Virtual Reality-Based Therapy Improves Balance, Quality of Life, and Mitigates Pain and Fear of Falling in Women with Bone Mineral Density Loss: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Study Selection: Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Variables
2.6. Methodological Quality, Risk of Bias, and Quality of Evidence Assessment
2.7. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of the Studies Included
3.3. Methodological Quality and Risk of Bias
3.4. Meta-Analysis
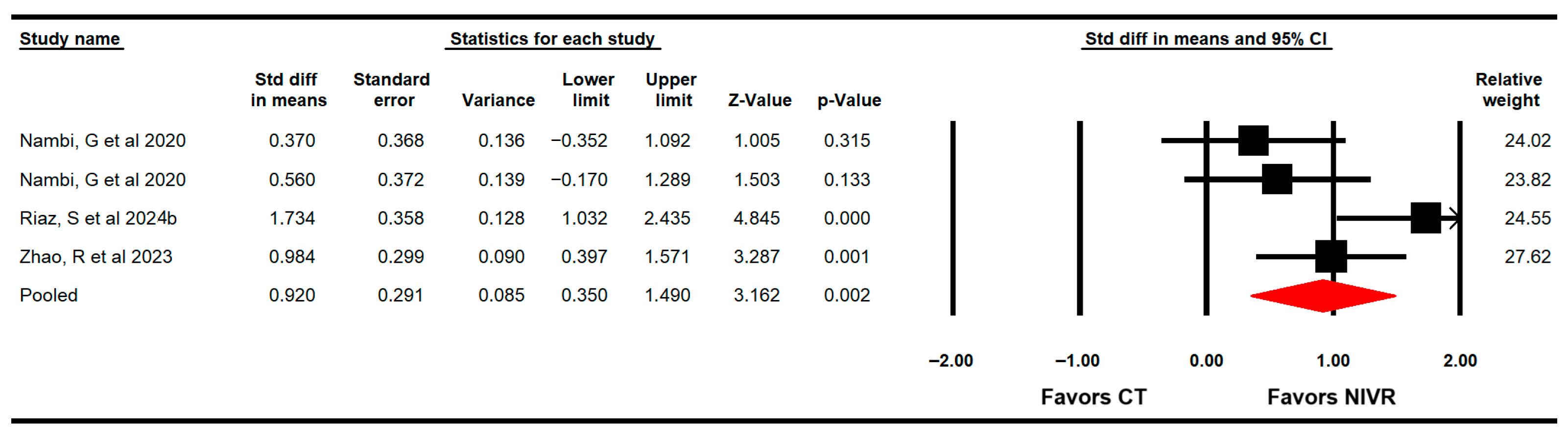
3.4.1. Bone Mineral Density
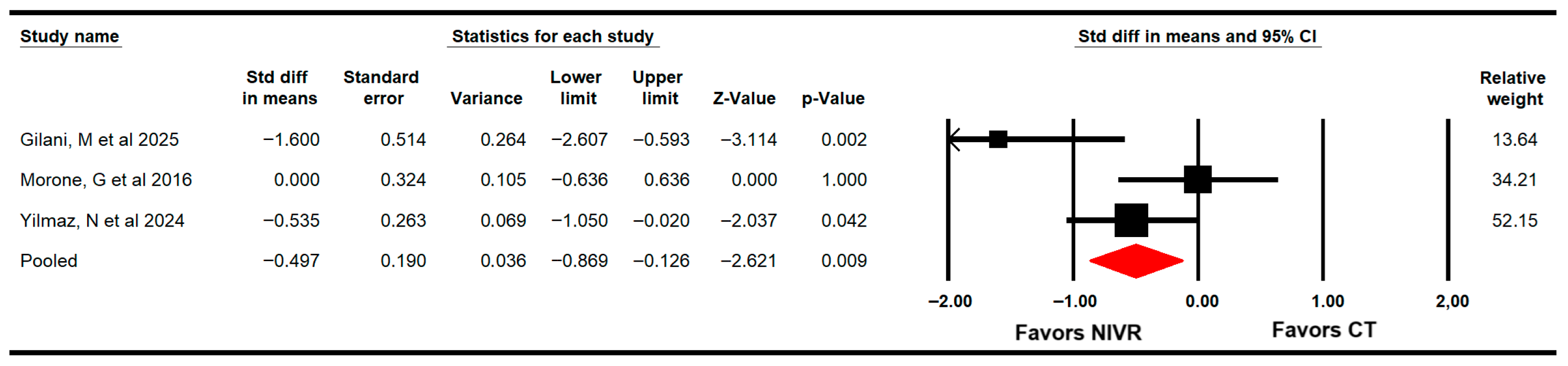
3.4.2. Functional Balance
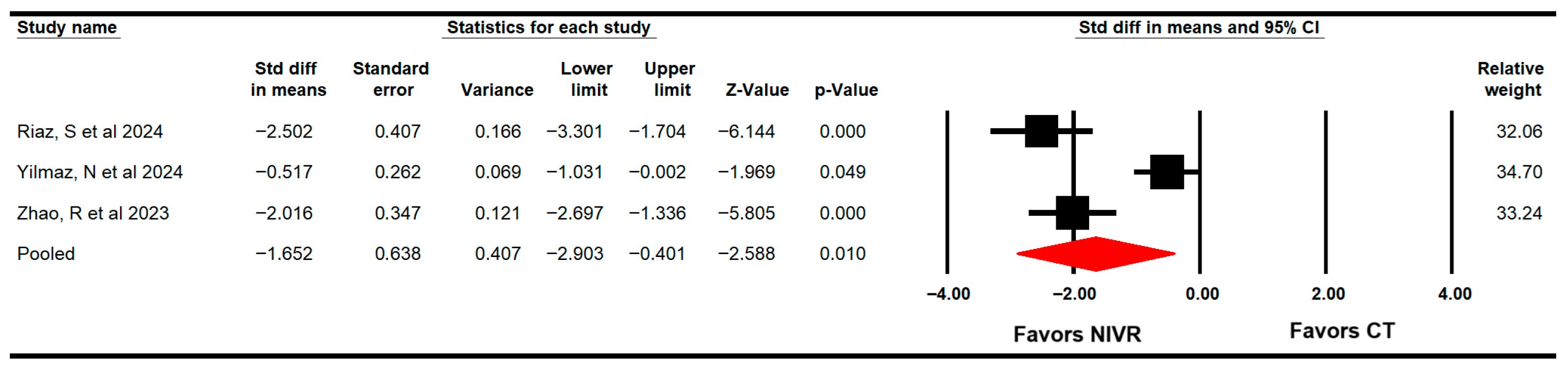
3.4.3. Dynamic Balance/Functional Mobility
3.4.4. Fear of Falling
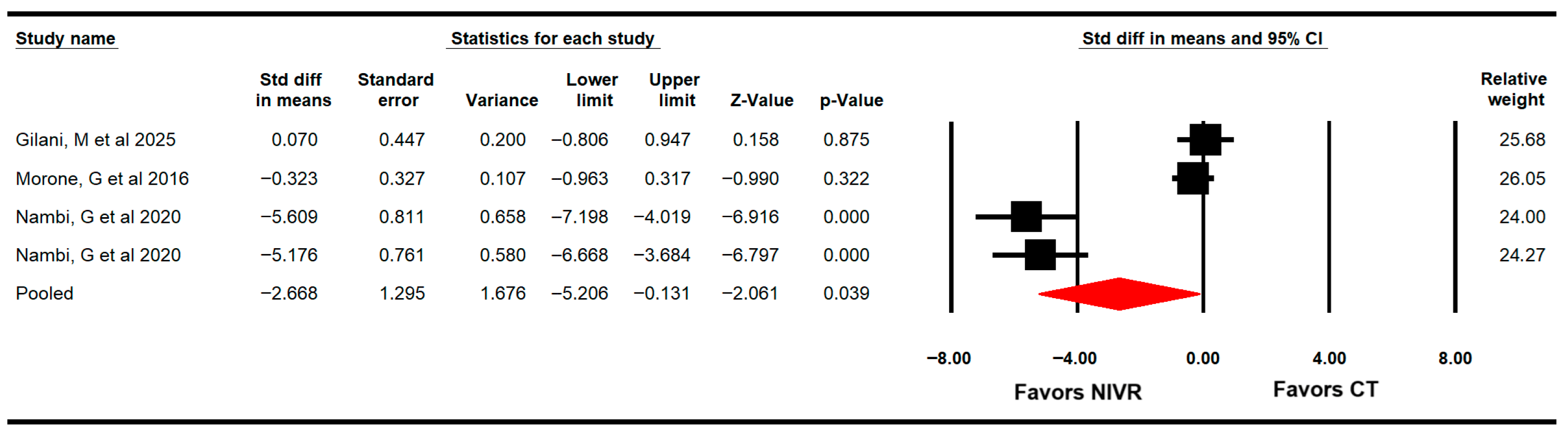
3.4.5. Pain
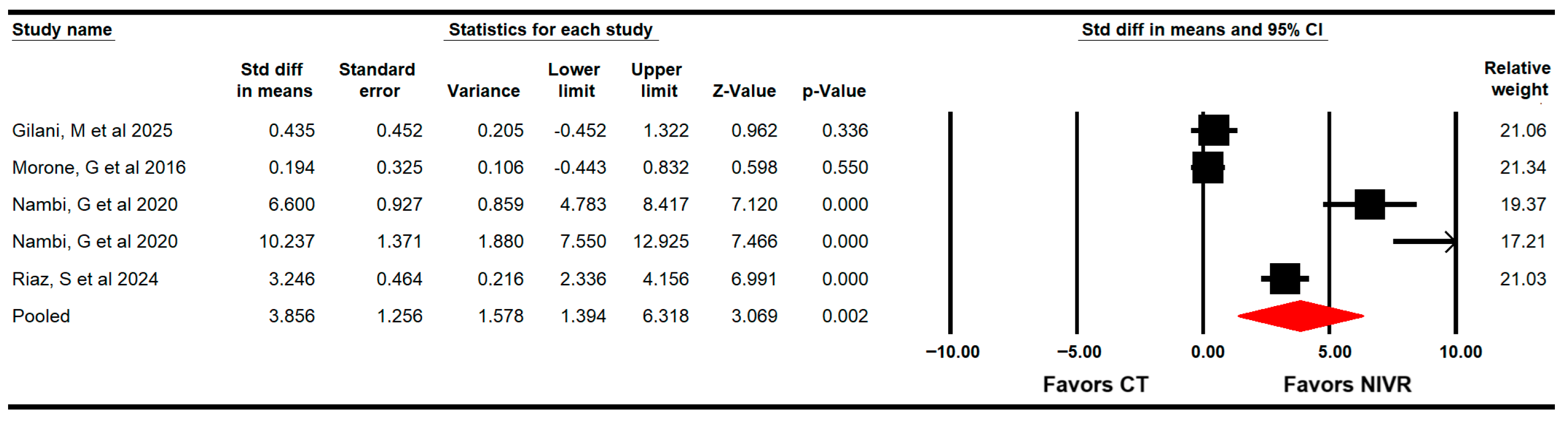
3.4.6. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | Bone mineral density |
| VRBT | Virtual reality-based therapy |
| NIVR | Non-immersive virtual reality |
| DLAs | Daily living activities |
| SRMA | Systematic review with meta-analysis |
| QoL | Quality of life |
| SMD | Standardized mean differences |
| 95% CI | 95% confidence interval |
| RCTs | Randomized controlled trials |
References
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Nicholson, W.K.; Silverstein, M.; Wong, J.B.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Jaén, C.R.; Krousel-Wood, M.; Lee, S.; Li, L.; et al. Screening for Osteoporosis to Prevent Fractures. JAMA 2025, 333, 498. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; McClung, M.R. Osteopenia: A Key Target for Fracture Prevention. Lancet Diabetes Endocrinol. 2024, 12, 856–864. [Google Scholar] [CrossRef]
- Kienberger, Y.; Sassmann, R.; Rieder, F.; Johansson, T.; Kässmann, H.; Pirich, C.; Wicker, A.; Niebauer, J. Effects of Whole Body Vibration in Postmenopausal Osteopenic Women on Bone Mineral Density, Muscle Strength, Postural Control and Quality of Life: The T-Bone Randomized Trial. Eur. J. Appl. Physiol. 2022, 122, 2331–2342. [Google Scholar] [CrossRef]
- Lorentzon, M.; Johansson, H.; Harvey, N.C.; Liu, E.; Vandenput, L.; McCloskey, E.V.; Kanis, J.A. Osteoporosis and Fractures in Women: The Burden of Disease. Climacteric 2022, 25, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Rasi, K.; Karinkanta, S.; Tokola, K.; Kannus, P.; Sievänen, H. Bone Mass and Strength and Fall-Related Fractures in Older Age. J. Osteoporos. 2019, 2019, 5134690. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An Estimate of the Worldwide Prevalence and Disability Associated with Osteoporotic Fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the Future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef]
