Improving OCTA Visualization of Macular Neovascularization via a Grayscale Inversion Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
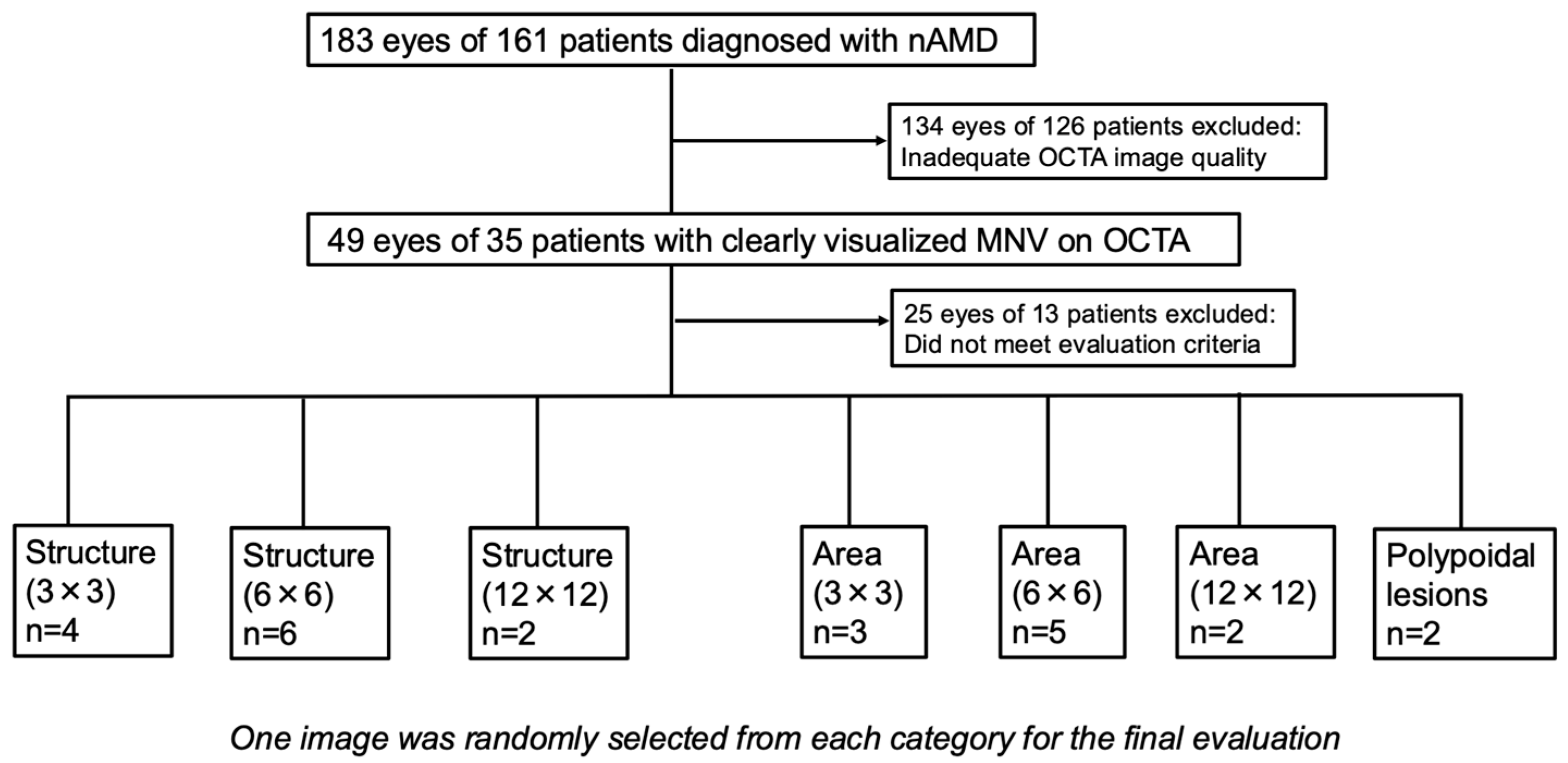
2.2. Subjects
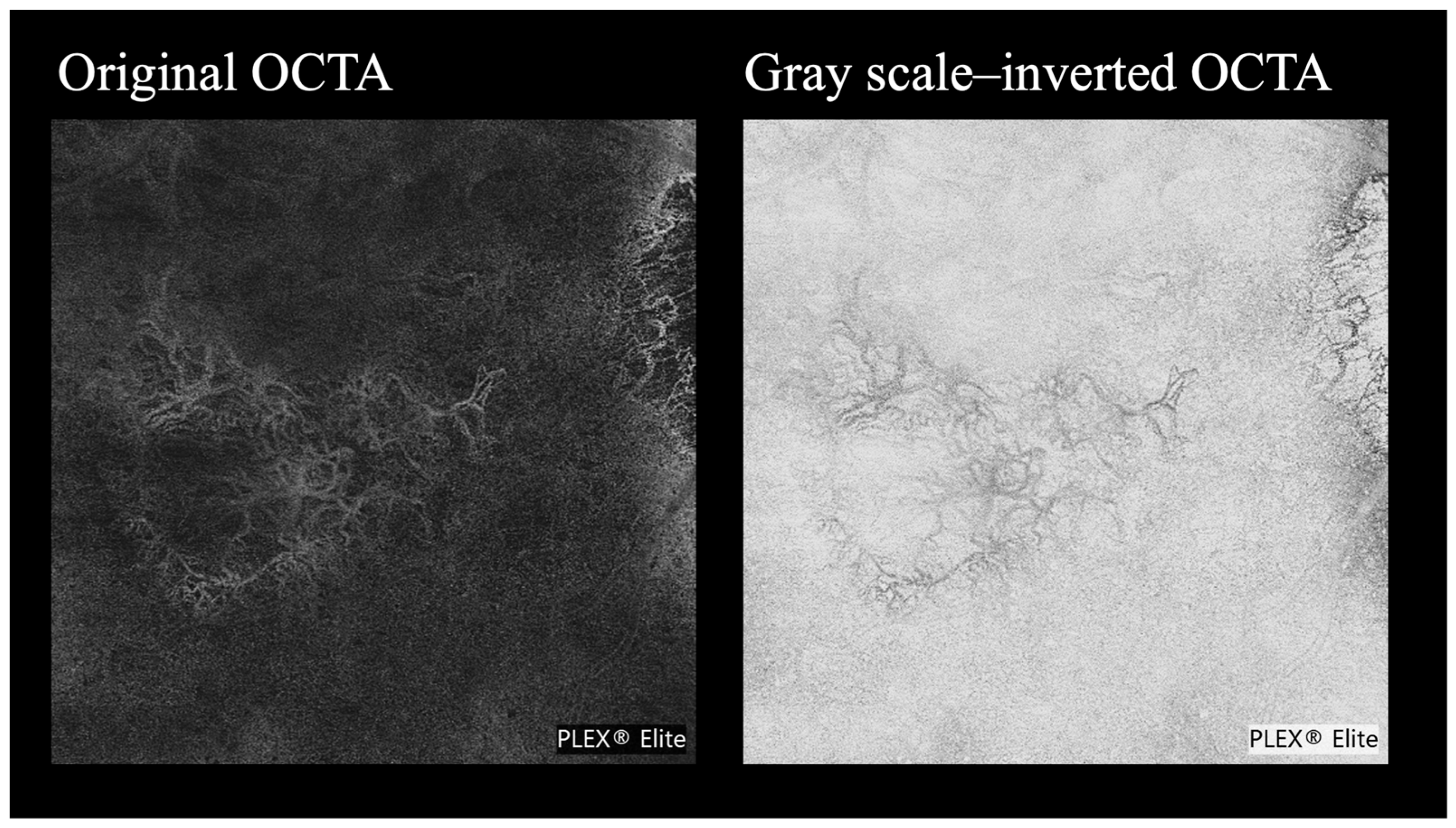
2.3. Image Selections and Evaluation Criteria
- Structure: how clearly can the overall vascular structure of the MNV, including peripheral branches, be visualized?
- Area: how clearly can the entire extent and margins of the MNV be understood?
- Polypoidal lesions: how clearly can the lesion’s shape, margins, and any branching features be understood?
