High-Dose 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration: Who Is Being Treated with This New Agent?
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Multimodal Imaging
2.3. Measurement of Central Subfield Thickness (CST)
2.4. Anti-VEGF Treatment
2.5. Statistical Analysis
3. Results
3.1. Reasons for Switching to High-Dose 8 mg Aflibercept
3.2. Pretreatment Characteristics
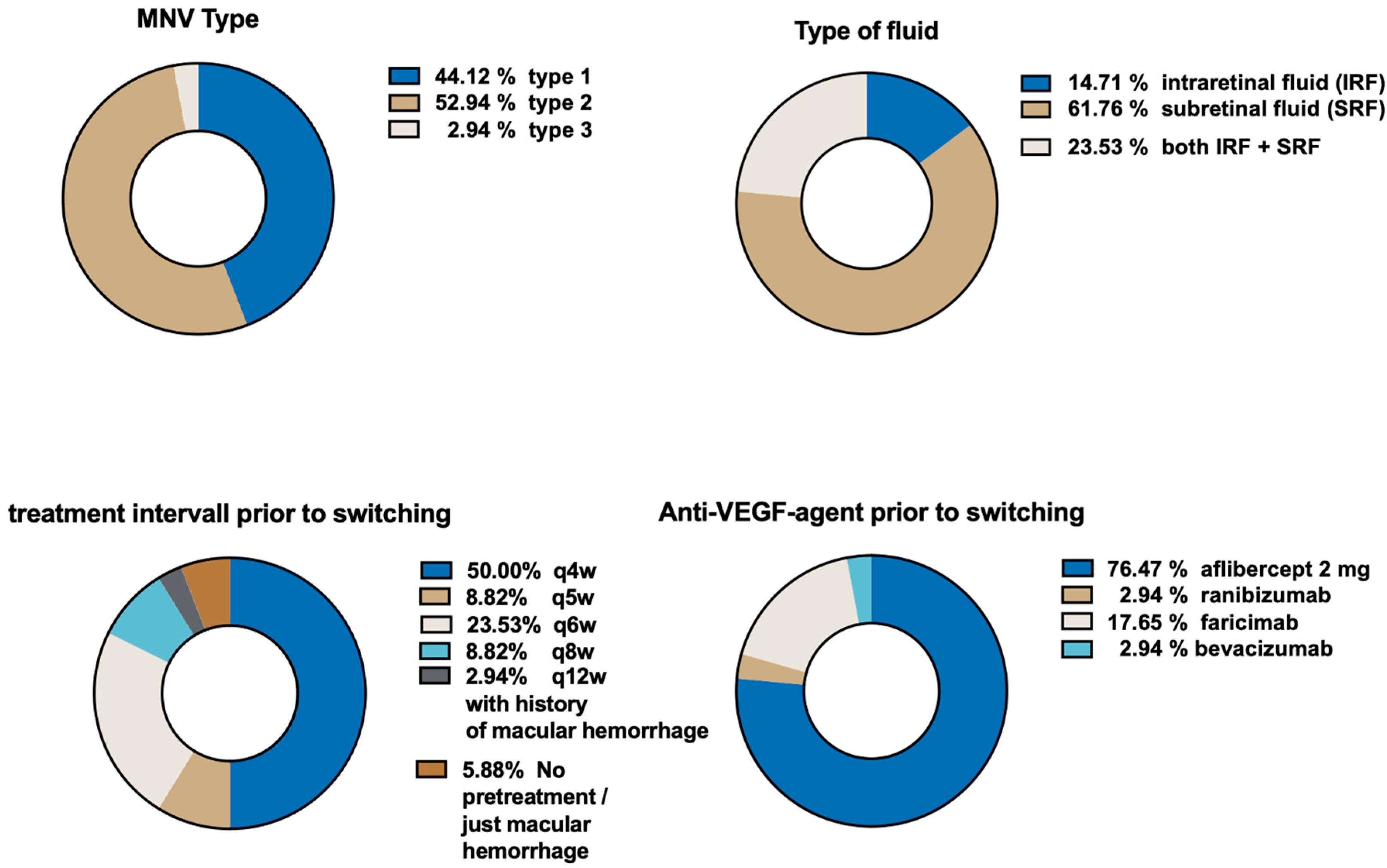
3.3. Macular Neovascularization (MNV) and Fluid Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freund, K.B.; Staurenghi, G.; Jung, J.J.; Zweifel, S.A.; Cozzi, M.; Hill, L.; Blotner, S.; Tsuboi, M.; Gune, S. Macular neovascularization lesion type and vision outcomes in neovascular age-related macular degeneration: Post hoc analysis of HARBOR. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2437–2447. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Freund, K.B.; Korobelnik, J.F.; Devenyi, R.; Framme, C.; Galic, J.; Herbert, E.; Hoerauf, H.; Lanzetta, P.; Michels, S.; Mitchell, P.; et al. Treat-and-Extend Regimens with Anti-Vegf Agents in Retinal Diseases: A Literature Review and Consensus Recommendations. Retina 2015, 35, 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.J.; Campain, A.; Barthelmes, D.; Simpson, J.M.; Guymer, R.H.; Hunyor, A.P.; McAllister, I.L.; Essex, R.W.; Morlet, N.; Gillies, M.C.; et al. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology 2015, 122, 1212–1219. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- New Eylea™ 8 mg Approved in E.U. News Release. Bayer AG. Published January 8. Available online: https://www.bayer.com/media/en-us/new-eylea-8-mg-approved-in-eu/# (accessed on 13 May 2024).