- Moreland, J.D.; Richardson, J.A.; Goldsmith, C.H.; Clase, C.M. Muscle Weakness and Falls in Older Adults: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2004, 52, 1121–1129. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and Its Association with Falls and Fractures in Older Adults: A Systematic Review and Meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Mahoney, J.R.; Cotton, K.; Verghese, J. Multisensory Integration Predicts Balance and Falls in Older Adults. J. Gerontol. Ser. A 2019, 74, 1429–1435. [Google Scholar] [CrossRef]
- Singh, R.R.; Maurya, P. Visual Impairment and Falls among Older Adults and Elderly: Evidence from Longitudinal Study of Ageing in India. BMC Public Health 2022, 22, 2324. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, L.G.; Perugini, A.J.; Fehrenbacher, J.C.; White, F.A.; Kacena, M.A. Assessment, Quantification, and Management of Fracture Pain: From Animals to the Clinic. Curr. Osteoporos. Rep. 2020, 18, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-K.; Lee, W.-J.; Chen, L.-Y.; Hwang, A.-C.; Lin, M.-H.; Peng, L.-N.; Chen, L.-K. Association between Frailty, Osteoporosis, Falls and Hip Fractures among Community-Dwelling People Aged 50 Years and Older in Taiwan: Results from I-Lan Longitudinal Aging Study. PLoS ONE 2015, 10, e0136968. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Prieto-Contreras, L.; Pérez-Ros, P. Factors Associated with Fear of Falling among Frail Older Adults. Geriatr. Nurs. 2021, 42, 1035–1041. [Google Scholar] [CrossRef]
- Golubić, A.; Šarabon, N.; Marković, G. Association between Trunk Muscle Strength and Static Balance in Older Women. J. Women Aging 2021, 33, 288–297. [Google Scholar] [CrossRef]
- Genev, I.K.; Tobin, M.K.; Zaidi, S.P.; Khan, S.R.; Amirouche, F.M.L.; Mehta, A.I. Spinal Compression Fracture Management. Glob. Spine J. 2017, 7, 71–82. [Google Scholar] [CrossRef]
- Zarinfar, Y.; Panahi, N.; Hosseinpour, M.; Sedokani, A.; Hajivalizadeh, S.; Nabipour, I.; Larijani, B.; Fahimfar, N.; Ostovar, A. The Association between Osteoporosis and Quality of Life among Older Adults in Southern Iran: Findings from the Bushehr Elderly Health Program. BMC Geriatr. 2024, 24, 766. [Google Scholar] [CrossRef]
- Rajabi, M.; Ostovar, A.; Sari, A.A.; Sajjadi-Jazi, S.M.; Mousavi, A.; Larijani, B.; Fahimfar, N.; Daroudi, R. Health-Related Quality of Life in Osteoporosis Patients with and without Fractures in Tehran, Iran. J. Bone Metab. 2023, 30, 37–46. [Google Scholar] [CrossRef]