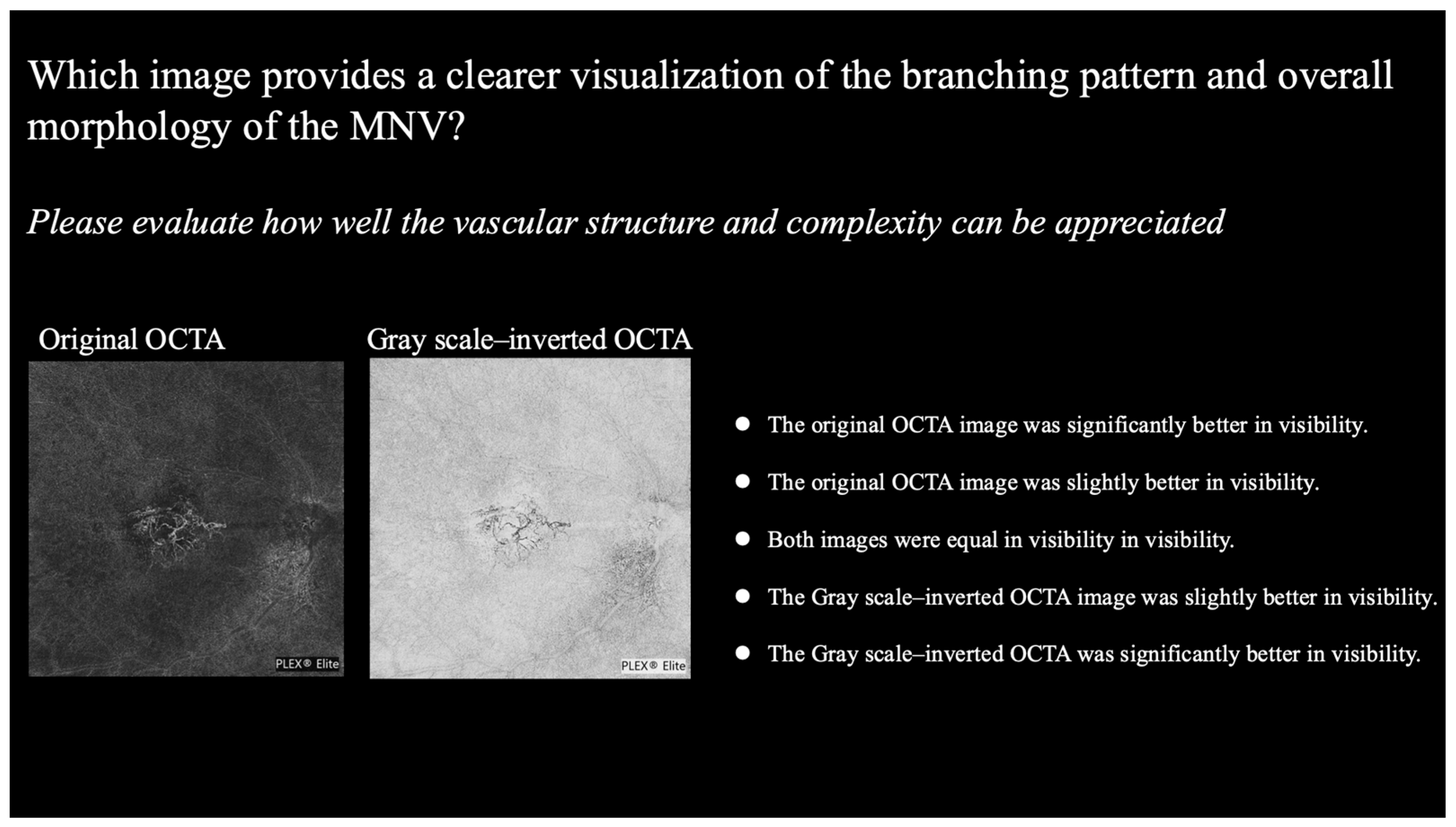
- For structure: which image provides a clearer visualization of the branching pattern and overall morphology of the MNV?
- For area: which image provides better visualization of the MNV boundaries and lesion area?
