Arrhythmia-Induced Cardiomyopathy in Atrial Fibrillation: Pathogenesis, Diagnosis, and Treatment
Abstract
1. Introduction
2. Epidemiology of AIC: Signals Amid Shared Aetiologies
3. Pathophysiology of AIC
3.1. AF-Mediated Remodeling
3.2. Metabolic–Adiposopathic Remodeling: Energetic Stress, Epicardial Adipose Tissue, and Cardiac Fibrosis
3.3. Inflammasome–Mitochondrial Crosstalk in AIC
3.4. Inherited Substrate and Ageing Biology
3.5. Sex-Specific Susceptibility and Recovery: Hormonal Modulation and Redox Biology in AIC
4. Diagnostic Evaluation and Management Pathways in AIC
| Author (Year) | Study Design | Population & AF Phenotype | AIC Diagnosis | Intervention/Comparator | Follow-up | Main Findings | Additional Findings |
|---|---|---|---|---|---|---|---|
| Schach et al., 2024 [27] | Prospective multicenter observational | Newly diagnosed LVSD (LVEF < 50%) with tachycardic AF/AFL (HR > 100 bpm); other causes excluded; analyzed n = 50 (age 68 ± 11, 66% male) | Post-rhythm control EF rise ≥ 15% absolute, or ≥10% with final EF ≥ 50%, adjudicated at 6 months | Guideline-directed rhythm control (ECV, PVI, CTI ablation, antiarrhythmics; combinations allowed) | Visits at 2, 4, 6 months after rhythm restoration | AIC diagnosed in 41/50 (82%); EF improved from 35.4 ± 8.2% to 57.2 ± 6.1% at 6 months; ~79% of EF gain occurred by 2 months; non-AIC rose from 37.0 ± 9.5% to 44.0 ± 7.8%; 90% of AIC reached EF > 50% by 6 months | Lower baseline LVEDD predicted AIC (cut-off ~56.5 mm; AUC 0.82); LGE presence/extent not discriminatory; NYHA and NT-proBNP improved in AIC |
| Assaf et al., 2025 [154] | Post hoc analysis of DECAAF II RCT database; persistent AF; first-time ablation (multicenter) | Persistent AF with LVSD (LVEF ≤ 50%); n = 119; baseline LVEF 39.1 ± 7.9%; continuous AF burden monitoring; HFpEF excluded | EF recovery to ≥50% post-ablation with ≥10% absolute increase or ≥15% absolute increase (retrospective definition) | Catheter ablation: PVI vs. PVI + MRI-guided substrate modification; arms pooled for AIC analyses | EF assessed at 3 months; AF burden monitored 12–18 months post-ablation (post-blanking) | AIC in 72/119 (60.5%); post-ablation LVEF 58.9 ± 4.7% vs. 44.0 ± 9.1% (non-AIC); ΔLVEF 19.9 ± 7.6% vs. 4.8 ± 7.5%; AF burden inversely correlated with LVEF (r = −0.23, p = 0.02); AF burden < 3.8% predicted AIC (AUC 0.706) | Lower LA septal fibrosis in AIC (12.2 ± 10.0% vs. 20.7 ± 11.4%, p < 0.001); septal fibrosis cutoff 15.8% predicts AIC (AUC 0.80) |
| Ahluwalia et al., 2024 [155] | Prospective observational; single-centre; control cohort with preserved LVEF | Persistent AF with LVSD (LVEF ≤ 50%) undergoing first-time CA; n = 43 enrolled, 41 re-evaluated; rate-controlled at baseline | LVEF recovery to ≥50% at 6 months post-CA in sinus rhythm; no alternative cause for LVSD | Catheter ablation (PVI as minimum; extra-PVI at operator discretion) vs. reference control group with preserved LVEF (pre/post characterization) | ≈6 months after CA (post-blanking); repeat CA restarted the 6-month clock | AIC in 34/41 (79.0%); LVEF 35 ± 10%→57 ± 4% (Δ ≈ 22 ± 9%); NT-proBNP remained elevated in 52.9%; 29.4% no peak VO2 improvement; 20.6% ventilatory inefficiency | GLS improved but remained abnormal in 58.8% with relative apical sparing; LARS impaired in 26.5%; higher short R–R burden in AIC vs. preserved LVEF (≈59% vs. 40%) |
| Prabhu et al., 2017 (CAMERA-MRI) [6] | Multicenter randomized trial (Australia); CA vs. medical rate control; CMR-guided endpoints | Persistent AF with idiopathic LVSD (LVEF ≤ 45%); n = 66 randomized (33 per arm) after excluding other causes; optimized rate control pre-CMR | Arrhythmia-mediated component inferred by EF recovery with sinus rhythm vs. persistent AF; LGE absence prespecified predictor | Catheter ablation (PVI + posterior wall isolation; ILR to quantify AF burden) vs. guideline-directed medical rate control | Primary endpoint at 6 months (CMR LVEF); clinical, echo, BNP, NYHA at 3 & 6 months | ΔLVEF +18.3% with CA vs. +4.4% with rate control (p < 0.0001); LVEF ≥ 50%: 58% vs. 9% (p = 0.0002); in CA, LGE− had greater ΔLVEF (22.3% vs. 11.6%) and higher normalization (73% vs. 29%); AF burden ~1.6% at 6 months in CA | LA volume and LVESV improved more with CA; BNP and NYHA improved; LGE extent inversely correlated with ΔLVEF; supports AF-mediated cardiomyopathy even with adequate rate control |
| Sugumar et al., 2020 (CAMERA-MRI long-term) [156] | Prospective long-term follow-up of RCT; CA vs. strict medical rate control; crossover allowed | Persistent AF with otherwise unexplained LVSD (baseline LVEF ~ 33%); n = 66 randomized; 62 completed long-term follow-up | AF-mediated component inferred by sustained LVEF recovery with rhythm control; LGE-negative status prespecified predictor of reversibility | Catheter ablation (PVI + posterior wall isolation in ~94%) vs. medical rate control; many crossed over to CA after 6 months | Mean 4.0 ± 0.9 years; continuous/serial rhythm monitoring for AF burden | ΔLVEF +16.4% with CA vs. +8.6% with MRC; LVEF 49.8% vs. 40.1% at ~4y; LVEF normalization 46.8% (CA) vs. 20% (MRC); AF burden inversely correlated with ΔLVEF; LGE− had larger ΔLVEF (≈18.9% vs. 9.8%) | Single-procedure success 43%; posterior wall isolation common; AF burden cutoff ~24% predicted ≥10% EF gain among recurrent persistent AF; procedure complications low |
| Hsu et al., 2004 [23] | Prospective cohort with matched procedural controls; single centre (Bordeaux) | AF with CHF/LVSD (LVEF < 45%); n = 58; controls: AF without CHF n = 58; mostly persistent/permanent AF | Ex juvantibus: marked LV recovery defined as ΔLVEF ≥ 20% or final LVEF ≥ 55% after AF ablation | Catheter ablation (PVI ± LA lines); comparator: AF ablation patients without CHF (procedural controls) | Clinical/echo at 1, 3, 6, 12 months post-ablation | In CHF group: mean ΔLVEF +21 ± 13%; LV diameters decreased (EDD −6 ± 6 mm, ESD −8 ± 7 mm); 72% achieved marked improvement; benefits seen even with adequate preablation rate control | Greatest EF gain within 3 months; arrhythmia recurrence attenuated recovery; subgroup with poor rate control & no structural disease had 92% marked improvement; nonrandomized design |
| Müller-Edenborn et al., 2019 [5] | Prospective observational; single centre (Freiburg–Bad Krozingen); SR restoration via electrical cardioversion | Idiopathic, non-ischaemic/non-valvular HFrEF with persistent/long-persistent AF; LVEF < 40%; n = 50; age 69 ± 11; 76% male | EF response after SR restoration: ≥15% absolute increase or normalization to >50% at Day 40 (ex juvantibus definition) | Electrical cardioversion; antiarrhythmics allowed; catheter ablation prohibited during study; comparator: AF recurrence vs. sustained SR | Serial TTE/MRI at baseline, Day 3, Day 40; handheld/24h ECG monitoring for recurrence | In sustained SR: LVEF 30 ± 7%→43 ± 9% (Day 3)→53 ± 9% (Day 40); 91% responders by Day 40; ICD eligibility dropped 76%→11% by Day 40 | Rapid remodeling: ↓LVESD, ↓LA size, improved MR; no clear clinical/echo/MRI predictors (including LGE); LV dilatation delayed but did not preclude recovery |
4.1. Integrative Assessment of AIC in Clinical Practice
4.2. Rhythm Control
4.3. Integrating Cardiometabolic Therapy to Modify the AIC Substrate
4.4. Predictors of Recovery After Rhythm Restoration
4.4.1. Clinical and Electrocardiographic Features
4.4.2. Echocardiographic Indices
4.4.3. Cardiac Magnetic Resonance
4.4.4. Advanced Quantitative Imaging and AI-Enabled Characterization
4.4.5. Composite Risk Stratification
4.5. Treatment Continuation, De-Escalation, and Surveillance in AIC
4.6. Evidence Gaps and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santhanakrishnan, R.; Wang, N.; Larson, M.G.; Magnani, J.W.; McManus, D.D.; Lubitz, S.A.; Ellinor, P.T.; Cheng, S.; Vasan, R.S.; Lee, D.S.; et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation 2016, 133, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, L.; Hu, Z.; Zhou, L.; Zhang, Z.; Xiong, Y.; Yao, Y. Causal Effects between Atrial Fibrillation and Heart Failure: Evidence from a Bidirectional Mendelian Randomization Study. BMC Med. Genom. 2023, 16, 187. [Google Scholar] [CrossRef]
- Zou, F.; Levine, H.; Mohanty, S.; Natale, A.; Di Biase, L. Atrial Fibrillation-Induced Cardiomyopathy. Card. Electrophysiol. Clin. 2025, 17, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Shoureshi, P.; Tan, A.Y.; Koneru, J.; Ellenbogen, K.A.; Kaszala, K.; Huizar, J.F. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 2214–2232. [Google Scholar] [CrossRef]
- Müller-Edenborn, B.; Minners, J.; Allgeier, J.; Burkhardt, T.; Lehrmann, H.; Ruile, P.; Merz, S.; Allgeier, M.; Neumann, F.-J.; Arentz, T.; et al. Rapid Improvement in Left Ventricular Function after Sinus Rhythm Restoration in Patients with Idiopathic Cardiomyopathy and Atrial Fibrillation. EP Europace 2019, 21, 871–878. [Google Scholar] [CrossRef]
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Dickow, J.; Kirchhof, P.; Van Houten, H.K.; Sangaralingham, L.R.; Dinshaw, L.H.W.; Friedman, P.A.; Packer, D.L.; Noseworthy, P.A.; Yao, X. Generalizability of the EAST-AFNET 4 Trial: Assessing Outcomes of Early Rhythm-Control Therapy in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e024214. [Google Scholar] [CrossRef]
- Pabel, S.; Knierim, M.; Stehle, T.; Alebrand, F.; Paulus, M.; Sieme, M.; Herwig, M.; Barsch, F.; Körtl, T.; Pöppl, A.; et al. Effects of Atrial Fibrillation on the Human Ventricle. Circ. Res. 2022, 130, 994–1010. [Google Scholar] [CrossRef]
- Pfenniger, A.; Yoo, S.; Arora, R. Oxidative Stress and Atrial Fibrillation. J. Mol. Cell. Cardiol. 2024, 196, 141–151. [Google Scholar] [CrossRef]
- Addison, D.; Farhad, H.; Shah, R.V.; Mayrhofer, T.; Abbasi, S.A.; John, R.M.; Michaud, G.F.; Jerosch-Herold, M.; Hoffmann, U.; Stevenson, W.G.; et al. Effect of Late Gadolinium Enhancement on the Recovery of Left Ventricular Systolic Function After Pulmonary Vein Isolation. J. Am. Heart Assoc. 2016, 5, e003570. [Google Scholar] [CrossRef]