- van der Leeuw, G.; Ayers, E.; Leveille, S.G.; Blankenstein, A.H.; van der Horst, H.E.; Verghese, J. The Effect of Pain on Major Cognitive Impairment in Older Adults. J. Pain 2018, 19, 1435–1444. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Y. Quality of Life in Postmenopausal Women with Osteoporosis: A Systematic Review and Meta-Analysis. Qual. Life Res. 2023, 32, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kuang, X.; Li, K.; Guo, X.; Deng, Q.; Li, D. Effects of Combined Calcium and Vitamin D Supplementation on Osteoporosis in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2020, 11, 10817–10827. [Google Scholar] [CrossRef]
- Anupama, D.S.; Norohna, J.A.; Acharya, K.KV.; Ravishankar; George, A. Effect of Exercise on Bone Mineral Density and Quality of Life among Postmenopausal Women with Osteoporosis without Fracture: A Systematic Review. Int. J. Orthop. Trauma Nurs. 2020, 39, 100796. [Google Scholar] [CrossRef]
- Rodrigues, I.B.; Armstrong, J.J.; Adachi, J.D.; MacDermid, J.C. Facilitators and Barriers to Exercise Adherence in Patients with Osteopenia and Osteoporosis: A Systematic Review. Osteoporos. Int. 2017, 28, 735–745. [Google Scholar] [CrossRef]
- Mangano, G.R.A.; Avola, M.; Blatti, C.; Caldaci, A.; Sapienza, M.; Chiaramonte, R.; Vecchio, M.; Pavone, V.; Testa, G. Non-Adherence to Anti-Osteoporosis Medication: Factors Influencing and Strategies to Overcome It. A Narratice Review. J. Clin. Med. 2022, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.L.; Keshner, E.A.; Levin, M.F. Virtual Reality for Physical and Motor Rehabilitation. In Virtual Reality Technologies for Health and Clinical Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Nieto-Escamez, F.; Cortés-Pérez, I.; Obrero-Gaitán, E.; Fusco, A. Virtual Reality Applications in Neurorehabilitation: Current Panorama and Challenges. Brain Sci. 2023, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a Non-Immersive Virtual Reality Component to Treadmill Training to Reduce Fall Risk in Older Adults (V-TIME): A Randomised Controlled Trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- Park, J.-H.; Jeon, H.-S.; Kim, J.-H.; Kim, Y.J.; Moon, G.A. Effectiveness of Non-Immersive Virtual Reality Exercises for Balance and Gait Improvement in Older Adults: A Meta-Analysis. Technol. Health Care 2024, 32, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Ibancos-Losada, M.d.R.; Zagalaz-Anula, N.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Nintendo® Wii Therapy Improves Upper Extremity Motor Function in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12343. [Google Scholar] [CrossRef] [PubMed]
- Opara Zupančič, M.; Šarabon, N. The Current State of Virtual Reality in the Management of Musculoskeletal Conditions and Associated Chronic Pain: Terminology, Technology, and Associations. Appl. Sci. 2025, 15, 2564. [Google Scholar] [CrossRef]