- For polypoidal lesions: which image provides a clearer visualization of the structure of the polypoidal lesion?
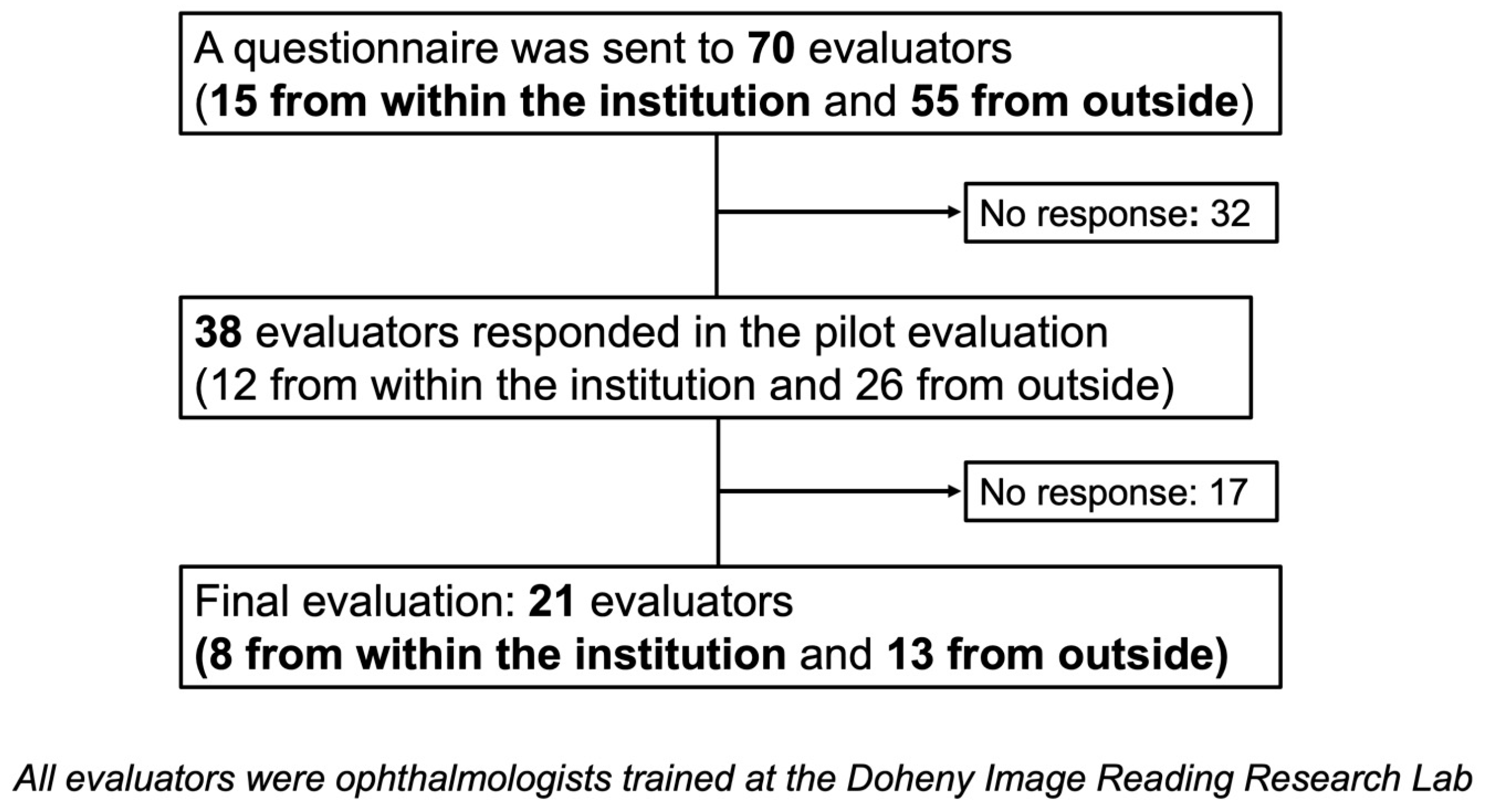
2.4. Pilot Evaluation
2.5. Final Evaluation
2.6. Statistical Analyses
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, J.; Tan, B.; Wong, D.; Garhöfer, G.; Liew, X.W.; Popa-Cherecheanu, A.; Chin, C.W.L.; Milea, D.; Chen, C.L.-H.; Schmetterer, L. Optical coherence tomography angiography of the retina and choroid in systemic diseases. Prog. Retin. Eye Res. 2024, 103, 101292. [Google Scholar] [CrossRef]
- Faatz, H.; Lommatzsch, A. Overview of the Use of Optical Coherence Tomography Angiography in Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2024, 13, 5042. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, O.W.; Oh, H.S.; Kim, S.H.; You, Y.S. Optical coherence tomography angiography in patients with polypoidal choroidalvasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, T.; Nozaki, M.; Yoshida, M.; Ogura, Y. Characteristics of polypoidal choroidal vasculopathy evaluated by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT324–OCT330. [Google Scholar] [CrossRef]
- Siggel, R.; Spital, C.; Lentzsch, A.; Liakopoulos, S. Optical coherence tomography angiography for the detection of macular neovascularization-comparison of en face versus cross-sectional view. Eye 2023, 37, 256–262. [Google Scholar] [CrossRef]
- Foulsham, W.; Chien, J.; Lenis, T.L.; Papakostas, T.D. Optical Coherence Tomography Angiography: Clinical Utility and Future Directions. J. Vitreoretin. Dis. 2022, 6, 229–242. [Google Scholar] [CrossRef]
- Arya, M.; Rashad, R.; Sorour, O.; Moult, E.M.; Fujimoto, J.G.; Waheed, N.K. Optical coherence tomography angiography (OCTA) flow speed mapping technology for retinal diseases. Expert Rev. Med. Devices 2018, 15, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Mino, T.; Moriguchi, Y.; Nagahama, H.; Tamura, M.; Oshima, Y.; Akiba, M.; Enaida, H. Developing quantitative analysis program of blood flow velocity according to vessel diameter for neovascular age-related macular degeneration using OCTA-VISTA. Sci. Rep. 2024, 14, 16352. [Google Scholar] [CrossRef]
- Lungren, M.P.; Samei, E.; Barnhart, H.; McAdams, H.P.; Leder, R.A.; Christensen, J.D.; Wylie, J.D.; Tan, J.W.; Li, X.; Hurwitz, L.M. Gray-scale inversion radiographic display for the detection of pulmonary nodules on chest radiographs. Clin. Imaging 2012, 36, 515–521. [Google Scholar] [CrossRef]