- Schworm, B.; Luft, N.; Keidel, L.F.; Herold, T.R.; Wolf, A.; Priglinger, S.G.; Siedlecki, J. Ranibizumab non-response in pachychoroid neovasculopathy: Effects of switching to aflibercept. Sci. Rep. 2020, 10, 8439. [Google Scholar] [CrossRef] [PubMed]
- Lanzetta, P.; Korobelnik, J.F.; Heier, J.S.; Leal, S.; Holz, F.G.; Clark, W.L.; Eichenbaum, D.; Iida, T.; Xiaodong, S.; Berliner, A.J.; et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet 2024, 403, 1141–1152. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Liberski, S.; Wichrowska, M.; Kocięcki, J. Aflibercept versus Faricimab in the Treatment of Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: A Review. Int. J. Mol. Sci. 2022, 23, 9424. [Google Scholar] [CrossRef] [PubMed]
- Monés, J.; Srivastava, S.K.; Jaffe, G.J.; Tadayoni, R.; Albini, T.A.; Kaiser, P.K.; Holz, F.G.; Korobelnik, J.-F.; Kim, I.K.; Pruente, C.; et al. Risk of Inflammation, Retinal Vasculitis, and Retinal Occlusion-Related Events with Brolucizumab: Post Hoc Review of HAWK and HARRIER. Ophthalmology 2021, 128, 1050–1059. [Google Scholar] [CrossRef]
- Witkin, A.J.; Hahn, P.; Murray, T.G.; Arevalo, J.F.; Blinder, K.J.; Choudhry, N.; Emerson, G.G.; Goldberg, R.A.; Kim, S.J.; Pearlman, J.; et al. Occlusive Retinal Vasculitis Following Intravitreal Brolucizumab. J. Vitreoretin. Dis. 2020, 4, 269–279. [Google Scholar] [CrossRef]
- Baumal, C.R.; Bodaghi, B.; Singer, M.; Tanzer, D.J.; Seres, A.; Joshi, M.R.; Feltgen, N.; Gale, R. Expert Opinion on Management of Intraocular Inflammation, Retinal Vasculitis, and Vascular Occlusion after Brolucizumab Treatment. Ophthalmol. Retina 2021, 5, 519–527. [Google Scholar] [CrossRef]
- Baumal, C.R.; Spaide, R.F.; Vajzovic, L.; Freund, K.B.; Walter, S.D.; John, V.; Rich, R.; Chaudhry, N.; Lakhanpal, R.R.; Oellers, P.R.; et al. Retinal Vasculitis and Intraocular Inflammation after Intravitreal Injection of Brolucizumab. Ophthalmology 2020, 127, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Haug, S.J.; Hien, D.L.; Uludag, G.; Ngoc, T.T.T.; Lajevardi, S.; Halim, M.S.; Sepah, Y.J.; Do, D.V.; Khanani, A.M. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am. J. Ophthalmol. Case Rep. 2020, 18, 100680. [Google Scholar] [CrossRef]
- Rush, R.B.; Rush, S.W. Intravitreal Faricimab for Aflibercept-Resistant Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2022, 16, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, F.; Lorger, A.; Hafner, M.; Klaas, J.E.; Schworm, B.; Kreutzer, T.C.; Priglinger, S.G.; Siedlecki, J. Retinal and choroidal efficacy of switching treatment to faricimab in recalcitrant neovascular age related macular degeneration. Sci. Rep. 2024, 14, 9600. [Google Scholar] [CrossRef] [PubMed]
- Machida, A.; Oishi, A.; Ikeda, J.; Kurihara, J.; Yoneda, A.; Tsuiki, E.; Hirata, Y.; Murakami, R.; Kitaoka, T. Factors Associated with Success of Switching to Faricimab for Neovascular Age-Related Macular Degeneration Refractory to Intravitreal Aflibercept. Life 2024, 14, 476. [Google Scholar] [CrossRef]
- Raimondi, R.; Falfeli, T.; Bogdanova-Bennet, A.; Varma, D.; Habib, M.; Kotagiri, A.; Steel, D.H.; Grinton, M. Outcomes of Treatment-Resistant Neovascular Age-Related Macular Degeneration Switched from Aflibercept to Faricimab. Ophthalmol. Retina 2023, 8, 537–544. [Google Scholar] [CrossRef]