- Reyat, J.S.; Sommerfeld, L.C.; O’Reilly, M.; Roth Cardoso, V.; Thiemann, E.; Khan, A.O.; O’Shea, C.; Harder, S.; Müller, C.; Barlow, J.; et al. PITX2 Deficiency Leads to Atrial Mitochondrial Dysfunction. Cardiovasc. Res. 2024, 120, 1907–1923. [Google Scholar] [CrossRef]
- Wang, M.-F.; Hou, C.; Jia, F.; Zhong, C.-H.; Xue, C.; Li, J.-J. Aging-Associated Atrial Fibrillation: A Comprehensive Review Focusing on the Potential Mechanisms. Aging Cell 2024, 23, e14309. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Bunch, T.J.; Steinberg, B.A. Revisiting Rate versus Rhythm Control in Atrial Fibrillation—Timing Matters. N. Engl. J. Med. 2020, 383, 1383–1384. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure: Results From the CABANA Trial. Circulation 2021, 143, 1377–1390. [Google Scholar] [CrossRef]
- Cersosimo, A.; Salerno, N.; Sabatino, J.; Scatteia, A.; Bisaccia, G.; De Rosa, S.; Dellegrottaglie, S.; Bucciarelli-Ducci, C.; Torella, D.; Leo, I. Underlying Mechanisms and Cardioprotective Effects of SGLT2i and GLP-1Ra: Insights from Cardiovascular Magnetic Resonance. Cardiovasc. Diabetol. 2024, 23, 94. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, N.; Zhang, X.; Zhu, Z.; Miao, Y.; Wu, Y.; Ling, J.; Li, C.; Gu, W.; Zhang, J.; et al. Association of Glucagon-like Peptide-1 Receptor Agonists with Atrial Fibrillation, Cardiac Arrest, and Ventricular Fibrillation: Casual Evidence from a Drug Target Mendelian Randomization. Diabetol. Metab. Syndr. 2025, 17, 179. [Google Scholar] [CrossRef]
- Stoll, L.; Lo, J.C. GLP-1 Receptor Agonists, the Holy Grail Preventing Atrial Fibrillation in Patients With T2D? JACC Basic Transl. Sci. 2023, 8, 937–938. [Google Scholar] [CrossRef]
- Bohne, L.J.; Jansen, H.J.; Dorey, T.W.; Daniel, I.M.; Jamieson, K.L.; Belke, D.D.; McRae, M.D.; Rose, R.A. Glucagon-Like Peptide-1 Protects Against Atrial Fibrillation and Atrial Remodeling in Type 2 Diabetic Mice. JACC Basic Transl. Sci. 2023, 8, 922–936. [Google Scholar] [CrossRef]
- Leo, I.; Salerno, N.; Figliozzi, S.; Cersosimo, A.; Ielapi, J.; Stankowski, K.; Bisaccia, G.; Dellegrottaglie, S.; Canino, G.; De Rosa, S.; et al. Effect of SGLT2 Inhibitors on Cardiac Structure and Function Assessed by Cardiac Magnetic Resonance: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2025, 24, 345. [Google Scholar] [CrossRef]
- Vinter, N.; Cordsen, P.; Johnsen, S.P.; Staerk, L.; Benjamin, E.J.; Frost, L.; Trinquart, L. Temporal Trends in Lifetime Risks of Atrial Fibrillation and Its Complications between 2000 and 2022: Danish, Nationwide, Population Based Cohort Study. BMJ 2024, 385, e077209. [Google Scholar] [CrossRef]
- Hsu, L.-F.; Jaïs, P.; Sanders, P.; Garrigue, S.; Hocini, M.; Sacher, F.; Takahashi, Y.; Rotter, M.; Pasquié, J.-L.; Scavée, C.; et al. Catheter Ablation for Atrial Fibrillation in Congestive Heart Failure. N. Engl. J. Med. 2004, 351, 2373–2383. [Google Scholar] [CrossRef]
- Bergonti, M.; Ascione, C.; Marcon, L.; Pambrun, T.; Della Rocca, D.G.; Ferrero, T.G.; Pannone, L.; Kühne, M.; Compagnucci, P.; Bonomi, A.; et al. Left Ventricular Functional Recovery after Atrial Fibrillation Catheter Ablation in Heart Failure: A Prediction Model. Eur. Heart J. 2023, 44, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Sossalla, S.; Vollmann, D. Arrhythmia-Induced Cardiomyopathy. Dtsch. Arztebl. Int. 2018, 115, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Pabel, S.; Sossalla, S. Atrial Fibrillation and Heart Failure: Novel Insights into the Chicken and Egg Dilemma. Eur. Heart J. 2022, 43, 3376–3378. [Google Scholar] [CrossRef]
- Schach, C.; Körtl, T.; Zeman, F.; Luttenberger, B.; Mühleck, F.; Baum, P.; Lavall, D.; Vosshage, N.H.; Resch, M.; Ripfel, S.; et al. Clinical Characterization of Arrhythmia-Induced Cardiomyopathy in Patients With Tachyarrhythmia and Idiopathic Heart Failure. JACC Clin. Electrophysiol. 2024, 10, 870–881. [Google Scholar] [CrossRef]
- Sohns, C.; Fox, H.; Marrouche, N.F.; Crijns, H.J.G.M.; Costard-Jaeckle, A.; Bergau, L.; Hindricks, G.; Dagres, N.; Sossalla, S.; Schramm, R.; et al. Catheter Ablation in End-Stage Heart Failure with Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1380–1389. [Google Scholar] [CrossRef]
- Willems, S.; Meyer, C.; de Bono, J.; Brandes, A.; Eckardt, L.; Elvan, A.; van Gelder, I.; Goette, A.; Gulizia, M.; Haegeli, L.; et al. Cabins, Castles, and Constant Hearts: Rhythm Control Therapy in Patients with Atrial Fibrillation. Eur. Heart J. 2019, 40, 3793–3799c. [Google Scholar] [CrossRef]
- Rillig, A.; Magnussen, C.; Ozga, A.-K.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Elvan, A.; et al. Early Rhythm Control Therapy in Patients With Atrial Fibrillation and Heart Failure. Circulation 2021, 144, 845–858. [Google Scholar] [CrossRef]
- Oraii, A.; McIntyre, W.F.; Parkash, R.; Kowalik, K.; Razeghi, G.; Benz, A.P.; Belley-Côté, E.P.; Conen, D.; Connolly, S.J.; Tang, A.S.L.; et al. Atrial Fibrillation Ablation in Heart Failure With Reduced vs Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. JAMA Cardiol. 2024, 9, 545–555. [Google Scholar] [CrossRef]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological Mechanisms of Atrial Fibrillation: A Translational Appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Stopps, T.P.; Ford, S.E.; de Bold, A.J. Rapid Ventricular Pacing in the Dog: Pathophysiologic Studies of Heart Failure. Circulation 1986, 74, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Hendrick, D.A.; Crawford, F.A.; Smith, A.C.; Hamada, Y.; Carabello, B.A. Chronic Supraventricular Tachycardia Causes Ventricular Dysfunction and Subendocardial Injury in Swine. Am. J. Physiol. 1990, 259, H218–H229. [Google Scholar] [CrossRef]
- Paelinck, B.; Vermeersch, P.; Stockman, D.; Convens, C.; Vaerenberg, M. Usefulness of Low-Dose Dobutamine Stress Echocardiography in Predicting Recovery of Poor Left Ventricular Function in Atrial Fibrillation Dilated Cardiomyopathy. Am. J. Cardiol. 1999, 83, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.P.; van Veldhuisen, D.J.; Crijns, H.J.; Lie, K.I. Reversion of Tachycardiomyopathy after Beta-Blocker. Lancet 1993, 341, 1667. [Google Scholar] [CrossRef]
- Kotecha, D.; Holmes, J.; Krum, H.; Altman, D.G.; Manzano, L.; Cleland, J.G.F.; Lip, G.Y.H.; Coats, A.J.S.; Andersson, B.; Kirchhof, P.; et al. Efficacy of β Blockers in Patients with Heart Failure plus Atrial Fibrillation: An Individual-Patient Data Meta-Analysis. Lancet 2014, 384, 2235–2243. [Google Scholar] [CrossRef]
- Li, D.; Shinagawa, K.; Pang, L.; Leung, T.K.; Cardin, S.; Wang, Z.; Nattel, S. Effects of Angiotensin-Converting Enzyme Inhibition on the Development of the Atrial Fibrillation Substrate in Dogs with Ventricular Tachypacing-Induced Congestive Heart Failure. Circulation 2001, 104, 2608–2614. [Google Scholar] [CrossRef]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial Fibrillation Begets Atrial Fibrillation. A Study in Awake Chronically Instrumented Goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial Cardiomyopathy Revisited-Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Hear. EP Europace 2024, 26, euae204. [Google Scholar] [CrossRef]
- Lenski, M.; Schleider, G.; Kohlhaas, M.; Adrian, L.; Adam, O.; Tian, Q.; Kaestner, L.; Lipp, P.; Lehrke, M.; Maack, C.; et al. Arrhythmia Causes Lipid Accumulation and Reduced Glucose Uptake. Basic Res. Cardiol. 2015, 110, 40. [Google Scholar] [CrossRef]
- Seibertz, F.; Rubio, T.; Springer, R.; Popp, F.; Ritter, M.; Liutkute, A.; Bartelt, L.; Stelzer, L.; Haghighi, F.; Pietras, J.; et al. Atrial Fibrillation-Associated Electrical Remodelling in Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes: A Novel Pathway for Antiarrhythmic Therapy Development. Cardiovasc. Res. 2023, 119, 2623–2637. [Google Scholar] [CrossRef]
- Guichard, J.-B.; Xiong, F.; Qi, X.-Y.; L’Heureux, N.; Hiram, R.; Xiao, J.; Naud, P.; Tardif, J.-C.; Da Costa, A.; Nattel, S. Role of Atrial Arrhythmia and Ventricular Response in Atrial Fibrillation Induced Atrial Remodelling. Cardiovasc. Res. 2021, 117, 462–471. [Google Scholar] [CrossRef] [PubMed]
- de Haan, S.; Greiser, M.; Harks, E.; Blaauw, Y.; van Hunnik, A.; Verheule, S.; Allessie, M.; Schotten, U. AVE0118, Blocker of the Transient Outward Current (Ito) and Ultrarapid Delayed Rectifier Current (IKur), Fully Restores Atrial Contractility after Cardioversion of Atrial Fibrillation in the Goat. Circulation 2006, 114, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef]
- Reilly, S.N.; Jayaram, R.; Nahar, K.; Antoniades, C.; Verheule, S.; Channon, K.M.; Alp, N.J.; Schotten, U.; Casadei, B. Atrial Sources of Reactive Oxygen Species Vary with the Duration and Substrate of Atrial Fibrillation: Implications for the Antiarrhythmic Effect of Statins. Circulation 2011, 124, 1107–1117. [Google Scholar] [CrossRef]
- Guichard, J.-B.; Naud, P.; Xiong, F.; Qi, X.; L’Heureux, N.; Hiram, R.; Tardif, J.-C.; Cartier, R.; Da Costa, A.; Nattel, S. Comparison of Atrial Remodeling Caused by Sustained Atrial Flutter Versus Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 374–388. [Google Scholar] [CrossRef]
- Paulus, M.G.; Renner, K.; Nickel, A.G.; Brochhausen, C.; Limm, K.; Zügner, E.; Baier, M.J.; Pabel, S.; Wallner, S.; Birner, C.; et al. Tachycardiomyopathy Entails a Dysfunctional Pattern of Interrelated Mitochondrial Functions. Basic Res. Cardiol. 2022, 117, 45. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Liu, A.; Eichhorn, C.; Ariga, R.; Levelt, E.; Clarke, W.T.; Rodgers, C.T.; Karamitsos, T.D.; Bashir, Y.; Ginks, M.; et al. Lone Atrial Fibrillation Is Associated With Impaired Left Ventricular Energetics That Persists Despite Successful Catheter Ablation. Circulation 2016, 134, 1068–1081. [Google Scholar] [CrossRef]