- Robinson, J.W.; Ryan, J.T.; McEntee, M.F.; Lewis, S.J.; Evanoff, M.G.; Rainford, L.A.; Brennan, P.C. Grey-scale inversion improves detection of lung nodules. Br. J. Radiol. 2013, 86, 27961545. [Google Scholar] [CrossRef]
- Park, J.B.; Cho, Y.S.; Choi, H.J. Diagnostic accuracy of the inverted grayscale rib series for detection of rib fracture in minor chest trauma. Am. J. Emerg. Med. 2015, 33, 548–552. [Google Scholar] [CrossRef]
- Blackwell, H.R. Contrast thresholds of the human eye. J. Opt. Soc. Am. 1946, 36, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sun, Y.; Hariri, S.; Zhou, Z.; Inamoto, Y.; Lee, S.J.; Shen, T.T.; Wang, R.K. Anterior segment optical coherence tomography evaluation of ocular graft-versus-host disease: A case study. Quant. Imaging Med. Surg. 2015, 5, 163–170. [Google Scholar]
- Told, R.; Reumueller, A.; Schranz, M.; Brugger, J.; Weigert, G.; Reiter, G.S.; Sacu, S.; Schmidt-Erfurth, U. OCTA Biomarker Search in Patients with nAMD: Influence of Retinal Fluid on Time-Dependent Biomarker Response. Curr. Eye Res. 2023, 48, 600–604. [Google Scholar] [CrossRef]
- Yanık, Ö.; Demirel, S.; Özcan, G.; Batıoğlu, F.; Özmert, E. Qualitative and quantitative comparisons of type 1 macular neovascularizations between pachychoroid neovasculopathy and neovascular age-related macular degeneration using optical coherence tomography angiography. Eye 2024, 38, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, S.-W.; Yun, C.; Oh, J. OCT Angiography Features of Neovascularization as Predictive Factors for Frequent Recurrence in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2020, 213, 109–119. [Google Scholar] [CrossRef]
- Takeuchi, J.; Kataoka, K.; Ito, Y.; Takayama, K.; Yasuma, T.; Kaneko, H.; Terasaki, H. Optical coherence tomography angiography to quantify choroidal neovascularization in response to aflibercept. Ophthalmologica 2018, 240, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, S.W.; Yun, C.; Oh, J.-H.; Oh, J. Predictive role of optical coherence tomography angiography for exudation recurrence in patients with type 1 neovascular age-related macular degeneration treated with pro-re-nata protocol. Eye 2023, 37, 34–41. [Google Scholar] [CrossRef]
- Querques, G.; Corvi, F.; Querques, L.; Souied, E.H.; Bandello, F. Optical Coherence Tomography Angiography of Choroidal Neovascularization Secondary to Pathologic Myopia. Dev. Ophthalmol. 2016, 56, 101–106. [Google Scholar]