- Chitjamnogchai, C.; Yuenyongchaiwat, K.; Sermsinsaithong, N.; Tavonudomgit, W.; Mahawong, L.; Buranapuntalug, S.; Thanawattano, C. Home-Based Virtual Reality Exercise and Resistance Training for Enhanced Cardiorespiratory Fitness in Community-Dwelling Older People with Sarcopenia: A Randomized, Double-Blind Controlled Trial. Life 2025, 15, 986. [Google Scholar] [CrossRef]
- Thuilier, E.; Carey, J.; Dempsey, M.; Dingliana, J.; Whelan, B.; Brennan, A. Virtual Rehabilitation for Patients with Osteoporosis or Other Musculoskeletal Disorders: A Systematic Review. Virtual Real 2024, 28, 93. [Google Scholar] [CrossRef]
- He, S.; Dong, S.; Lin, X.; Wang, Z.; Diao, Y.; Gao, X. The Effectiveness of Virtual Reality in People with Osteoporosis or Osteopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Physiol. 2025, 16, 1612882. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley Blackwell & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.Z.; Moseley, A.M.; Maher, C.G.; Nascimento, D.P.; Costa, L.d.C.M.; Costa, L.O. Methodologic Quality and Statistical Reporting of Physical Therapy Randomized Controlled Trials Relevant to Musculoskeletal Conditions. Arch. Phys. Med. Rehabil. 2018, 99, 129–136. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T. Grading Quality of Evidence and Strength of Recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A Checklist Designed to Aid Consistency and Reproducibility of GRADE Assessments: Development and Pilot Validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Software, Version 4; Biostat: Englewood, NJ, USA, 2023. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Kinney, A.R.; Eakman, A.M.; Graham, J.E. Novel Effect Size Interpretation Guidelines and an Evaluation of Statistical Power in Rehabilitation Research. Arch. Phys. Med. Rehabil. 2020, 101, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Rücker, G.; Schwarzer, G. Beyond the Forest Plot: The Drapery Plot. Res. Synth. Methods 2021, 12, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M. Funnel Plots for Detecting Bias in Meta-Analysis: Guidelines on Choice of Axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.; Greenland, S.; Lash, T. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thompson, S.; Altman, D. Statistical Heterogeneity in Systematic Reviews of Clinical Trials: A Critical Appraisal of Guidelines and Practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Gilani, M.; Torkaman, G.; Bahrami, F.; Bayat, N. Virtual Reality Exergaming Capability to Change Muscle Strategy During the Limits of Stability Test and Reduce Fear of Falling in Primary Osteoporotic Women. Games Health J. 2023, 12, 310–322. [Google Scholar] [CrossRef]