- Billing, A.M.; Kim, Y.C.; Gullaksen, S.; Schrage, B.; Raabe, J.; Hutzfeldt, A.; Demir, F.; Kovalenko, E.; Lassé, M.; Dugourd, A.; et al. Metabolic Communication by SGLT2 Inhibition. Circulation 2024, 149, 860–884. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Pond, A.L.; Lamorgese, M.; Rossie, S.S.; McCarthy, P.M.; Nerbonne, J.M. Atrial L-Type Ca2+ Currents and Human Atrial Fibrillation. Circ. Res. 1999, 85, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Grammatika Pavlidou, N.; Dobrev, S.; Beneke, K.; Reinhardt, F.; Pecha, S.; Jacquet, E.; Abu-Taha, I.H.; Schmidt, C.; Voigt, N.; Kamler, M.; et al. Phosphodiesterase 8 Governs CAMP/PKA-Dependent Reduction of L-Type Calcium Current in Human Atrial Fibrillation: A Novel Arrhythmogenic Mechanism. Eur. Heart J. 2023, 44, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Rivet-Bastide, M.; Vandecasteele, G.; Hatem, S.; Verde, I.; Bénardeau, A.; Mercadier, J.J.; Fischmeister, R. CGMP-Stimulated Cyclic Nucleotide Phosphodiesterase Regulates the Basal Calcium Current in Human Atrial Myocytes. J. Clin. Investig. 1997, 99, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Christ, T.; Boknik, P.; Wöhrl, S.; Wettwer, E.; Graf, E.M.; Bosch, R.F.; Knaut, M.; Schmitz, W.; Ravens, U.; Dobrev, D. L-Type Ca2+ Current Downregulation in Chronic Human Atrial Fibrillation Is Associated with Increased Activity of Protein Phosphatases. Circulation 2004, 110, 2651–2657. [Google Scholar] [CrossRef]
- Heijman, J.; Zhou, X.; Morotti, S.; Molina, C.E.; Abu-Taha, I.H.; Tekook, M.; Jespersen, T.; Zhang, Y.; Dobrev, S.; Milting, H.; et al. Enhanced Ca2+-Dependent SK-Channel Gating and Membrane Trafficking in Human Atrial Fibrillation. Circ. Res. 2023, 132, e116–e133. [Google Scholar] [CrossRef]
- Reyes Gaido, O.E.; Nkashama, L.J.; Schole, K.L.; Wang, Q.; Umapathi, P.; Mesubi, O.O.; Konstantinidis, K.; Luczak, E.D.; Anderson, M.E. CaMKII as a Therapeutic Target in Cardiovascular Disease. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 249–272. [Google Scholar] [CrossRef]
- Tessier, S.; Karczewski, P.; Krause, E.G.; Pansard, Y.; Acar, C.; Lang-Lazdunski, M.; Mercadier, J.J.; Hatem, S.N. Regulation of the Transient Outward K(+) Current by Ca(2+)/Calmodulin-Dependent Protein Kinases II in Human Atrial Myocytes. Circ. Res. 1999, 85, 810–819. [Google Scholar] [CrossRef]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients with Atrial Fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef]
- Dinanian, S.; Boixel, C.; Juin, C.; Hulot, J.-S.; Coulombe, A.; Rücker-Martin, C.; Bonnet, N.; Le Grand, B.; Slama, M.; Mercadier, J.-J.; et al. Downregulation of the Calcium Current in Human Right Atrial Myocytes from Patients in Sinus Rhythm but with a High Risk of Atrial Fibrillation. Eur. Heart J. 2008, 29, 1190–1197. [Google Scholar] [CrossRef]
- Deroubaix, E.; Folliguet, T.; Rücker-Martin, C.; Dinanian, S.; Boixel, C.; Validire, P.; Daniel, P.; Capderou, A.; Hatem, S.N. Moderate and Chronic Hemodynamic Overload of Sheep Atria Induces Reversible Cellular Electrophysiologic Abnormalities and Atrial Vulnerability. J. Am. Coll. Cardiol. 2004, 44, 1918–1926. [Google Scholar] [CrossRef]
- Le Grand, B.L.; Hatem, S.; Deroubaix, E.; Couétil, J.P.; Coraboeuf, E. Depressed Transient Outward and Calcium Currents in Dilated Human Atria. Cardiovasc. Res. 1994, 28, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Syeda, F.; Holmes, A.P.; Yu, T.Y.; Tull, S.; Kuhlmann, S.M.; Pavlovic, D.; Betney, D.; Riley, G.; Kucera, J.P.; Jousset, F.; et al. PITX2 Modulates Atrial Membrane Potential and the Antiarrhythmic Effects of Sodium-Channel Blockers. J. Am. Coll. Cardiol. 2016, 68, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Lemoine, M.D.; Mearini, G.; Koivumäki, J.; Sani, J.; Schwedhelm, E.; Kirchhof, P.; Ghalawinji, A.; Stoll, M.; Hansen, A.; et al. PITX2 Knockout Induces Key Findings of Electrical Remodeling as Seen in Persistent Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2023, 16, e011602. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, M.; van Marion, D.M.S.; Wüst, R.C.I.; Houtkooper, R.H.; Zhang, D.; Groot, N.M.S.d.; Henning, R.H.; Brundel, B.J.J.M. Mitochondrial Dysfunction Underlies Cardiomyocyte Remodeling in Experimental and Clinical Atrial Fibrillation. Cells 2019, 8, 1202. [Google Scholar] [CrossRef]
- Roselli, C.; Chaffin, M.D.; Weng, L.-C.; Aeschbacher, S.; Ahlberg, G.; Albert, C.M.; Almgren, P.; Alonso, A.; Anderson, C.D.; Aragam, K.G.; et al. Multi-Ethnic Genome-Wide Association Study for Atrial Fibrillation. Nat. Genet. 2018, 50, 1225–1233. [Google Scholar] [CrossRef]
- Zhang, M.; Hill, M.C.; Kadow, Z.A.; Suh, J.H.; Tucker, N.R.; Hall, A.W.; Tran, T.T.; Swinton, P.S.; Leach, J.P.; Margulies, K.B.; et al. Long-Range Pitx2c Enhancer-Promoter Interactions Prevent Predisposition to Atrial Fibrillation. Proc. Natl. Acad. Sci. USA 2019, 116, 22692–22698. [Google Scholar] [CrossRef]
- van Ouwerkerk, A.F.; Hall, A.W.; Kadow, Z.A.; Lazarevic, S.; Reyat, J.S.; Tucker, N.R.; Nadadur, R.D.; Bosada, F.M.; Bianchi, V.; Ellinor, P.T.; et al. Epigenetic and Transcriptional Networks Underlying Atrial Fibrillation. Circ. Res. 2020, 127, 34–50. [Google Scholar] [CrossRef]
- Reyat, J.S.; Chua, W.; Cardoso, V.R.; Witten, A.; Kastner, P.M.; Kabir, S.N.; Sinner, M.F.; Wesselink, R.; Holmes, A.P.; Pavlovic, D.; et al. Reduced Left Atrial Cardiomyocyte PITX2 and Elevated Circulating BMP10 Predict Atrial Fibrillation after Ablation. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Winters, J.; Isaacs, A.; Zeemering, S.; Kawczynski, M.; Maesen, B.; Maessen, J.; Bidar, E.; Boukens, B.; Hermans, B.; van Hunnik, A.; et al. Heart Failure, Female Sex, and Atrial Fibrillation Are the Main Drivers of Human Atrial Cardiomyopathy: Results From the CATCH ME Consortium. J. Am. Heart Assoc. 2023, 12, e031220. [Google Scholar] [CrossRef]
- Fabritz, L.; Chua, W.; Cardoso, V.R.; Al-Taie, C.; Borof, K.; Suling, A.; Krause, L.; Kany, S.; Magnussen, C.; Wegscheider, K.; et al. Blood-Based Cardiometabolic Phenotypes in Atrial Fibrillation and Their Associated Risk: EAST-AFNET 4 Biomolecule Study. Cardiovasc. Res. 2024, 120, 855–868. [Google Scholar] [CrossRef]
- Chua, W.; Khashaba, A.; Canagarajah, H.; Nielsen, J.C.; di Biase, L.; Haeusler, K.G.; Hindricks, G.; Mont, L.; Piccini, J.; Schnabel, R.B.; et al. Disturbed Atrial Metabolism, Shear Stress, and Cardiac Load Contribute to Atrial Fibrillation after Ablation: AXAFA Biomolecule Study. EP Europace 2024, 26, euae028. [Google Scholar] [CrossRef]
- Palà, E.; Bustamante, A.; Pagola, J.; Juega, J.; Francisco-Pascual, J.; Penalba, A.; Rodriguez, M.; De Lera Alfonso, M.; Arenillas, J.F.; Cabezas, J.A.; et al. Blood-Based Biomarkers to Search for Atrial Fibrillation in High-Risk Asymptomatic Individuals and Cryptogenic Stroke Patients. Front. Cardiovasc. Med. 2022, 9, 908053. [Google Scholar] [CrossRef]
- Fabritz, L.; Al-Taie, C.; Borof, K.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Roth Cardoso, V.; Chua, W.; van Elferen, S.; Eckardt, L.; et al. Biomarker-Based Prediction of Sinus Rhythm in Atrial Fibrillation Patients: The EAST-AFNET 4 Biomolecule Study. Eur. Heart J. 2024, 45, 5002–5019. [Google Scholar] [CrossRef]
- Hennings, E.; Blum, S.; Aeschbacher, S.; Coslovsky, M.; Knecht, S.; Eken, C.; Lischer, M.; Paladini, R.E.; Krisai, P.; Reichlin, T.; et al. Bone Morphogenetic Protein 10-A Novel Biomarker to Predict Adverse Outcomes in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2023, 12, e028255. [Google Scholar] [CrossRef]
- Chua, W.; Cardoso, V.R.; Guasch, E.; Sinner, M.F.; Al-Taie, C.; Brady, P.; Casadei, B.; Crijns, H.J.G.M.; Dudink, E.A.M.P.; Hatem, S.N.; et al. An Angiopoietin 2, FGF23, and BMP10 Biomarker Signature Differentiates Atrial Fibrillation from Other Concomitant Cardiovascular Conditions. Sci. Rep. 2023, 13, 16743. [Google Scholar] [CrossRef] [PubMed]
- Staszewsky, L.; Meessen, J.M.T.A.; Novelli, D.; Wienhues-Thelen, U.-H.; Disertori, M.; Maggioni, A.P.; Masson, S.; Tognoni, G.; Franzosi, M.G.; Lucci, D.; et al. Total NT-ProBNP, a Novel Biomarker Related to Recurrent Atrial Fibrillation. BMC Cardiovasc. Disord. 2021, 21, 553. [Google Scholar] [CrossRef] [PubMed]
- Fabritz, L.; Guasch, E.; Antoniades, C.; Bardinet, I.; Benninger, G.; Betts, T.R.; Brand, E.; Breithardt, G.; Bucklar-Suchankova, G.; Camm, A.J.; et al. Expert Consensus Document: Defining the Major Health Modifiers Causing Atrial Fibrillation: A Roadmap to Underpin Personalized Prevention and Treatment. Nat. Rev. Cardiol. 2016, 13, 230–237. [Google Scholar] [CrossRef]
- Lkhagva, B.; Lee, T.-W.; Lin, Y.-K.; Chen, Y.-C.; Chung, C.-C.; Higa, S.; Chen, Y.-J. Disturbed Cardiac Metabolism Triggers Atrial Arrhythmogenesis in Diabetes Mellitus: Energy Substrate Alternate as a Potential Therapeutic Intervention. Cells 2022, 11, 2915. [Google Scholar] [CrossRef]
- Opacic, D.; van Bragt, K.A.; Nasrallah, H.M.; Schotten, U.; Verheule, S. Atrial Metabolism and Tissue Perfusion as Determinants of Electrical and Structural Remodelling in Atrial Fibrillation. Cardiovasc. Res. 2016, 109, 527–541. [Google Scholar] [CrossRef]
- Muszyński, P.; Bonda, T.A. Mitochondrial Dysfunction in Atrial Fibrillation-Mechanisms and Pharmacological Interventions. J. Clin. Med. 2021, 10, 2385. [Google Scholar] [CrossRef]
- da Menezes, S.A., Jr.; de França-e-Silva, A.L.G.; de Oliveira, J.M.; da Silva, D.M. Developing Pharmacological Therapies for Atrial Fibrillation Targeting Mitochondrial Dysfunction and Oxidative Stress: A Scoping Review. Int. J. Mol. Sci. 2023, 25, 535. [Google Scholar] [CrossRef]
