptional Gene Silencing via Histone Deacetylation in S. Cerevisiae. Cell 2007, 131, 706–717. [Google Scholar] [CrossRef]
- Chaves-Arquero, B.; Pérez-Cañadillas, J.M. The Nrd1–Nab3–Sen1 Transcription Termination Complex from a Structural Perspective. Biochem. Soc. Trans. 2023, 51, 1257–1269. [Google Scholar] [CrossRef]
- Bowring, B.G.; Sethiya, P.; Desmarini, D.; Lev, S.; Tran Le, L.; Bahn, Y.-S.; Lee, S.-H.; Toh-e, A.; Proschogo, N.; Savage, T.; et al. Dysregulating PHO Signaling via the CDK Machinery Differentially Impacts Energy Metabolism, Calcineurin Signaling, and Virulence in Cryptococcus neoformans. mBio 2023, 14, e03551-22. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, K.; Li, S.; Cao, L.; Nan, Y.; Li, Q.; Li, W.; Hong, Z. A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 5501. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Comte-Miserez, V.; Zhang, M.; Yu, X.; Chen, Q.; Jessen, H.J.; Mayer, A.; Wu, S.; Ye, S. Cryo-EM Structure of the Polyphosphate Polymerase VTC Reveals Coupling of Polymer Synthesis to Membrane Transit. EMBO J. 2023, 42, e113320. [Google Scholar] [CrossRef]
- Tomashevsky, A.; Kulakovskaya, E.; Trilisenko, L.; Kulakovskiy, I.V.; Kulakovskaya, T.; Fedorov, A.; Eldarov, M. VTC4 Polyphosphate Polymerase Knockout Increases Stress Resistance of Saccharomyces cerevisiae Cells. Biology 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Gerasimaitė, R.; Mayer, A. Ppn2, a Novel Zn2+-Dependent Polyphosphatase in the Acidocalcisome-like Yeast Vacuole. J. Cell Sci. 2017, 130, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, I.A.; Ryazanova, L.; Ledova, L.; Zvonarev, A.; Valiakhmetov, A.; Suntsova, M.; Modestov, A.; Buzdin, A.; Lyabin, D.N.; Kulakovskiy, I.V.; et al. Ppn2 Polyphosphatase Improves the Ability of S. Cerevisiae to Grow in Mild Alkaline Medium. J. Fungi 2024, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, N.; Ryazanova, L.; Ledova, L.; Trilisenko, L.; Kulakovskaya, T. Stress Resistance of Saccharomyces cerevisiae Strains Overexpressing Yeast Polyphosphatases. Stresses 2022, 2, 17–25. [Google Scholar] [CrossRef]
- Hürlimann, H.C.; Stadler-Waibel, M.; Werner, T.P.; Freimoser, F.M. Pho91 Is a Vacuolar Phosphate Transporter That Regulates Phosphate and Polyphosphate Metabolism in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 4438–4445. [Google Scholar] [CrossRef]
- Schneider, S.; Kühlbrandt, W.; Yildiz, Ö. Complementary Structures of the Yeast Phosphate Transporter Pho90 Provide Insights into Its Transport Mechanism. Structure 2024, 32, 979–988.e4. [Google Scholar] [CrossRef]
- Farofonova, V.; Andreeva, N.; Kulakovskaya, E.; Karginov, A.; Agaphonov, M.; Kulakovskaya, T. Multiple Effects of the PHO91 Gene Knockout in Ogataea Parapolymorpha. Folia Microbiol. 2023, 68, 587–593. [Google Scholar] [CrossRef]
- He, Q.; Zhang, R.; Tury, S.; Courgnaud, V.; Liu, F.; Battini, J.; Li, B.; Chen, Q. Structural Basis of Phosphate Export by Human XPR1. Nat. Commun. 2025, 16, 683. [Google Scholar] [CrossRef]
- Kulakovskaya, E.; Zvonarev, A.; Kulakovskaya, T. PHM6 and PHM7 Genes Are Essential for Phosphate Surplus in the Cells of Saccharomyces cerevisiae. Arch. Microbiol. 2023, 205, 47. [Google Scholar] [CrossRef]
- Kim, G.-D.; Qiu, D.; Jessen, H.J.; Mayer, A. Metabolic Consequences of Polyphosphate Synthesis and Imminent Phosphate Limitation. mBio 2023, 14, e00102-23. [Google Scholar] [CrossRef]
- Schoeppe, R.; Waldmann, M.; Jessen, H.J.; Renné, T. An Update on Polyphosphate In Vivo Activities. Biomolecules 2024, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Popova, Y.; Thayumanavan, P.; Lonati, E.; Agrochão, M.; Thevelein, J.M. Transport and Signaling through the Phosphate-Binding Site of the Yeast Pho84 Phosphate Transceptor. Proc. Natl. Acad. Sci. USA 2010, 107, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Couso, I.; Pérez-Pérez, M.E.; Ford, M.M.; Martínez-Force, E.; Hicks, L.M.; Umen, J.G.; Crespo, J.L. Phosphorus Availability Regulates TORC1 Signaling via LST8 in Chlamydomonas. Plant Cell 2020, 32, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Malliakas, C.D.; Zhou, Q.; Gu, A.Z.; Aristilde, L. Molecular Coordination, Structure, and Stability of Metal-Polyphosphate Complexes Resolved by Molecular Modeling and X-Ray Scattering: Structural Insights on the Biological Fate of Polyphosphate. Environ. Sci. Technol. 2021, 55, 14185–14193. [Google Scholar] [CrossRef]