- Morone, G.; Paolucci, T.; Luziatelli, S.; Iosa, M.; Piermattei, C.; Zangrando, F.; Paolucci, S.; Vulpiani, M.C.; Saraceni, V.M.; Baldari, C.; et al. Wii Fit Is Effective in Women with Bone Loss Condition Associated with Balance Disorders: A Randomized Controlled Trial. Aging Clin. Exp. Res. 2016, 28, 1187–1193. [Google Scholar] [CrossRef]
- Riaz, S.; Shakil Ur Rehman, S.; Hassan, D.; Hafeez, S. Gamified Exercise with Kinect: Can Kinect-Based Virtual Reality Training Improve Physical Performance and Quality of Life in Postmenopausal Women with Osteopenia? A Randomized Controlled Trial. Sensors 2024, 24, 3577. [Google Scholar] [CrossRef]
- Yilmaz, N.; Kösehasanoğulları, M. The Effectiveness of Virtual Reality Exercise Games on Balance Functions and Fear of Falling in Women with Osteoporosis. Rheumatol. Int. 2024, 44, 1071–1076. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, X.; Guan, J.; Zhang, C.; Zhu, K. The Effect of Virtual Reality Technology on Anti-Fall Ability and Bone Mineral Density of the Elderly with Osteoporosis in an Elderly Care Institution. Eur. J. Med. Res. 2023, 28, 204. [Google Scholar] [CrossRef]
- Nambi, G.; Kamal-Abdelbasset, W.; Alsaid-Moawd, S.; Reda-Sakr, H.; Emam-Elnegamy, T.; Saji-George, J. Effect of Virtual Reality Training on Post-Menopausal Osteoporotic Women. Arch. Pharm. Pract. 2020, 11, 6–12. [Google Scholar]
- Riaz, S.; Shakil Ur Rehman, S.; Hafeez, S.; Hassan, D. Effects of Kinect-Based Virtual Reality Training on Bone Mineral Density and Fracture Risk in Postmenopausal Women with Osteopenia: A Randomized Controlled Trial. Sci. Rep. 2024, 14, 6650. [Google Scholar] [CrossRef]
- Negm, A.M.; Papaioannou, A. Diagnosis of Osteosarcopenia—Clinical. In Osteosarcopenia; Duque, G., Troen, B.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–204. [Google Scholar]
- Kerschan-Schindl, K.; Hasenoehrl, T. Exercise in the Prevention of Age-Related Fragility Fractures (Narrative Review). Gerontology 2025, 71, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B. Postural Orientation and Equilibrium: What Do We Need to Know about Neural Control of Balance to Prevent Falls? Age Ageing 2006, 35 (Suppl 2), ii7–ii11. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Chang, S.-T.; Lee, C.-H.; Liou, I.-H.; Cherng, R.-J. Effects of Virtual Reality on the Balance Performance of Older Adults: A Systematic Review and Meta-Analysis. J. Phys. Ther. Sci. 2024, 36, 2024–2027. [Google Scholar] [CrossRef]