- Verheule, S.; Sato, T.; Everett, T., 4th; Engle, S.K.; Otten, D.; Rubart-von der Lohe, M.; Nakajima, H.O.; Nakajima, H.; Field, L.J.; Olgin, J.E. Increased Vulnerability to Atrial Fibrillation in Transgenic Mice with Selective Atrial Fibrosis Caused by Overexpression of TGF-Beta1. Circ. Res. 2004, 94, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.; Purmah, Y.; Cardoso, V.R.; Gkoutos, G.V.; Tull, S.P.; Neculau, G.; Thomas, M.R.; Kotecha, D.; Lip, G.Y.H.; Kirchhof, P.; et al. Data-Driven Discovery and Validation of Circulating Blood-Based Biomarkers Associated with Prevalent Atrial Fibrillation. Eur. Heart J. 2019, 40, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.; Law, J.P.; Cardoso, V.R.; Purmah, Y.; Neculau, G.; Jawad-Ul-Qamar, M.; Russell, K.; Turner, A.; Tull, S.P.; Nehaj, F.; et al. Quantification of Fibroblast Growth Factor 23 and N-Terminal pro-B-Type Natriuretic Peptide to Identify Patients with Atrial Fibrillation Using a High-Throughput Platform: A Validation Study. PLoS Med. 2021, 18, e1003405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, Y. Circulating Galectin-3 and Atrial Fibrillation Recurrence after Catheter Ablation: A Meta-Analysis. Cardiovasc. Ther. 2019, 2019, 4148129. [Google Scholar] [CrossRef]
- Hernández-Romero, D.; Vílchez, J.A.; Lahoz, Á.; Romero-Aniorte, A.I.; Jover, E.; García-Alberola, A.; Jara-Rubio, R.; Martínez, C.M.; Valdés, M.; Marín, F. Galectin-3 as a Marker of Interstitial Atrial Remodelling Involved in Atrial Fibrillation. Sci. Rep. 2017, 7, 40378. [Google Scholar] [CrossRef]
- Suthahar, N.; Lau, E.S.; Blaha, M.J.; Paniagua, S.M.; Larson, M.G.; Psaty, B.M.; Benjamin, E.J.; Allison, M.A.; Bartz, T.M.; Januzzi, J.L.J.; et al. Sex-Specific Associations of Cardiovascular Risk Factors and Biomarkers With Incident Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 1455–1465. [Google Scholar] [CrossRef]
- González-Ferrero, T.; Bergonti, M.; López-Canoa, J.N.; Arias, F.G.-R.; Eiras Penas, S.; Spera, F.; González-Maestro, A.; Minguito-Carazo, C.; Martínez-Sande, J.L.; González-Melchor, L.; et al. Atrial Fibrillation Ablation in Patients with Arrhythmia-Induced Cardiomyopathy: A Prospective Multicentre Study. ESC Heart Fail. 2023, 10, 3055–3066. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial Adipose Tissue in Contemporary Cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Chilukoti, R.K.; Giese, A.; Malenke, W.; Homuth, G.; Bukowska, A.; Goette, A.; Felix, S.B.; Kanaan, J.; Wollert, H.-G.; Evert, K.; et al. Atrial Fibrillation and Rapid Acute Pacing Regulate Adipocyte/Adipositas-Related Gene Expression in the Atria. Int. J. Cardiol. 2015, 187, 604–613. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Farahmand, P.; Rücker-Martin, C.; Dilanian, G.; Fradet, M.; Sawaki, D.; Derumeaux, G.; LePrince, P.; Clément, K.; et al. Atrial Natriuretic Peptide Regulates Adipose Tissue Accumulation in Adult Atria. Proc. Natl. Acad. Sci. USA 2017, 114, E771–E780. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Margaritis, M.; Verheule, S.; Recalde, A.; Sanna, F.; Herdman, L.; Psarros, C.; Nasrallah, H.; Coutinho, P.; Akoumianakis, I.; et al. Mutual Regulation of Epicardial Adipose Tissue and Myocardial Redox State by PPAR-γ/Adiponectin Signalling. Circ. Res. 2016, 118, 842–855. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Zhang, P.; Chen, Y.; Li, C.; Chen, J.; Wang, Y.; Li, Y. The Crucial Role of Activin A/ALK4 Pathway in the Pathogenesis of Ang-II-Induced Atrial Fibrosis and Vulnerability to Atrial Fibrillation. Basic Res. Cardiol. 2017, 112, 47. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human Epicardial Adipose Tissue Induces Fibrosis of the Atrial Myocardium through the Secretion of Adipo-Fibrokines. Eur. Heart J. 2015, 36, 795–805a. [Google Scholar] [CrossRef] [PubMed]
- Lamounier-Zepter, V.; Look, C.; Alvarez, J.; Christ, T.; Ravens, U.; Schunck, W.-H.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Morano, I. Adipocyte Fatty Acid-Binding Protein Suppresses Cardiomyocyte Contraction: A New Link between Obesity and Heart Disease. Circ. Res. 2009, 105, 326–334. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Pucéat, M.; et al. Reactivation of the Epicardium at the Origin of Myocardial Fibro-Fatty Infiltration During the Atrial Cardiomyopathy. Circ. Res. 2020, 126, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- De Coster, T.; Claus, P.; Kazbanov, I.V.; Haemers, P.; Willems, R.; Sipido, K.R.; Panfilov, A.V. Arrhythmogenicity of Fibro-Fatty Infiltrations. Sci. Rep. 2018, 8, 2050. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.A.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J. Am. Coll. Cardiol. 2020, 76, 1197–1211. [Google Scholar] [CrossRef]
- Gharaviri, A.; Bidar, E.; Potse, M.; Zeemering, S.; Verheule, S.; Pezzuto, S.; Krause, R.; Maessen, J.G.; Auricchio, A.; Schotten, U. Epicardial Fibrosis Explains Increased Endo-Epicardial Dissociation and Epicardial Breakthroughs in Human Atrial Fibrillation. Front. Physiol. 2020, 11, 68. [Google Scholar] [CrossRef]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial Fibrillation Is Associated with the Fibrotic Remodelling of Adipose Tissue in the Subepicardium of Human and Sheep Atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef]
- Momot, K.; Krauz, K.; Pruc, M.; Szarpak, L.; Rodkiewicz, D.; Mamcarz, A. Association Between Left Atrial Epicardial Adipose Tissue Attenuation Assessed by Cardiac Computed Tomography and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4771. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, I.; Kousta, M.; Kossyvakis, C.; Paraskevaidis, N.T.; Vrachatis, D.; Deftereos, S.; Giannopoulos, G. Epicardial Adipose Tissue and Atrial Fibrillation Recurrence Following Catheter Ablation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 6369. [Google Scholar] [CrossRef] [PubMed]
- Ernault, A.C.; Meijborg, V.M.F.; Coronel, R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1730–1745. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.N., 3rd; Taylor, R.R.; Pool, P.E.; Whipple, G.H.; Covell, J.W.; Ross, J.J.; Braunwald, E. Congestive Heart Failure Following Chronic Tachycardia. Am. Heart J. 1971, 81, 790–798. [Google Scholar] [CrossRef]
- Zeemering, S.; Isaacs, A.; Winters, J.; Maesen, B.; Bidar, E.; Dimopoulou, C.; Guasch, E.; Batlle, M.; Haase, D.; Hatem, S.N.; et al. Atrial Fibrillation in the Presence and Absence of Heart Failure Enhances Expression of Genes Involved in Cardiomyocyte Structure, Conduction Properties, Fibrosis, Inflammation, and Endothelial Dysfunction. Heart Rhythm. 2022, 19, 2115–2124. [Google Scholar] [CrossRef]
- Mueller, K.A.L.; Heinzmann, D.; Klingel, K.; Fallier-Becker, P.; Kandolf, R.; Kilias, A.; Walker-Allgaier, B.; Borst, O.; Kumbrink, J.; Kirchner, T.; et al. Histopathological and Immunological Characteristics of Tachycardia-Induced Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 69, 2160–2172. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Ktenopoulos, N.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: Mechanistic Pathways and Emerging Treatment Concepts. J. Clin. Med. 2025, 14, 3250. [Google Scholar] [CrossRef]
- Körtl, T.; Stehle, T.; Riedl, D.; Trausel, J.; Rebs, S.; Pabel, S.; Paulus, M.; Holzamer, A.; Marrouche, N.; Maier, L.S.; et al. Atrial Fibrillation Burden Specifically Determines Human Ventricular Cellular Remodeling. JACC Clin. Electrophysiol. 2022, 8, 1357–1366. [Google Scholar] [CrossRef]
- Ling, L.; Khammy, O.; Byrne, M.; Amirahmadi, F.; Foster, A.; Li, G.; Zhang, L.; dos Remedios, C.; Chen, C.; Kaye, D.M. Irregular Rhythm Adversely Influences Calcium Handling in Ventricular Myocardium: Implications for the Interaction between Heart Failure and Atrial Fibrillation. Circ. Heart Fail. 2012, 5, 786–793. [Google Scholar] [CrossRef]
- Slawik, J.; Adrian, L.; Hohl, M.; Lothschütz, S.; Laufs, U.; Böhm, M. Irregular Pacing of Ventricular Cardiomyocytes Induces Pro-Fibrotic Signalling Involving Paracrine Effects of Transforming Growth Factor Beta and Connective Tissue Growth Factor. Eur. J. Heart Fail. 2019, 21, 482–491. [Google Scholar] [CrossRef]
- Chelu, M.G.; Sarma, S.; Sood, S.; Wang, S.; van Oort, R.J.; Skapura, D.G.; Li, N.; Santonastasi, M.; Müller, F.U.; Schmitz, W.; et al. Calmodulin Kinase II-Mediated Sarcoplasmic Reticulum Ca2+ Leak Promotes Atrial Fibrillation in Mice. J. Clin. Investig. 2009, 119, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Ernst, P.; Bidwell, P.A.; Dora, M.; Thomas, D.D.; Kamdar, F. Cardiac Calcium Regulation in Human Induced Pluripotent Stem Cell Cardiomyocytes: Implications for Disease Modeling and Maturation. Front. Cell Dev. Biol. 2022, 10, 986107. [Google Scholar] [CrossRef] [PubMed]
- Mo, B.; Ding, Y.; Ji, Q. NLRP3 Inflammasome in Cardiovascular Diseases: An Update. Front. Immunol. 2025, 16, 1550226. [Google Scholar] [CrossRef]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory Signalling in Atrial Cardiomyocytes: A Novel Unifying Principle in Atrial Fibrillation Pathophysiology. Nat. Rev. Cardiol. 2023, 20, 145–167. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Theofilis, P.; Milaras, N.; Vlachakis, P.K.; Grigoriou, K.; Patoulias, D.; Karamitsos, T.; Antoniadis, A.P.; Fragakis, N.; et al. Inflammasome Signaling in Cardiac Arrhythmias: Linking Inflammation, Fibrosis, and Electrical Remodeling. Int. J. Mol. Sci. 2025, 26, 5954. [Google Scholar] [CrossRef]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.-X.; Marks, A.R. Mitochondrial Oxidative Stress Promotes Atrial Fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Nattel, S.; Lip, G.Y.H.; Ren, J. Inflammasome Signaling in Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2349–2366. [Google Scholar] [CrossRef]
- Li, L.; Coarfa, C.; Yuan, Y.; Abu-Taha, I.; Wang, X.; Song, J.; Zeng, Y.; Chen, X.; Koirala, A.; Grimm, S.L.; et al. Fibroblast-Restricted Inflammasome Activation Promotes Atrial Fibrillation and Heart Failure With Diastolic Dysfunction. JACC Basic Transl. Sci. 2025, 10. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Arnar, D.O.; Helgadottir, A.; Gretarsdottir, S.; Holm, H.; Sigurdsson, A.; Jonasdottir, A.; Baker, A.; Thorleifsson, G.; Kristjansson, K.; et al. Variants Conferring Risk of Atrial Fibrillation on Chromosome 4q25. Nature 2007, 448, 353–357. [Google Scholar] [CrossRef]