- Peng, X.; Ma, C.; Feng, Y.; Zhang, B.; Zhu, M.; Ma, T.; Yu, Q.; Li, M. Phosphate Starvation by Energy Metabolism Disturbance in Candida albicansvip1Δ/Δ Induces Lipid Droplet Accumulation and Cell Membrane Damage. Molecules 2022, 27, 686. [Google Scholar] [CrossRef]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.-X.; Dong, D.-F. Soil Phosphorus Transformation and Plant Uptake Driven by Phosphate-Solubilizing Microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.; Liu, S.; Guo, L.; Zhang, C.; Ye, X.; Tian, D. Phosphate Solubilizing Fungi Enhance Insoluble Phosphate Dissolution via Organic Acid Production: Mechanisms and Applications. Front. Microbiol. 2025, 16, 1600231. [Google Scholar] [CrossRef]
- Vera-Morales, M.; López Medina, S.E.; Naranjo-Morán, J.; Quevedo, A.; Ratti, M.F. Nematophagous Fungi: A Review of Their Phosphorus Solubilization Potential. Microorganisms 2023, 11, 137. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungal Biomineralization. Curr. Biol. 2021, 31, R1557–R1563. [Google Scholar] [CrossRef]
- Watkinson, S.; Bebber, D.; Darrah, P.; Fricker, M.; Tlalka, M.; Boddy, L. The Role of Wood Decay Fungi in the Carbon and Nitrogen Dynamics of the Forest Floor. In Fungi in Biogeochemical Cycles; Gadd, G.M., Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 151–181. ISBN 978-0-521-84579-3. [Google Scholar]
- Tian, D.; Chen, H.; De Oliveira Mendes, G.; Feng, Y. Editorial: Biotechnology of Phosphate Solubilizing Microorganisms for Metabolites Regulation: Present and Future. Front. Bioeng. Biotechnol. 2023, 11, 1258741. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia Abarca, G.; Del Carmen Perea Rojas, Y.; De La Cruz Elizondo, Y.; García Guzman, K.Y. Selection and Characterization of Phosphate-Solubilizing Fungi and Their Effects on Coffee Plantations. Plants 2023, 12, 3395. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, C.; Agnolucci, M.; Avio, L.; Giovannini, L.; Palla, M.; Turrini, A.; Giovannetti, M. Mycorrhizal Symbionts and Associated Bacteria: Potent Allies to Improve Plant Phosphorus Availability and Food Security. Front. Microbiol. 2022, 12, 797381. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, B.; Zheng, S.; Zhang, X.; Wang, X.; Dong, W.; Xie, Q.; Wang, G.; Xiao, Y.; Chen, F.; et al. A Phosphate Starvation Response-Centered Network Regulates Mycorrhizal Symbiosis. Cell 2021, 184, 5527–5540.e18. [Google Scholar] [CrossRef]
- Das, D.; Gutjahr, C. Old Dog, New Trick: The PHR-SPX System Regulates Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2022, 15, 225–227. [Google Scholar] [CrossRef]
- Srivastava, R.; Roychowdhury, A.; Kumar, R. Host SPX-PHR Regulatory Circuit: The Molecular Dynamo Steering Mycorrhization in Plants. Plant Cell Rep. 2022, 41, 1329–1332. [Google Scholar] [CrossRef]
- Zhang, S.; Nie, Y.; Fan, X.; Wei, W.; Chen, H.; Xie, X.; Tang, M. A Transcriptional Activator from Rhizophagus irregularis Regulates Phosphate Uptake and Homeostasis in AM Symbiosis during Phosphorous Starvation. Front. Microbiol. 2023, 13, 1114089. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.R.R.; Roychowdhury, A.; Srivastava, R.; Akash; Gaganan, G.A.; Parida, A.P.; Kumar, R. Silencing of SlSPX1 and SlSPX2 Promote Growth and Root Mycorrhization in Tomato (Solanum lycopersicum L.) Seedlings. Plant Sci. 2023, 333, 111723. [Google Scholar] [CrossRef]
- Brazhnikova, Y.V.; Shaposhnikov, A.I.; Sazanova, A.L.; Belimov, A.A.; Mukasheva, T.D.; Ignatova, L.V. Phosphate Mobilization by Culturable Fungi and Their Capacity to Increase Soil P Availability and Promote Barley Growth. Curr. Microbiol. 2022, 79, 240. [Google Scholar] [CrossRef]