- García-López, H.; Obrero-Gaitán, E.; Castro-Sánchez, A.M.; Lara-Palomo, I.C.; Nieto-Escamez, F.A.; Cortés-Pérez, I. Non-Immersive Virtual Reality to Improve Balance and Reduce Risk of Falls in People Diagnosed with Parkinson’s Disease: A Systematic Review. Brain Sci. 2021, 11, 1435. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.K.R.; Marques, A.P.; Andrade, K.F.A.; de Assis, J.C.S.; Brito, A.L.; Souza, G.S.; Callegari, B. Virtual Reality in Improving Anticipatory Postural Adjustments to Step Initiation in Individuals with Knee Osteoarthritis: A Randomized Controlled Trial. Games Health J. 2024, 13, 100–108. [Google Scholar] [CrossRef]
- Gofredo, M.; Baglio, F.; De Icco, R.; Proietti, S.; Maggioni, G.; Turolla, A.; Pournajaf, S.; Jonsdottir, J.; Zeni, F.; Federico, S.; et al. Efficacy of Non-Immersive Virtual Reality-Based Telerehabilitation on Postural Stability in Parkinson’s Disease: A Multicenter Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2024, 59, 689–696. [Google Scholar] [CrossRef]
- Saragih, I.D.; Chen, Y.; Suarilah, I.; Susanto, H.; Lee, B. Virtual Reality Intervention for Fall Prevention in Older Adults: A Meta-Analysis. J. Nurs. Sch. 2025, 57, 759–775. [Google Scholar] [CrossRef]
- Rodríguez-Almagro, D.; Achalandabaso-Ochoa, A.; Ibáñez-Vera, A.J.; Góngora-Rodríguez, J.; Rodríguez-Huguet, M. Effectiveness of Virtual Reality Therapy on Balance and Gait in the Elderly: A Systematic Review. Healthcare 2024, 12, 158. [Google Scholar] [CrossRef]
- Saby, A.; Alvarez, A.; Smolins, D.; Petros, J.; Nguyen, L.; Trujillo, M.; Aygün, O. Effects of Embodiment in Virtual Reality for Treatment of Chronic Pain: Pilot Open-Label Study. JMIR Form. Res. 2024, 8, e34162. [Google Scholar] [CrossRef]
- Maheta, B.; Kraft, A.; Interrante, N.; Fereydooni, S.; Bailenson, J.; Beams, B.; Keny, C.; Osborne, T.; Giannitrapani, K.; Lorenz, K. Using Virtual Reality to Improve Outcomes Related to Quality of Life Among Older Adults With Serious Illnesses: Systematic Review of Randomized Controlled Trials. J. Med. Internet Res. 2025, 27, e54452. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, L.; Bharadwaj, L.; Nissen, K.; Estey, J. Non-Immersive Virtual Reality Exercise Can Increase Exercise in Older Adults Living in the Community and in Long-Term Care: A Randomized Controlled Trial. Clin. Interv. Aging 2025, 20, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.; Fuentes, J.; da Costa, B.R.; Saltaji, H.; Ha, C.; Cummings, G.G. Blinding in Physical Therapy Trials and Its Association with Treatment Effects. Am. J. Phys. Med. Rehabil. 2017, 96, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.; Saltaji, H.; da Costa, B.R.; Fuentes, J.; Ha, C.; Cummings, G.G. What Is the Influence of Randomisation Sequence Generation and Allocation Concealment on Treatment Effects of Physical Therapy Trials? A Meta-Epidemiological Study. BMJ Open 2015, 5, e008562. [Google Scholar] [CrossRef]