- Kany, S.; Jurgens, S.J.; Rämö, J.T.; Christophersen, I.E.; Rienstra, M.; Chung, M.K.; Olesen, M.S.; Ackerman, M.J.; McNally, E.M.; Semsarian, C.; et al. Genetic Testing in Early-Onset Atrial Fibrillation. Eur. Heart J. 2024, 45, 3111–3123. [Google Scholar] [CrossRef]
- Kirchhof, P.; Kahr, P.C.; Kaese, S.; Piccini, I.; Vokshi, I.; Scheld, H.-H.; Rotering, H.; Fortmueller, L.; Laakmann, S.; Verheule, S.; et al. PITX2c Is Expressed in the Adult Left Atrium, and Reducing Pitx2c Expression Promotes Atrial Fibrillation Inducibility and Complex Changes in Gene Expression. Circ. Cardiovasc. Genet. 2011, 4, 123–133. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Hernández-Torres, F.; Daimi, H.; Serra, S.A.; Herraiz, A.; Hove-Madsen, L.; Aránega, A.; Franco, D. Pitx2 Impairs Calcium Handling in a Dose-Dependent Manner by Modulating Wnt Signalling. Cardiovasc. Res. 2016, 109, 55–66. [Google Scholar] [CrossRef]
- Ramírez de Acuña, F.; Hernandez-Torres, F.; Rodriguez-Outeiriño, L.; Dominguez, J.N.; Matias-Valiente, L.; Sanchez-Fernandez, C.; Franco, D.; Aranega, A.E. Pitx2 Differentially Regulates the Distinct Phases of Myogenic Program and Delineates Satellite Cell Lineages During Muscle Development. Front. Cell Dev. Biol. 2022, 10, 940622. [Google Scholar] [CrossRef]
- Kany, S.; Al-Taie, C.; Roselli, C.; Pirruccello, J.P.; Borof, K.; Reinbold, C.; Suling, A.; Krause, L.; Reissmann, B.; Schnabel, R.B.; et al. Association of Genetic Risk and Outcomes in Patients with Atrial Fibrillation: Interactions with Early Rhythm Control in the EAST-AFNET4 Trial. Cardiovasc. Res. 2023, 119, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.G.; Cappola, T.P.; Day, S.M. Genetic Testing in Early-Onset Atrial Fibrillation. JAMA Cardiol. 2025, 10, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, M.; Qian, J.; Li, B.; Tu, X.; Xu, C.; Li, S.; Chen, S.; Zhao, Y.; Huang, Y.; et al. Identification of Rare Variants in TNNI3 with Atrial Fibrillation in a Chinese GeneID Population. Mol. Genet. Genom. 2016, 291, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.S.; et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef]
- Maron, B.J. Hypertrophic Cardiomyopathy: A Systematic Review. JAMA 2002, 287, 1308–1320. [Google Scholar] [CrossRef]
- Kany, S.; Reissmann, B.; Metzner, A.; Kirchhof, P.; Darbar, D.; Schnabel, R.B. Genetics of Atrial Fibrillation-Practical Applications for Clinical Management: If Not Now, When and How? Cardiovasc. Res. 2021, 117, 1718–1731. [Google Scholar] [CrossRef]
- Aragam, K.G.; Chaffin, M.; Levinson, R.T.; McDermott, G.; Choi, S.H.; Shoemaker, M.B.; Haas, M.E.; Weng, L.-C.; Lindsay, M.E.; Smith, J.G.; et al. Phenotypic Refinement of Heart Failure in a National Biobank Facilitates Genetic Discovery. Circulation 2019, 139, 489–501. [Google Scholar] [CrossRef]
- Olson, T.M.; Michels, V.V.; Ballew, J.D.; Reyna, S.P.; Karst, M.L.; Herron, K.J.; Horton, S.C.; Rodeheffer, R.J.; Anderson, J.L. Sodium Channel Mutations and Susceptibility to Heart Failure and Atrial Fibrillation. JAMA 2005, 293, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, L.C.; Holmes, A.P.; Yu, T.Y.; O’Shea, C.; Kavanagh, D.M.; Pike, J.M.; Wright, T.; Syeda, F.; Aljehani, A.; Kew, T.; et al. Reduced Plakoglobin Increases the Risk of Sodium Current Defects and Atrial Conduction Abnormalities in Response to Androgenic Anabolic Steroid Abuse. J. Physiol. 2024, 602, 4409–4436. [Google Scholar] [CrossRef] [PubMed]
- Chalazan, B.; Mol, D.; Darbar, F.A.; Ornelas-Loredo, A.; Al-Azzam, B.; Chen, Y.; Tofovic, D.; Sridhar, A.; Alzahrani, Z.; Ellinor, P.; et al. Association of Rare Genetic Variants and Early-Onset Atrial Fibrillation in Ethnic Minority Individuals. JAMA Cardiol. 2021, 6, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Keil, L.; Berisha, F.; Knappe, D.; Kubisch, C.; Shoukier, M.; Kirchhof, P.; Fabritz, L.; Hellenbroich, Y.; Woitschach, R.; Magnussen, C. LMNA Mutation in a Family with a Strong History of Sudden Cardiac Death. Genes 2022, 13, 169. [Google Scholar] [CrossRef]
- Nakano, Y.; Ochi, H.; Sairaku, A.; Onohara, Y.; Tokuyama, T.; Motoda, C.; Matsumura, H.; Tomomori, S.; Amioka, M.; Hironobe, N.; et al. HCN4 Gene Polymorphisms Are Associated With Occurrence of Tachycardia-Induced Cardiomyopathy in Patients with Atrial Fibrillation. Circ. Genom. Precis. Med. 2018, 11, e001980. [Google Scholar] [CrossRef]
- Linz, D.; Andrade, J.G.; Arbelo, E.; Boriani, G.; Breithardt, G.; Camm, A.J.; Caso, V.; Nielsen, J.C.; De Melis, M.; De Potter, T.; et al. Longer and Better Lives for Patients with Atrial Fibrillation: The 9th AFNET/EHRA Consensus Conference. EP Europace 2024, 26, euae070. [Google Scholar] [CrossRef]
- Spach, M.S.; Dolber, P.C. Relating Extracellular Potentials and Their Derivatives to Anisotropic Propagation at a Microscopic Level in Human Cardiac Muscle. Evidence for Electrical Uncoupling of Side-to-Side Fiber Connections with Increasing Age. Circ. Res. 1986, 58, 356–371. [Google Scholar] [CrossRef]
- Alvarez-Franco, A.; Rouco, R.; Ramirez, R.J.; Guerrero-Serna, G.; Tiana, M.; Cogliati, S.; Kaur, K.; Saeed, M.; Magni, R.; Enriquez, J.A.; et al. Transcriptome and Proteome Mapping in the Sheep Atria Reveal Molecular Featurets of Atrial Fibrillation Progression. Cardiovasc. Res. 2021, 117, 1760–1775. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Li, J.; Liu, J.; Baks-Te Bulte, L.; Wiersma, M.; Malik, N.-U.-A.; van Marion, D.M.S.; Tolouee, M.; Hoogstra-Berends, F.; et al. DNA Damage-Induced PARP1 Activation Confers Cardiomyocyte Dysfunction through NAD+ Depletion in Experimental Atrial Fibrillation. Nat. Commun. 2019, 10, 1307. [Google Scholar] [CrossRef]
- Burstein, B.; Libby, E.; Calderone, A.; Nattel, S. Differential Behaviors of Atrial versus Ventricular Fibroblasts: A Potential Role for Platelet-Derived Growth Factor in Atrial-Ventricular Remodeling Differences. Circulation 2008, 117, 1630–1641. [Google Scholar] [CrossRef]
- Aimé-Sempé, C.; Folliguet, T.; Rücker-Martin, C.; Krajewska, M.; Krajewska, S.; Heimburger, M.; Aubier, M.; Mercadier, J.J.; Reed, J.C.; Hatem, S.N. Myocardial Cell Death in Fibrillating and Dilated Human Right Atria. J. Am. Coll. Cardiol. 1999, 34, 1577–1586. [Google Scholar] [CrossRef]
- Akoum, N.; Mahnkopf, C.; Kholmovski, E.G.; Brachmann, J.; Marrouche, N.F. Age and Sex Differences in Atrial Fibrosis among Patients with Atrial Fibrillation. EP Europace 2018, 20, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.R.; Nalliah, C.J.; Lee, G.; Voskoboinik, A.; Chieng, D.; Prabhu, S.; Parameswaran, R.; Sugumar, H.; Al-Kaisey, A.; McLellan, A.; et al. Sex-Related Differences in Atrial Remodeling in Patients With Atrial Fibrillation: Relationship to Ablation Outcomes. Circ. Arrhythm. Electrophysiol. 2022, 15, e009925. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Sun, H.; Xiong, S.; Luo, Y.; Tang, Y.; Zhang, Z.; Liu, H. Sex-Related Differences in Left Atrial Substrate among Patients with Atrial Fibrillation: Evidence from High-Density Voltage Mapping. Eur. J. Med. Res. 2024, 29, 354. [Google Scholar] [CrossRef] [PubMed]
- Van Leuven, O.; Bergonti, M.; Spera, F.R.; Ferrero, T.G.; Nsahlai, M.; Bilotta, G.; Tijskens, M.; Boris, W.; Saenen, J.; Huybrechts, W.; et al. Gender-Related Differences in Atrial Substrate in Patients with Atrial Fibrillation. Am. J. Cardiol. 2023, 203, 451–458. [Google Scholar] [CrossRef]
- Marzak, H.; Ringele, R.; Matsushita, K.; Marchandot, B.; Fitouchi, S.; Cardi, T.; Kanso, M.; Schatz, A.; Hammann, J.; Ohlmann, P.; et al. Impact of Gender on Left Atrial Low-Voltage Zones in Patients with Persistent Atrial Fibrillation: Results of a Voltage-Guided Ablation. Front. Cardiovasc. Med. 2023, 10, 1229345. [Google Scholar] [CrossRef]
- Younes, H.; Sohns, C.; Akoum, N.; Feng, H.; Tsakiris, E.; El Hajjar, A.H.; Donnellan, E.; Pandey, A.C.; Lim, C.; Bidaoui, G.; et al. Sex-Specific Outcomes and Left Atrial Remodeling Following Catheter Ablation of Persistent Atrial Fibrillation: Results from the DECAAF II Trial. J. Interv. Card. Electrophysiol. 2024, 67, 1843–1850. [Google Scholar] [CrossRef]
- Du, W.; Zhu, W.; Yang, H.; Dong, Q.; Fei, Y.; Li, X.; Li, S.; Han, B. Different Impact of Female Gender on the Outcome of Catheter Ablation between Paroxysmal and Persistent Atrial Fibrillation. BMC Cardiovasc. Disord. 2025, 25, 364. [Google Scholar] [CrossRef]
- Segan, L.; Chieng, D.; Crowley, R.; William, J.; Sugumar, H.; Ling, L.-H.; Hawson, J.; Prabhu, S.; Voskoboinik, A.; Morton, J.B.; et al. Sex-Specific Outcomes after Catheter Ablation for Persistent AF. Heart Rhythm 2024, 21, 762–770. [Google Scholar] [CrossRef]
- Ehdaie, A.; Cingolani, E.; Shehata, M.; Wang, X.; Curtis, A.B.; Chugh, S.S. Sex Differences in Cardiac Arrhythmias: Clinical and Research Implications. Circ. Arrhythm. Electrophysiol. 2018, 11, e005680. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Lagranha, C.J.; Silva, T.L.A.; Silva, S.C.A.; Braz, G.R.F.; da Silva, A.I.; Fernandes, M.P.; Sellitti, D.F. Protective Effects of Estrogen against Cardiovascular Disease Mediated via Oxidative Stress in the Brain. Life Sci. 2018, 192, 190–198. [Google Scholar] [CrossRef]
- Lu, B.; Huang, B.; Wang, Y.; Ma, G.; Cai, Z. Mitochondrial Calcium Homeostasis Mediated by Estradiol Contributes to Atrial Fibrillation Protection. Biochem. Biophys. Res. Commun. 2025, 772, 152050. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.; Feng, H.; Bsoul, M.; Bidaoui, G.; Younes, H.; Massad, C.; Mekhael, M.; Noujaim, C.; Kreidieh, O.; Rao, S.; et al. Characterization of Arrhythmia-Induced Cardiomyopathy Using Magnetic Resonance Imaging in Patients with Persistent Atrial Fibrillation and Left Ventricular Systolic Dysfunction—Insights from DECAAF II. Eur. J. Heart Fail. 2025, 27, 1622–1632. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Honarbakhsh, S.; Abbass, H.; Joshi, A.; Chow, A.W.C.; Dhinoja, M.; Petersen, S.E.; Hunter, R.J.; Lloyd, G.; Schilling, R.J. Characterisation of Patients Who Develop Atrial Fibrillation-Induced Cardiomyopathy. Open Heart 2024, 11, e002955. [Google Scholar] [CrossRef]