- Bruyère, E.; Miere, A.; Cohen, S.Y.; Martiano, D.; Sikorav, A.; Popeanga, A.; Semoun, O.; Querques, G.; Souied, E.H. Neovascularization Secondary to High Myopia Imaged by Optical Coherence Tomography Angiography. Retina 2017, 37, 2095–2101. [Google Scholar] [CrossRef]
- Kimura, M.; Sakurada, Y.; Fukuda, Y.; Matsubara, M.; Kotoda, Y.; Kasai, Y.; Sugiyama, A.; Kikushima, W.; Tsuru, D.V.; Kashiwagi, K. Association of Polyp Regression after Loading Phase with 12-Month Outcomes of Eyes with Polypoidal Choroidal Vasculopathy. Pharmaceuticals 2024, 17, 687. [Google Scholar] [CrossRef]
- Kim, D.G.; Kwak, H.D.; Jang, J.Y.; Ji, Y.; Lee, S.H.; Lee, E.K.; Park, K.H.; Kim, J.H.; Lee, J.S.; Song, Y.; et al. Long-term efficacy and safety of brolucizumab in neovascular age-related macular degeneration: A multicentre retrospective real-world study. Acta Ophthalmol. 2024, 102, e1018–e1028. [Google Scholar]
- Bo, Q.; Zhang, M.; Chen, J.; Jia, H.; Shen, M.; Sun, M.; Xu, M.; Feng, J.; Yan, Q.; Yu, Y.; et al. Progression of Polypoidal Lesions Associated with Exudative Recurrence in Polypoidal Choroidal Vasculopathy. Ophthalmology 2023, 130, 167–178. [Google Scholar]
- Lee, S.H.; Park, H.S.; Han, J.W. Efficacy of Brolucizumab in Polyp Regression of Treatment-Naive Polypoidal Choroidal Vasculopathy and Its Effect on 1-Year Treatment Outcome. Korean J. Ophthalmol. 2024, 38, 185–193. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kobayashi, K.; Aoki, S.; Miyamoto, H.; Imaizumi, H. Comparative analysis of polypoidal choroidal vasculopathy with and without hemorrhage treated by anti-VEGF monotherapy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Schottenhamml, J.; Hohberger, B.; Mardin, C.Y. Applications of Artificial Intelligence in Optical Coherence Tomography Angiography Imaging. Klin. Monatsblätter Augenheilkd. 2022, 239, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, M.; Cennamo, G.; Di Antonio, L.; Lupidi, M.; Parravano, M.; Pellegrini, M.; Veritti, D.; Vujosevic, S.; Savastano, M.C. Practical guidance for imaging biomarkers in exudative age-related macular degeneration. Surv. Ophthalmol. 2023, 68, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Kataoka, K.; Takeuchi, J.; Fujita, A.; Kaneko, H.; Shimizu, H.; Ito, Y.; Terasaki, H.; Vavvas, D.G. Vascular maturity of type 1 and type 2 choroidal neovascularization evaluated by optical coherence tomography angiography. PLoS ONE 2019, 14, e0216304. [Google Scholar] [CrossRef]
| Evaluation Factor | Inverted >> Original (+2) | Inverted > Original (+1) | Inverted = Original (0) | Inverted < Original (−1) | Inverted << Original (−2) | p-Value | Mean ± SD |