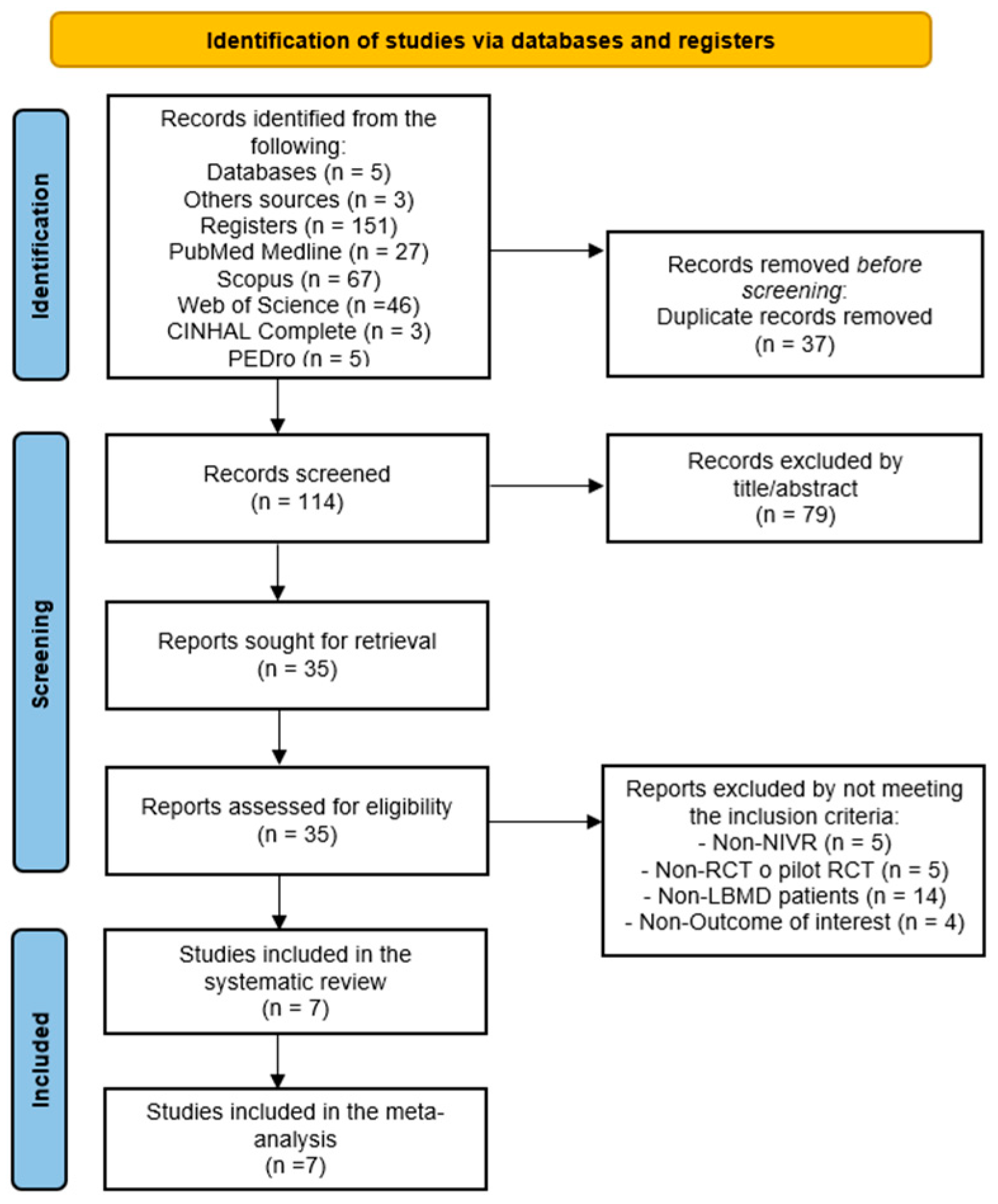

| Database | Search Strategy |
|---|---|
| PubMed Medline | (Osteoporosis[mh] or osteoporosis[tiab] or low bone density[tiab] or osteoporosis postmenopausal[mh] or osteoporosis postmenopausal[tiab] or osteopenia[tiab]) and (virtual reality[mh] or virtual reality[tiab] or exergaming[mh] or exergame *[tiab] or virtual reality exposure therapy[mh] or virtual reality exposure therapy[tiab] or wii[tiab] or Nintendo[tiab] or videogame *[tiab] or non-immersive virtual reality[tiab] or kinect[tiab] or virtual environment[tiab] or seious game[tiab]) |
| SCOPUS | (“Osteoporosis” or “low bone density” or “osteoporosis postmenopausal” or “osteopenia”) and (“virtual reality” or “exergaming” or “virtual reality exposure therapy” or “wii” or “Nintendo” or “videogamame” or “immersive virtual reality” or “Kinect” or “virtual environment” or “seious game”) |
| Web of Science | TOPIC(*Osteoporosis* or *low bone density* or *osteoporosis postmenopausal* or *osteopenia*) and TOPIC(*virtual reality* or *exergaming* or *virtual reality exposure therapy* or *wii* or *Nintendo* or *videogamame* or *immersive virtual reality* or *Kinect* or *virtual environment* or *seious game*) |
| CINAHL Complete | AB (Osteoporosis or low bone density or osteoporosis postmenopausal or osteopenia) and AB (virtual reality or exergaming or virtual reality exposure therapy or Wii or Nintendo or videogame or immersive virtual reality or kinect or virtual environment or serious game) |
| PEDro | Osteoporosis and virtual reality |
| Study | Participants (N, Diagnosis, Age) | VR Group | Control group | Outcomes |
|---|---|---|---|---|
| N, Characteristics of the Intervention | N, Characteristics of the Intervention | Variable (Test) | ||
| Gilani, M et al., 2023 (Iran) [60] Non-blinded RCT Setting: Physical Therapy Department at Tarbiat Modares University Funding: Yes | 20 women diagnosed with primary osteoporosis Mean age: 58.9 ± 0.6 years | 10 women performed strength and balance training in non-immersive virtual reality using the Xbox Kinect 360 system, 60 min per day, 3 days per week for 6 weeks. | 10 women performed conventional training with balance and strength exercises, 60 min a day, 3 days a week for 6 weeks. | Fear of falling (FES-I) Quality of life (Qualeffo-41) Pain (Qualeffo-41) |