- Sugumar, H.; Prabhu, S.; Costello, B.; Chieng, D.; Azzopardi, S.; Voskoboinik, A.; Parameswaran, R.; Wong, G.R.; Anderson, R.; Al-Kaisey, A.M.; et al. Catheter Ablation Versus Medication in Atrial Fibrillation and Systolic Dysfunction: Late Outcomes of CAMERA-MRI Study. JACC Clin. Electrophysiol. 2020, 6, 1721–1731. [Google Scholar] [CrossRef]
- Pierucci, N.; Mariani, M.V.; Iannetti, G.; Maffei, L.; Coluccio, A.; Laviola, D.; Palombi, M.; Trivigno, S.; Spadafora, L.; Chourda, E.; et al. Atrial Cardiomyopathy: New Pathophysiological and Clinical Aspects. Minerva Cardiol. Angiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Lim, L.K.E.; Tan, Y.K.; Goh, C.; Teo, Y.H.; Ho, J.S.Y.; Dalakoti, M.; Chan, M.Y.Y.; Sia, C.-H.; Yeo, L.L.L.; et al. Assessment of Left Atrial Fibrosis by Cardiac Magnetic Resonance Imaging in Ischemic Stroke Patients Without Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2024, 13, e033059. [Google Scholar] [CrossRef]
- Seah, A.; Li, T.Y.W.; Sari, N.Y.; Lee, C.-H.; Yeo, T.-C.; Yip, J.W.L.; Lim, Y.C.; Poh, K.-K.; Kong, W.K.F.; Lin, W.; et al. The Prognostic Implication of Left Atrial Strain Parameters with Conventional Left Atrial Parameters for the Prediction of Adverse Outcomes in Asian Patients with Hypertrophic Cardiomyopathy-An Echocardiographic Study. J. Cardiovasc. Dev. Dis. 2025, 12, 261. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Jirataiporn, K.; Yindeengam, A.; Songsangjinda, T. Cardiac Magnetic Resonance Left Atrial Strain in the Prediction of Death, Ischemic Stroke, and Heart Failure. J. Am. Heart Assoc. 2024, 13, e034336. [Google Scholar] [CrossRef]
- Kamel, H.; Bartz, T.M.; Elkind, M.S.V.; Okin, P.M.; Thacker, E.L.; Patton, K.K.; Stein, P.K.; deFilippi, C.R.; Gottesman, R.F.; Heckbert, S.R.; et al. Atrial Cardiopathy and the Risk of Ischemic Stroke in the CHS (Cardiovascular Health Study). Stroke 2018, 49, 980–986. [Google Scholar] [CrossRef]
- Chin, A.; Badri, M.; Ntusi, N.B.; Okreglicki, A. The Clinical, Electrocardiographic and Echocardiographic Characteristics and Long-Term Outcome of Patients with Tachycardia-Induced Cardiomyopathy. Cardiovasc. J. Afr. 2012, 23, 136–142. [Google Scholar] [CrossRef]
- Becher, N.; Metzner, A.; Toennis, T.; Kirchhof, P.; Schnabel, R.B. Atrial Fibrillation Burden: A New Outcome Predictor and Therapeutic Target. Eur. Heart J. 2024, 45, 2824–2838. [Google Scholar] [CrossRef]
- Orlov, O.S.; Asfour, A.; Bogdanova, A.A.; Shchekochikhin, D.Y.; Akselrod, A.S.; Nesterov, A.P.; Andreev, D.A. Predictors of tachycardia-induced cardiomyopathy in patients with first-time decompensation of chro-nic heart failure with reduced left ventricular ejection fraction of nonischemic etiology and persistent atrial tachyarrhythmia. Kardiologiia 2022, 62, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Serban, T.; du Fay de Lavallaz, J.; Mannhart, D.; Pfister, O.; van der Stouwe, J.G.; Kaufmann, B.A.; Knecht, S.; Kühne, M.; Sticherling, C.; Badertscher, P. Echocardiographic Pattern of Left Ventricular Function Recovery in Tachycardia-Induced Cardiomyopathy Patients. ESC Heart Fail. 2023, 10, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Schach, C.; Körtl, T.; Wachter, R.; Maier, L.S.; Sossalla, S. Echocardiographic Evaluation of LV Function in Patients with Tachyarrhythmia and Reduced Left Ventricular Function in Response to Rhythm Restoration. J. Clin. Med. 2021, 10, 3706. [Google Scholar] [CrossRef] [PubMed]
- Bunting, K.V.; Gill, S.K.; Sitch, A.; Mehta, S.; O’Connor, K.; Lip, G.Y.; Kirchhof, P.; Strauss, V.Y.; Rahimi, K.; Camm, A.J.; et al. Improving the Diagnosis of Heart Failure in Patients with Atrial Fibrillation. Heart 2021, 107, 902–908. [Google Scholar] [CrossRef]
- Ling, L.; Kalman, J.M.; Ellims, A.H.; Iles, L.M.; Medi, C.; Sherratt, C.; Kaye, D.M.; Hare, J.L.; Kistler, P.M.; Taylor, A.J. Diffuse Ventricular Fibrosis Is a Late Outcome of Tachycardia-Mediated Cardiomyopathy after Successful Ablation. Circ. Arrhythm. Electrophysiol. 2013, 6, 697–704. [Google Scholar] [CrossRef]
- Cavus, E.; Schneider, J.N.; di Carluccio, E.; Ziegler, A.; Haack, A.; Ojeda, F.; Chevalier, C.; Jahnke, C.; Riedl, K.A.; Radunski, U.K.; et al. Unrecognized Myocardial Scar by Late-Gadolinium-Enhancement Cardiovascular Magnetic Resonance: Insights from the Population-Based Hamburg City Health Study. J. Cardiovasc. Magn. Reson. 2024, 26, 101008. [Google Scholar] [CrossRef]
- Karakasis, P.; Vlachakis, P.K.; Theofilis, P.; Ktenopoulos, N.; Patoulias, D.; Fyntanidou, B.; Antoniadis, A.P.; Fragakis, N. Atrial Cardiomyopathy in Atrial Fibrillation: A Multimodal Diagnostic Framework. Diagnostics 2025, 15, 1207. [Google Scholar] [CrossRef]
- Orlov, O.; Asfour, A.; Shchekochikhin, D.; Magomedova, Z.; Bogdanova, A.; Komarova, A.; Podianov, M.; Gromyko, G.; Pershina, E.; Nesterov, A.; et al. Cardiac Magnetic Resonance in Patients with Suspected Tachycardia-Induced Cardiomyopathy: The Impact of Late Gadolinium Enhancement and Epicardial Fat Tissue. J. Pers. Med. 2023, 13, 1440. [Google Scholar] [CrossRef]
- Brady, P.F.; Chua, W.; Nehaj, F.; Connolly, D.L.; Khashaba, A.; Purmah, Y.J.V.; Ul-Qamar, M.J.; Thomas, M.R.; Varma, C.; Schnabel, R.B.; et al. Interactions Between Atrial Fibrillation and Natriuretic Peptide in Predicting Heart Failure Hospitalization or Cardiovascular Death. J. Am. Heart Assoc. 2022, 11, e022833. [Google Scholar] [CrossRef]
- Nia, A.M.; Gassanov, N.; Dahlem, K.M.; Caglayan, E.; Hellmich, M.; Erdmann, E.; Er, F. Diagnostic Accuracy of NT-ProBNP Ratio (BNP-R) for Early Diagnosis of Tachycardia-Mediated Cardiomyopathy: A Pilot Study. Clin. Res. Cardiol. 2011, 100, 887–896. [Google Scholar] [CrossRef]
- Supanekar, N.; Gilge, J.L.; Ahmed, A.; Patel, P.J. Post-Ablation P Wave Characteristics Correlate with Recurrent Atrial Fibrillation in the ABCD-AF Cohort. J. Interv. Card. Electrophysiol. 2022, 64, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Givertz, M.M.; Ho, C.Y.; Judge, D.P.; Kantor, P.F.; McBride, K.L.; Morales, A.; Taylor, M.R.G.; Vatta, M.; Ware, S.M. Genetic Evaluation of Cardiomyopathy-A Heart Failure Society of America Practice Guideline. J. Card. Fail. 2018, 24, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Jameson, H.S.; Hanley, A.; Hill, M.C.; Xiao, L.; Ye, J.; Bapat, A.; Ronzier, E.; Hall, A.W.; Hucker, W.J.; Clauss, S.; et al. Loss of the Atrial Fibrillation-Related Gene, Zfhx3, Results in Atrial Dilation and Arrhythmias. Circ. Res. 2023, 133, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Dobrev, D.; Nattel, S. Atrial Fibrillation: Pathophysiology, Genetic and Epigenetic Mechanisms. Lancet Reg. Health Eur. 2024, 37, 100785. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, J.; Ruan, X.; Li, Y.; Abramowitz, S.A.; Wang, L.; Jiang, F.; Xiong, Y.; Levin, M.G.; Voight, B.F.; et al. Cross-Population GWAS and Proteomics Improve Risk Prediction and Reveal Mechanisms in Atrial Fibrillation. Nat. Commun. 2025, 16, 6426. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Milaras, N.; Vlachakis, P.K.; Patoulias, D.; Karamitsos, T.; Antoniadis, A.P.; Fragakis, N. Epigenetic Drivers of Atrial Fibrillation: Mechanisms, Biomarkers, and Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 5253. [Google Scholar] [CrossRef]
- Parikh, V.N.; Day, S.M.; Lakdawala, N.K.; Adler, E.D.; Olivotto, I.; Seidman, C.E.; Ho, C.Y. Advances in the Study and Treatment of Genetic Cardiomyopathies. Cell 2025, 188, 901–918. [Google Scholar] [CrossRef]
- Chao, T.; Ge, Y.; Sun, J.; Wang, C. Research Landscape of Genetics in Dilated Cardiomyopathy: Insight from a Bibliometric Analysis. Front. Cardiovasc. Med. 2024, 11, 1362551. [Google Scholar] [CrossRef]
- Ebrahim, M.A.; Ali, N.M.; Albash, B.Y.; Al Sayegh, A.H.; Ahmad, N.B.; Voß, S.; Klag, F.; Groß, J.; Holler, S.; Walhorn, V.; et al. Phenotypic Diversity Caused by the DES Missense Mutation p.R127P (c.380G>C) Contributing to Significant Cardiac Mortality and Morbidity Associated With a Desmin Filament Assembly Defect. Circ. Genom. Precis. Med. 2025, 18, e004896. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Milaras, N.; Kalinderi, K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Gene Therapy for Cardiac Arrhythmias: Mechanisms, Modalities and Therapeutic Applications. Med. Sci. 2025, 13, 102. [Google Scholar] [CrossRef]
- Imai, Y.; Kusano, K.; Aiba, T.; Ako, J.; Asano, Y.; Harada-Shiba, M.; Kataoka, M.; Kosho, T.; Kubo, T.; Matsumura, T.; et al. JCS/JCC/JSPCCS 2024 Guideline on Genetic Testing and Counseling in Cardiovascular Disease. J. Cardiol. 2025, 85, 115–176. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2023, 149, 109–279. [Google Scholar] [CrossRef] [PubMed]
- Monda, E.; Bakalakos, A.; Cannie, D.; O’Mahony, C.; Syrris, P.; Kaski, J.P.; Limongelli, G.; Elliott, P.M. Prevalence of Pathogenic Variants in Cardiomyopathy-Associated Genes in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2024, 12, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Borlaug, B.A.; Bailey, A.L.; Eckman, P.M.; Guglin, M.; Hall, S.; Montgomery, M.; Ramani, G.; Khazanie, P. Practical Guidance for Hemodynamic Assessment by Right Heart Catheterization in Management of Heart Failure. JACC Heart Fail. 2024, 12, 1141–1156. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. EP Europace 2024, 26, euae043. [Google Scholar] [CrossRef]
- Linz, D.; Chaldoupi, S.-M. Early Rhythm Management in Patients With Atrial Fibrillation: From Symptom Control to Adverse Outcome Reduction. JACC Clin. Electrophysiol. 2024, 10, 1406–1408. [Google Scholar] [CrossRef]