- Szigiato, A.; Mohan, N.; Talcott, K.E.; Mammo, D.A.; Babiuch, A.S.; Kaiser, P.K.; Ehlers, J.P.; Rachitskaya, A.; Yuan, A.; Srivastava, S.K.; et al. Short-Term Outcomes of Faricimab in Patients with Neovascular Age-Related Macular Degeneration on Prior Anti-VEGF Therapy. Ophthalmol. Retina 2024, 8, 10–17. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Brown, D.M.; Reed, K.; Berliner, A.J.; Gerstenblith, A.T.; Breazna, A.; Abraham, P.; Fein, J.G.; Chu, K.W.; Clark, W.L.; et al. Effect of High-Dose Intravitreal Aflibercept, 8 mg, in Patients with Neovascular Age-Related Macular Degeneration: The Phase 2 CANDELA Randomized Clinical Trial. JAMA Ophthalmol. 2023, 141, 834–842. [Google Scholar] [CrossRef]
- Fung, A.E.; Lalwani, G.A.; Rosenfeld, P.J.; Dubovy, S.R.; Michels, S.; Feuer, W.J.; Puliafito, C.A.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Busbee, B.G.; Ho, A.C.; Brown, D.M.; Heier, J.S.; Suñer, I.J.; Li, Z.; Rubio, R.G.; Lai, P. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013, 120, 1046–1056. [Google Scholar] [CrossRef]
- You, Q.S.; Gaber, R.; Meshi, A.; Ramkumar, H.L.; Alam, M.; Muftuoglu, I.K.; Freeman, W.R. High-Dose High-Frequency Aflibercept For Recalcitrant Neovascular Age-Related Macular Degeneration. Retina 2018, 38, 1156–1165. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Roberts, C.L.; Saggau, D.D.; Alliman, K.J. High-Dose Aflibercept for Neovascular AMD and DME in Suboptimal Responders to Standard-Dose Aflibercept. J. Vitreoretin. Dis. 2023, 7, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chu, W.; Lin, P.; Xu, H.; Chen, X. Switching to the aflibercept (3 mg) therapy for treatment-resistant wet age-related macular degeneration: 1-year outcomes. Medicine 2024, 103, e37839. [Google Scholar] [CrossRef] [PubMed]
| Parameter | |
| Eyes (n) | 34 |
| Right/Left | 13/21 |
| Patients (n) | 31 |
| Female/Male | 22/9 |
| Mean age (years) | 78.6 ± 8.9 |
| Mean injections before switching | |
| –total | 34.5 ± 26.3 |
| –per year | 8.0 ± 2.0 |
| Last treatment before switching | |
| –Ranibizumab | 1 (3%) |
| –Aflibercept 2 mg | 26 (76%) |
| –Faricimab | 6 (18%) |
| –Bevacizumab | 1 (3%) |
| Mean injection interval (weeks) at switch | 5.2 ± 2.0 |
| Mean CST (µm) at switch | 420 ± 136 |
| Mean visual acuity (logMAR) at switch | 0.4 ± 0.4 |
| Reasons for swtiching | |
| recalcitrant nAMD despite q4w | |
| q4w | 17 (50.0%) |
| wish to extend treatment interval above | 15 (44%) |
| q5w | 3 (20.0%) |
| q6w | 8 (53.3%) |
| q8w | 3 (20.0%) |
| q12w | 1 (6.7%) |
| macular hemorrhage | 2 (6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liesenhoff, C.; Meyrl, C.; Krause, D.; Eckardt, F.; Lorger, A.; Deiters, V.; Schiefelbein, J.; Klaas, J.E.; Schworm, B.; Priglinger, S.G.; et al. High-Dose 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration: Who Is Being Treated with This New Agent? Life 2025, 15, 1657. https://doi.org/10.3390/life15111657
Liesenhoff C, Meyrl C, Krause D, Eckardt F, Lorger A, Deiters V, Schiefelbein J, Klaas JE, Schworm B, Priglinger SG, et al. High-Dose 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration: Who Is Being Treated with This New Agent? Life. 2025; 15(11):1657. https://doi.org/10.3390/life15111657
Chicago/Turabian StyleLiesenhoff, Caspar, Carolin Meyrl, Daniel Krause, Franziska Eckardt, Anna Lorger, Viktoria Deiters, Johannes Schiefelbein, Julian Elias Klaas, Benedikt Schworm, Siegfried G. Priglinger, and et al. 2025. "High-Dose 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration: Who Is Being Treated with This New Agent?" Life 15, no. 11: 1657. https://doi.org/10.3390/life15111657
APA StyleLiesenhoff, C., Meyrl, C., Krause, D., Eckardt, F., Lorger, A., Deiters, V., Schiefelbein, J., Klaas, J. E., Schworm, B., Priglinger, S. G., & Siedlecki, J. (2025). High-Dose 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration: Who Is Being Treated with This New Agent? Life, 15(11), 1657. https://doi.org/10.3390/life15111657