|---|---|---|---|---|---|---|---|
| Structure (3 × 3) | 0 | 10 | 1 | 5 | 5 | 0.793 | −0.19 ± 1.25 |
| Structure (6 × 6) | 4 | 9 | 6 | 1 | 1 | <0.01 | 0.67 ± 1.02 |
| Structure (12 × 12) | 4 | 9 | 5 | 2 | 1 | <0.05 | 0.62 ± 1.07 |
| Area (3 × 3) | 0 | 3 | 9 | 6 | 3 | 0.895 | −0.33 ± 1.11 |
| Area (6 × 6) | 4 | 4 | 4 | 8 | 1 | 0.328 | 0.60 ± 1.26 |
| Area (12 × 12) | 0 | 1 | 13 | 6 | 1 | 0.986 | −0.33 ± 0.66 |
| Polypoidal lesions | 3 | 7 | 7 | 4 | 0 | <0.05 | 0.43 ± 0.98 |
| Evaluation Factor | Positive Rate (%) | Negative Rate (%) | Neutral Rate (%) |
|---|---|---|---|
| Structure (3 × 3) | 47.6 | 47.6 | 4.8 |
| Structure (6 × 6) | 61.9 | 9.5 | 28.6 |
| Structure (12 × 12) | 61.9 | 14.3 | 23.8 |
| Area (3 × 3) | 14.3 | 42.9 | 42.9 |
| Area (6 × 6) | 38.1 | 42.9 | 19.0 |
| Area (12 × 12) | 4.8 | 33.3 | 61.9 |
| Polypoidal lesions | 47.6 | 19.0 | 33.3 |
| Evaluation Factor | Inverted >> Original (+2) | Inverted > Original (+1) | Inverted = Original (0) | Inverted < Original (−1) | Inverted << Original (−2) | p-Value |
|---|---|---|---|---|---|---|
| Area (3 × 3) | 0 | 3 | 9 | 6 | 3 | 0.121 |
| Area (6 × 6) | 4 | 4 | 4 | 8 | 1 | 0.69 |
| Area (12 × 12) | 0 | 1 | 13 | 6 | 1 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chujo, S.; Chung, Y.-C.; Quarta, A.; Kwak, H.; Soylu, C.; Abbasgholizadeh, R.; Alhelaly, M.; Rattu, R.; Corradetti, G.; Nittala, M.G.; et al. Improving OCTA Visualization of Macular Neovascularization via a Grayscale Inversion Method. Life 2025, 15, 1512. https://doi.org/10.3390/life15101512
Chujo S, Chung Y-C, Quarta A, Kwak H, Soylu C, Abbasgholizadeh R, Alhelaly M, Rattu R, Corradetti G, Nittala MG, et al. Improving OCTA Visualization of Macular Neovascularization via a Grayscale Inversion Method. Life. 2025; 15(10):1512. https://doi.org/10.3390/life15101512
Chicago/Turabian StyleChujo, Shinichiro, Yu-Chien Chung, Alberto Quarta, Hyunduck Kwak, Ceren Soylu, Rouzbeh Abbasgholizadeh, Mai Alhelaly, Raiyna Rattu, Giulia Corradetti, Muneeswar Gupta Nittala, and et al. 2025. "Improving OCTA Visualization of Macular Neovascularization via a Grayscale Inversion Method" Life 15, no. 10: 1512. https://doi.org/10.3390/life15101512
APA StyleChujo, S., Chung, Y.-C., Quarta, A., Kwak, H., Soylu, C., Abbasgholizadeh, R., Alhelaly, M., Rattu, R., Corradetti, G., Nittala, M. G., & Sadda, S. R. (2025). Improving OCTA Visualization of Macular Neovascularization via a Grayscale Inversion Method. Life, 15(10), 1512. https://doi.org/10.3390/life15101512