- Pamporis, K.; Karakasis, P.; Sagris, M.; Theofilis, P.; Milaras, N.; Pantelidaki, A.; Mourouzis, I.; Fragakis, N.; Vlachos, K.; Kordalis, A.; et al. Prevalence of Asymptomatic Atrial Fibrillation and Risk Factors Associated with Asymptomatic Status: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2025, zwaf138. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major Clinical Outcomes in Symptomatic vs. Asymptomatic Atrial Fibrillation: A Meta-Analysis. Eur. Heart J. 2024, 46, 1189–1202. [Google Scholar] [CrossRef]
- Merino, J.L.; Tamargo, J.; Blomström-Lundqvist, C.; Boriani, G.; Crijns, H.J.G.M.; Dobrev, D.; Goette, A.; Hohnloser, S.H.; Naccarelli, G.V.; Reiffel, J.A.; et al. Practical Compendium of Antiarrhythmic Drugs: A Clinical Consensus Statement of the European Heart Rhythm Association of the ESC. EP Europace 2025, 27, euaf076. [Google Scholar] [CrossRef]
- Rillig, A.; Eckardt, L.; Borof, K.; Camm, A.J.; Crijns, H.J.G.M.; Goette, A.; Breithardt, G.; Lemoine, M.D.; Metzner, A.; Rottner, L.; et al. Safety and Efficacy of Long-Term Sodium Channel Blocker Therapy for Early Rhythm Control: The EAST-AFNET 4 Trial. EP Europace 2024, 26, euae121. [Google Scholar] [CrossRef]
- Reichlin, T.; Kueffer, T.; Badertscher, P.; Jüni, P.; Knecht, S.; Thalmann, G.; Kozhuharov, N.; Krisai, P.; Jufer, C.; Maurhofer, J.; et al. Pulsed Field or Cryoballoon Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- William, J.; Chieng, D.; Curtin, A.G.; Sugumar, H.; Ling, L.H.; Segan, L.; Crowley, R.; Iyer, A.; Prabhu, S.; Voskoboinik, A.; et al. Radiofrequency Catheter Ablation of Persistent Atrial Fibrillation by Pulmonary Vein Isolation with or without Left Atrial Posterior Wall Isolation: Long-Term Outcomes of the CAPLA Trial. Eur. Heart J. 2025, 46, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 305–315. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Ferrick, K.J. Atrial Fibrillation Ablation: Safety in Numbers. J. Am. Coll. Cardiol. 2023, 81, 2100–2102. [Google Scholar] [CrossRef]
- Benali, K.; Khairy, P.; Hammache, N.; Petzl, A.; Da Costa, A.; Verma, A.; Andrade, J.G.; Macle, L. Procedure-Related Complications of Catheter Ablation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2023, 81, 2089–2099. [Google Scholar] [CrossRef]
- Karakasis, P.; Tzeis, S.; Pamporis, K.; Schuermans, A.; Theofilis, P.; Milaras, N.; Tsiachris, D.; Efremidis, M.; Antoniadis, A.P.; Fragakis, N. Impact of Catheter Ablation Timing According to Duration of Atrial Fibrillation History on Arrhythmia Recurrences and Clinical Outcomes: A Meta-Analysis. EP Europace 2025, 27, euaf110. [Google Scholar] [CrossRef]
- Eckardt, L.; Sehner, S.; Suling, A.; Borof, K.; Breithardt, G.; Crijns, H.; Goette, A.; Wegscheider, K.; Zapf, A.; Camm, J.; et al. Attaining Sinus Rhythm Mediates Improved Outcome with Early Rhythm Control Therapy of Atrial Fibrillation: The EAST-AFNET 4 Trial. Eur. Heart J. 2022, 43, 4127–4144. [Google Scholar] [CrossRef]
- Corley, S.D.; Epstein, A.E.; DiMarco, J.P.; Domanski, M.J.; Geller, N.; Greene, H.L.; Josephson, R.A.; Kellen, J.C.; Klein, R.C.; Krahn, A.D.; et al. Relationships between Sinus Rhythm, Treatment, and Survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004, 109, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Metzner, A.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Elvan, A.; Goette, A.; Haegeli, L.M.; et al. Anticoagulation, Therapy of Concomitant Conditions, and Early Rhythm Control Therapy: A Detailed Analysis of Treatment Patterns in the EAST-AFNET 4 Trial. EP Europace 2022, 24, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Bunting, K.V.; Gill, S.K.; Mehta, S.; Stanbury, M.; Jones, J.C.; Haynes, S.; Calvert, M.J.; Deeks, J.J.; Steeds, R.P.; et al. Effect of Digoxin vs Bisoprolol for Heart Rate Control in Atrial Fibrillation on Patient-Reported Quality of Life: The RATE-AF Randomized Clinical Trial. JAMA 2020, 324, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. EP Europace 2022, 24, 71–164. [Google Scholar] [CrossRef]
- Brignole, M.; Pentimalli, F.; Palmisano, P.; Landolina, M.; Quartieri, F.; Occhetta, E.; Calò, L.; Mascia, G.; Mont, L.; Vernooy, K.; et al. AV Junction Ablation and Cardiac Resynchronization for Patients with Permanent Atrial Fibrillation and Narrow QRS: The APAF-CRT Mortality Trial. Eur. Heart J. 2021, 42, 4731–4739. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.M.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation 2020, 141, 1227–1234. [Google Scholar] [CrossRef]
- Jaiswal, V.; Ang, S.P.; Kumar, D.; Deb, N.; Jaiswal, A.; Joshi, A.; Nasir, Y.M.; Bandyopadhyay, D.; Michos, E.D.; Benjamin, E.J.; et al. Sodium-Glucose Cotransporter-2 Inhibitors and Arrhythmias: A Meta-Analysis of 38 Randomized Controlled Trials. JACC Adv. 2025, 4, 101615. [Google Scholar] [CrossRef]
- Mariani, M.V.; Manzi, G.; Pierucci, N.; Laviola, D.; Piro, A.; D’Amato, A.; Filomena, D.; Matteucci, A.; Severino, P.; Miraldi, F.; et al. SGLT2i Effect on Atrial Fibrillation: A Network Meta-Analysis of Randomized Controlled Trials. J. Cardiovasc. Electrophysiol. 2024, 35, 1754–1765. [Google Scholar] [CrossRef]
- Adamo, M.; Gardner, R.S.; McDonagh, T.A.; Metra, M. The “Ten Commandments” of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2022, 43, 440–441. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Fabritz, L.; Crijns, H.J.G.M.; Guasch, E.; Goette, A.; Häusler, K.G.; Kotecha, D.; Lewalter, T.; Meyer, C.; Potpara, T.S.; Rienstra, M.; et al. Dynamic Risk Assessment to Improve Quality of Care in Patients with Atrial Fibrillation: The 7th AFNET/EHRA Consensus Conference. EP Europace 2021, 23, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmann, M.W.; Preckel, B.; Koolwijk, P.; van Hinsbergh, V.W.M.; et al. Empagliflozin and Dapagliflozin Reduce ROS Generation and Restore NO Bioavailability in Tumor Necrosis Factor α-Stimulated Human Coronary Arterial Endothelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 53, 865–886. [Google Scholar] [CrossRef]
- Koizumi, T.; Watanabe, M.; Yokota, T.; Tsuda, M.; Handa, H.; Koya, J.; Nishino, K.; Tatsuta, D.; Natsui, H.; Kadosaka, T.; et al. Empagliflozin Suppresses Mitochondrial Reactive Oxygen Species Generation and Mitigates the Inducibility of Atrial Fibrillation in Diabetic Rats. Front. Cardiovasc. Med. 2023, 10, 1005408. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin Alleviates Cardiac Fibrosis through Suppressing EndMT and Fibroblast Activation via AMPKα/TGF-β/Smad Signalling in Type 2 Diabetic Rats. J. Cell. Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef]
- Sukhanov, S.; Higashi, Y.; Yoshida, T.; Mummidi, S.; Aroor, A.R.; Jeffrey Russell, J.; Bender, S.B.; DeMarco, V.G.; Chandrasekar, B. The SGLT2 Inhibitor Empagliflozin Attenuates Interleukin-17A-Induced Human Aortic Smooth Muscle Cell Proliferation and Migration by Targeting TRAF3IP2/ROS/NLRP3/Caspase-1-Dependent IL-1β and IL-18 Secretion. Cell. Signal. 2021, 77, 109825. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 Inhibition Modulates NLRP3 Inflammasome Activity via Ketones and Insulin in Diabetes with Cardiovascular Disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Wang, Q. Effects and Mechanisms of SGLT2 Inhibitors on the NLRP3 Inflammasome, with a Focus on Atherosclerosis. Front. Endocrinol. 2022, 13, 992937. [Google Scholar] [CrossRef]
- Dyck, J.R.B.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac Mechanisms of the Beneficial Effects of SGLT2 Inhibitors in Heart Failure: Evidence for Potential off-Target Effects. J. Mol. Cell. Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef]
- Packer, M. SGLT2 Inhibitors: Role in Protective Reprogramming of Cardiac Nutrient Transport and Metabolism. Nat. Rev. Cardiol. 2023, 20, 443–462. [Google Scholar] [CrossRef]
- Søndergaard, E.; Lauritzen, E.S.; Lauritsen, K.M.; Åkerblom, A.; Nuutila, P.; Oldgren, J.; Gormsen, L.C. SGLT2 Inhibition Reduces Myocardial Oxygen Consumption. Metab. Open 2022, 15, 100207. [Google Scholar] [CrossRef]
- El-Saied, S.B.; El-Sherbeny, W.S.; El-Sharkawy, S.I. Impact of Sodium Glucose Co-Transporter-2 Inhibitors on Left Atrial Functions in Patients with Type-2 Diabetes and Heart Failure with Mildly Reduced Ejection Fraction. Int. J. Cardiol. Heart Vasc. 2024, 50, 101329. [Google Scholar] [CrossRef] [PubMed]
- Stachteas, P.; Nasoufidou, A.; Karagiannidis, E.; Patoulias, D.; Karakasis, P.; Alexiou, S.; Samaras, A.; Zormpas, G.; Stavropoulos, G.; Tsalikakis, D.; et al. The Role of Sodium Glucose Co-Transporter 2 Inhibitors in Atrial Fibrillation: A Comprehensive Review. J. Clin. Med. 2024, 13, 5408. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, G.Y.; Maeng, H.J.; Kim, H.; Bae, J.H.; Kim, K.M.; Lim, S. Effects of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination on the Atherosclerosis-Related Process in a Type 2 Diabetes Mouse Model. Endocrinol. Metab. 2021, 36, 157–170. [Google Scholar] [CrossRef]
- Yadav, P.; Khurana, A.; Bhatti, J.S.; Weiskirchen, R.; Navik, U. Glucagon-like Peptide 1 and Fibroblast Growth Factor-21 in Non-Alcoholic Steatohepatitis: An Experimental to Clinical Perspective. Pharmacol. Res. 2022, 184, 106426. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-Inflammatory Role of Glucagon-like Peptide 1 Receptor Agonists and Its Clinical Implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222370. [Google Scholar] [CrossRef]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-like Peptide-1: A Multi-Faceted Anti-Inflammatory Agent. Front. Immunol. 2023, 14, 1148209. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Theofilis, P.; Pamporis, K.; Sagris, M.; Vlachakis, P.K.; Koufakis, T.; Antoniadis, A.P.; Fragakis, N. GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes. Int. J. Mol. Sci. 2025, 26, 3050. [Google Scholar] [CrossRef]