- Kushwaha, A.S.; Ahmad, I.; Lata, S.; Padalia, K.; Yadav, A.K.; Kumar, M. Mycorrhizal Fungus Serendipita Indica-Associated Acid Phosphatase Rescues the Phosphate Nutrition with Reduced Arsenic Uptake in the Host Plant under Arsenic Stress. Ecotoxicol. Environ. Saf. 2024, 269, 115783. [Google Scholar] [CrossRef]
- Kushwaha, A.S.; Thakur, R.S.; Patel, D.K.; Kumar, M. Impact of Arsenic on Phosphate Solubilization, Acquisition and Poly-Phosphate Accumulation in Endophytic Fungus Serendipita Indica. Microbiol. Res. 2022, 259, 127014. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, R.; Zhang, C.; Yang, J.; Lyu, L.; Shi, Z.; Man, Y.B.; Wu, F. Selenium Uptake and Accumulation in Winter Wheat as Affected by Level of Phosphate Application and Arbuscular Mycorrhizal Fungi. J. Hazard. Mater. 2022, 433, 128762. [Google Scholar] [CrossRef]
- Martín-Cardoso, H.; Bundó, M.; Val-Torregrosa, B.; San Segundo, B. Phosphate Accumulation in Rice Leaves Promotes Fungal Pathogenicity and Represses Host Immune Responses during Pathogen Infection. Front. Plant Sci. 2024, 14, 1330349. [Google Scholar] [CrossRef] [PubMed]
- McCombe, C.L.; Wegner, A.; Wirtz, L.; Zamora, C.S.; Casanova, F.; Aditya, S.; Greenwood, J.R.; De Paula, S.; England, E.; Shang, S.; et al. Plant Pathogenic Fungi Hijack Phosphate Signaling with Conserved Enzymatic Effectors. Science 2025, 387, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, Y.; Wang, S.; Hu, S. A Study of Organic Acid Production in Contrasts between Two Phosphate Solubilizing Fungi: Penicillium Oxalicum and Aspergillus Niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef]
- Ferreyra-Suarez, D.; García-Depraect, O.; Castro-Muñoz, R. A Review on Fungal-Based Biopesticides and Biofertilizers Production. Ecotoxicol. Environ. Saf. 2024, 283, 116945. [Google Scholar] [CrossRef]
- García-Berumen, J.A.; Flores De La Torre, J.A.; De Los Santos-Villalobos, S.; Espinoza-Canales, A.; Echavarría-Cháirez, F.G.; Gutiérrez-Bañuelos, H. Phosphorus Dynamics and Sustainable Agriculture: The Role of Microbial Solubilization and Innovations in Nutrient Management. Curr. Res. Microb. Sci. 2025, 8, 100326. [Google Scholar] [CrossRef]
- Wang, C.; Pan, G.; Lu, X.; Qi, W. Phosphorus Solubilizing Microorganisms: Potential Promoters of Agricultural and Environmental Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1181078. [Google Scholar] [CrossRef] [PubMed]
- Eichler-Löbermann, B.; Blossei, J.; Kim, D.-G. Microbial Strategies to Alleviate Phosphorus Deficiency in African Smallholder Farms: Inoculation and Soil Microbiome Enhancement. Plant Soil 2025. [Google Scholar] [CrossRef]
- Köhler, J.R.; Acosta-Zaldívar, M.; Qi, W. Phosphate in Virulence of Candida albicans and Candida glabrata. J. Fungi 2020, 6, 40. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Ning, Y.; Wang, S.; Li, F.; Cao, X.; Wang, Q.; Ren, A. Multilayered Regulation of Fungal Phosphate Metabolism: From Molecular Mechanisms to Ecological Roles in the Global Phosphorus Cycle. Life 2025, 15, 1676. https://doi.org/10.3390/life15111676
Tan Y, Ning Y, Wang S, Li F, Cao X, Wang Q, Ren A. Multilayered Regulation of Fungal Phosphate Metabolism: From Molecular Mechanisms to Ecological Roles in the Global Phosphorus Cycle. Life. 2025; 15(11):1676. https://doi.org/10.3390/life15111676
Chicago/Turabian StyleTan, Yanan, Yanda Ning, Siyi Wang, Faqin Li, Xuewei Cao, Qin Wang, and Ang Ren. 2025. "Multilayered Regulation of Fungal Phosphate Metabolism: From Molecular Mechanisms to Ecological Roles in the Global Phosphorus Cycle" Life 15, no. 11: 1676. https://doi.org/10.3390/life15111676
APA StyleTan, Y., Ning, Y., Wang, S., Li, F., Cao, X., Wang, Q., & Ren, A. (2025). Multilayered Regulation of Fungal Phosphate Metabolism: From Molecular Mechanisms to Ecological Roles in the Global Phosphorus Cycle. Life, 15(11), 1676. https://doi.org/10.3390/life15111676