- Li, Q.; Lin, Y.; Wang, S.; Zhang, L.; Guo, L. GLP-1 Inhibits High-Glucose-Induced Oxidative Injury of Vascular Endothelial Cells. Sci. Rep. 2017, 7, 8008. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.-S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Solomon, S.D.; Ostrominski, J.W.; Wang, X.; Shah, S.J.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Kitzman, D.W.; Verma, S.; Abildstrøm, S.Z.; et al. Effect of Semaglutide on Cardiac Structure and Function in Patients With Obesity-Related Heart Failure. J. Am. Coll. Cardiol. 2024, 84, 1587–1602. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Borlaug, B.A.; Zile, M.R.; Ruff, D.; DiMaria, J.M.; Menon, V.; Ou, Y.; Zarante, A.M.; Hurt, K.C.; Murakami, M.; et al. Tirzepatide Reduces LV Mass and Paracardiac Adipose Tissue in Obesity-Related Heart Failure: SUMMIT CMR Substudy. J. Am. Coll. Cardiol. 2024, 85, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-Analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Marinelli, E.A.; Arbelo, E.; Boriani, G.; Boveda, S.; Buckley, C.M.; Camm, A.J.; Casadei, B.; Chua, W.; Dagres, N.; et al. Early Diagnosis and Better Rhythm Management to Improve Outcomes in Patients with Atrial Fibrillation: The 8th AFNET/EHRA Consensus Conference. EP Europace 2023, 25, 6–27. [Google Scholar] [CrossRef]
- Prabhu, S.; Costello, B.T.; Taylor, A.J.; Gutman, S.J.; Voskoboinik, A.; McLellan, A.J.A.; Peck, K.Y.; Sugumar, H.; Iles, L.; Pathik, B.; et al. Regression of Diffuse Ventricular Fibrosis Following Restoration of Sinus Rhythm with Catheter Ablation in Patients With Atrial Fibrillation and Systolic Dysfunction: A Substudy of the CAMERA MRI Trial. JACC Clin. Electrophysiol. 2018, 4, 999–1007. [Google Scholar] [CrossRef]
- Borges-Rosa, J.; Sousa, P.A.; António, N.; Elvas, L.; Gonçalves, L. Predictors of Systolic Function Recovery after Atrial Fibrillation Ablation in Heart Failure Patients. Rev. Port. Cardiol. 2024, 43, 587–596. [Google Scholar] [CrossRef]
- Bergonti, M.; Spera, F.; Tijskens, M.; Bonomi, A.; Saenen, J.; Huybrechts, W.; Miljoen, H.; Wittock, A.; Casella, M.; Tondo, C.; et al. A New Prediction Model for Left Ventricular Systolic Function Recovery after Catheter Ablation of Atrial Fibrillation in Patients with Heart Failure: The ANTWOORD Study. Int. J. Cardiol. 2022, 358, 45–50. [Google Scholar] [CrossRef]
- Kusunose, K.; Torii, Y.; Yamada, H.; Nishio, S.; Hirata, Y.; Seno, H.; Saijo, Y.; Ise, T.; Yamaguchi, K.; Tobiume, T.; et al. Clinical Utility of Longitudinal Strain to Predict Functional Recovery in Patients With Tachyarrhythmia and Reduced LVEF. JACC Cardiovasc. Imaging 2017, 10, 118–126. [Google Scholar] [CrossRef]
- Schach, C.; Lavall, D.; Voßhage, N.; Körtl, T.; Meindl, C.; Ücer, E.; Hamer, O.; Maier, L.S.; Wachter, R.; Sossalla, S. Time to Recovery from Systolic Dysfunction Correlates with Left Ventricular Fibrosis in Arrhythmia-Induced Cardiomyopathy. Life 2024, 14, 330. [Google Scholar] [CrossRef]
- Barbaroux, H.; Loecher, M.; Brackenier, Y.; Kunze, K.P.; Neji, R.; Pennell, D.J.; Ennis, D.B.; Nielles-Vallespin, S.; Scott, A.D.; Young, A.A. DENSE-SIM: A Modular Pipeline for the Evaluation of Cine Displacement Encoding with Stimulated Echoes Images with Sub-Voxel Ground-Truth Strain. J. Cardiovasc. Magn. Reson. 2025, 27, 101866. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Chung, Y.; Choe, Y.H. Deep Learning for Classification of Late Gadolinium Enhancement Lesions Based on the 16-Segment Left Ventricular Model. Phys. Medica 2024, 117, 103193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, J.; Zhou, Z.; Liang, Y.; Chen, Z.; Chen, X.; Zhang, X. RAS Dataset: A 3D Cardiac LGE-MRI Dataset for Segmentation of Right Atrial Cavity. Sci. Data 2024, 11, 401. [Google Scholar] [CrossRef]
- Crawley, R.; Amirrajab, S.; Lustermans, D.; Holtackers, R.J.; Plein, S.; Veta, M.; Breeuwer, M.; Chiribiri, A.; Scannell, C.M. Automated Cardiovascular MR Myocardial Scar Quantification with Unsupervised Domain Adaptation. Eur. Radiol. Exp. 2024, 8, 93. [Google Scholar] [CrossRef]
- Holste, G.; Oikonomou, E.K.; Tokodi, M.; Kovács, A.; Wang, Z.; Khera, R. Complete AI-Enabled Echocardiography Interpretation With Multitask Deep Learning. JAMA 2025, 334, 306–318. [Google Scholar] [CrossRef]
- Sahashi, Y.; Ieki, H.; Yuan, V.; Christensen, M.; Vukadinovic, M.; Binder-Rodriguez, C.; Rhee, J.; Zou, J.Y.; He, B.; Cheng, P.; et al. Artificial Intelligence Automation of Echocardiographic Measurements. J. Am. Coll. Cardiol. 2025, 86, 964–978. [Google Scholar] [CrossRef]
- Jabbour, J.P.; Palombi, M.; Bonanni, M.; Matteucci, A.; Arcari, L.; Pierucci, N.; La Fazia, V.M.; Lavalle, C.; Mariani, M.V. The Role of Cardiac Magnetic Resonance in Characterizing Atrial Cardiomyopathy and Guiding Substrate Ablation in Atrial Fibrillation: A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 114. [Google Scholar] [CrossRef]
- Baeßler, B.; Engelhardt, S.; Hekalo, A.; Hennemuth, A.; Hüllebrand, M.; Laube, A.; Scherer, C.; Tölle, M.; Wech, T. Perfect Match: Radiomics and Artificial Intelligence in Cardiac Imaging. Circ. Cardiovasc. Imaging 2024, 17, e015490. [Google Scholar] [CrossRef]
- Amyar, A.; Al-Deiri, D.; Sroubek, J.; Kiang, A.; Ghanbari, F.; Nakamori, S.; Rodriguez, J.; Kramer, D.B.; Manning, W.J.; Kwon, D.; et al. Radiomic Cardiac MRI Signatures for Predicting Ventricular Arrhythmias in Patients With Nonischemic Dilated Cardiomyopathy. JACC Adv. 2025, 4, 101684. [Google Scholar] [CrossRef]
- Paciorek, A.M.; von Schacky, C.E.; Foreman, S.C.; Gassert, F.G.; Gassert, F.T.; Kirschke, J.S.; Laugwitz, K.-L.; Geith, T.; Hadamitzky, M.; Nadjiri, J. Automated Assessment of Cardiac Pathologies on Cardiac MRI Using T1-Mapping and Late Gadolinium Phase Sensitive Inversion Recovery Sequences with Deep Learning. BMC Med. Imaging 2024, 24, 43. [Google Scholar] [CrossRef]
- Tsampasian, V.; Cameron, D.; Sobhan, R.; Bazoukis, G.; Vassiliou, V.S. Phosphorus Magnetic Resonance Spectroscopy (31P MRS) and Cardiovascular Disease: The Importance of Energy. Medicina 2023, 59, 174. [Google Scholar] [CrossRef]
- Rosengren, S.; Skibsted Clemmensen, T.; Hvitfeldt Poulsen, S.; Tolbod, L.; Harms, H.J.; Wikström, G.; Kero, T.; Thyrsted Ladefoged, B.; Sörensen, J. Outcome Prediction by Myocardial External Efficiency from (11) C-Acetate Positron Emission Tomography in Cardiac Amyloidosis. ESC Heart Fail. 2024, 11, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-G.; Kim, H.Y.; Park, K.S.; Kim, J.; Moon, J.B.; Lee, N.; Park, H.; Cho, J.Y.; Yoon, H.J.; Ahn, Y.; et al. Myocardial Perfusion and Oxidative Metabolism in Healthy Subjects: Sex-Specific Insights from Vasodilatory Stress (11)C-Acetate PET. EJNMMI Res. 2025, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Varrenti, M.; Bonvicini, E.; Baroni, M.; Gigli, L.; Carbonaro, M.; Garofani, I.; Colombo, G.; Vargiu, S.; De Filippo, V.; Giordano, F.; et al. Arrhythmia-Induced Cardiomyopathy: Predictors of Improvement in Left Ventricular Systolic Function After Catheter Ablation. J. Clin. Med. 2025, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart FailureDeveloped by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Halliday, B.P.; Wassall, R.; Lota, A.S.; Khalique, Z.; Gregson, J.; Newsome, S.; Jackson, R.; Rahneva, T.; Wage, R.; Smith, G.; et al. Withdrawal of Pharmacological Treatment for Heart Failure in Patients with Recovered Dilated Cardiomyopathy (TRED-HF): An Open-Label, Pilot, Randomised Trial. Lancet 2019, 393, 61–73. [Google Scholar] [CrossRef]
- Hasdemir, C.; Ulucan, C.; Yavuzgil, O.; Yuksel, A.; Kartal, Y.; Simsek, E.; Musayev, O.; Kayikcioglu, M.; Payzin, S.; Kultursay, H.; et al. Tachycardia-Induced Cardiomyopathy in Patients with Idiopathic Ventricular Arrhythmias: The Incidence, Clinical and Electrophysiologic Characteristics, and the Predictors. J. Cardiovasc. Electrophysiol. 2011, 22, 663–668. [Google Scholar] [CrossRef]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Sagris, M.; Pamporis, K.; Stachteas, P.; Sidiropoulos, G.; Vlachakis, P.K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Artificial Intelligence in Atrial Fibrillation: From Early Detection to Precision Therapy. J. Clin. Med. 2025, 14, 2627. [Google Scholar] [CrossRef]
- Karakasis, P.; Antoniadis, A.P.; Theofilis, P.; Vlachakis, P.K.; Milaras, N.; Patoulias, D.; Karamitsos, T.; Fragakis, N. Digital Twin Models in Atrial Fibrillation: Charting the Future of Precision Therapy? J. Pers. Med. 2025, 15, 256. [Google Scholar] [CrossRef]
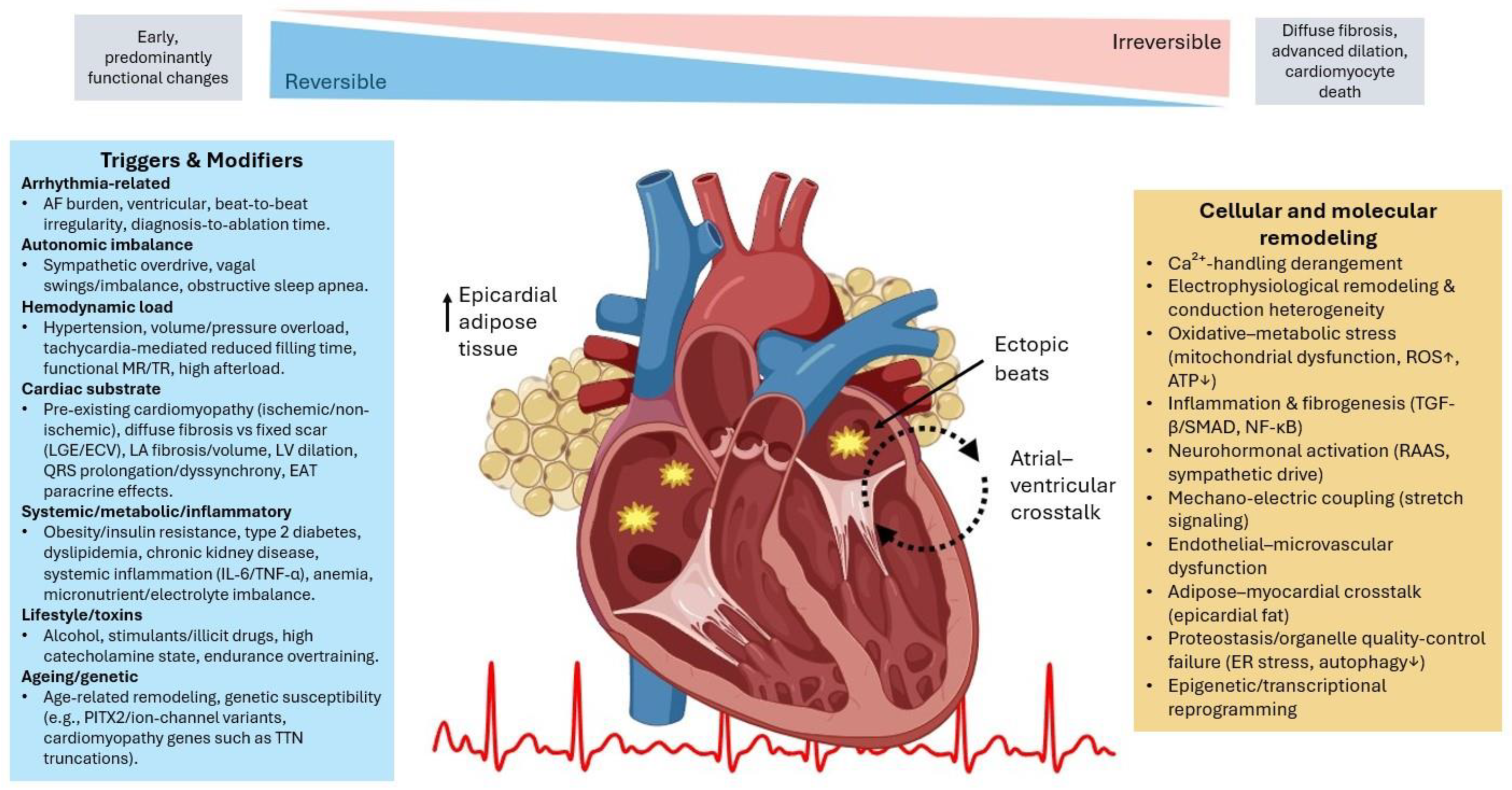
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Apostolos, A.; Ktenopoulos, N.; Grigoriou, K.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Arrhythmia-Induced Cardiomyopathy in Atrial Fibrillation: Pathogenesis, Diagnosis, and Treatment. Life 2025, 15, 1675. https://doi.org/10.3390/life15111675
Karakasis P, Theofilis P, Vlachakis PK, Apostolos A, Ktenopoulos N, Grigoriou K, Patoulias D, Antoniadis AP, Fragakis N. Arrhythmia-Induced Cardiomyopathy in Atrial Fibrillation: Pathogenesis, Diagnosis, and Treatment. Life. 2025; 15(11):1675. https://doi.org/10.3390/life15111675
Chicago/Turabian StyleKarakasis, Paschalis, Panagiotis Theofilis, Panayotis K. Vlachakis, Anastasios Apostolos, Nikolaos Ktenopoulos, Konstantinos Grigoriou, Dimitrios Patoulias, Antonios P. Antoniadis, and Nikolaos Fragakis. 2025. "Arrhythmia-Induced Cardiomyopathy in Atrial Fibrillation: Pathogenesis, Diagnosis, and Treatment" Life 15, no. 11: 1675. https://doi.org/10.3390/life15111675
APA StyleKarakasis, P., Theofilis, P., Vlachakis, P. K., Apostolos, A., Ktenopoulos, N., Grigoriou, K., Patoulias, D., Antoniadis, A. P., & Fragakis, N. (2025). Arrhythmia-Induced Cardiomyopathy in Atrial Fibrillation: Pathogenesis, Diagnosis, and Treatment. Life, 15(11), 1675. https://doi.org/10.3390/life15111675