| Morone, G et al., 2016 (Italy) [61] Single-blinded RCT Setting: Physical Medicine and Rehabilitation, Policlinico Umberto I Funding: NR | 38 women diagnosed with bone loss condition Mean age: 68.9 ± 1.6 years | 19 women performed balance and strength training using the non-immersive virtual reality system Nintendo Wii, 60 min a day, 2 days a week for 8 weeks. | 19 women performed conventional training with balance and strength exercises, 60 min a day, 2 days a week for 8 weeks. | Fear of falling (FES-I) Quality of life (SF-36) Pain (VAS) Functional balance (BBS) |
| Nambi, G et al., 2020 (Saudi Arabia) [65] Double-blinded RCT Setting: Physical Therapy Department Funding: Yes | 45 women diagnosed with osteoporosis Mean age: 56.9 ± 0.9 years | 15 women performed non-immersive virtual reality training with the Pro-Kin system for 45 min a day, 4 days a week, for 12 weeks. The training consisted of weight-bearing hip and knee mobility exercises. | Control group 1: 15 women performed aerobic exercise training for 30 min, 4 days a week, for 12 weeks. Control group 2: 15 women did not perform any specific exercise protocol for 12 weeks. | Quality of life (Quality of Life questionnaire) Pain (Quality of Life questionnaire) Bone mineral density (dual energy X-ray absorptiometry) |
| Riaz, S et al., 2024a (Pakistan) [64] Double-blinded RCT Setting: Riphah Rehabilitation Center Funding: No. | 43 women diagnosed with osteopenia Mean age: 58.1 ± 0.2 years | 22 women performed non-immersive virtual reality with Xbox Kinect, 45 min per session, 3 days a week for 24 weeks + outdoor walk 30 min per day, 7 days a week for 24 weeks. | 21 women performed outdoor walks for 30 min a day, 7 days a week, for 24 weeks. | Quality of life (ECOS-16) Dynamic balance/mobility (TUG) |
| Riaz, S et al., 2024b (Pakistan) [66] Double-blinded RCT Setting: Riphah Rehabilitation Center Funding: No. | 43 women diagnosed with osteopenia Mean age: 58.1 ± 0.2 years | 22 women performed non-immersive virtual reality with Xbox Kinect, 45 min per session, 3 days a week for 24 weeks + outdoor walk 30 min per day, 7 days a week for 24 weeks. | 21 women performed outdoor walks for 30 min a day, 7 days a week. for 24 weeks. | Bone mineral density (dual energy X-ray absorptiometry) |
| Yilmaz, N et al., 2024 (Turkey) [63] Non-blinded RCT Setting: NR. Funding: Yes. | 60 women diagnosed with osteoporosis Mean age: 50 ± 4.1 years | 30 women performed balance exercises with the non-immersive virtual reality system Nintendo Wii balance board, 45 min per session, 3 days a week for 12 weeks + walking and upper and lower limb mobility exercises as a warm-up. | 30 women performed mobility, strengthening, stretching, and balance exercises, 45 min per session, 3 days a week for 12 weeks + walking and upper and lower limb exercises as a warm-up. | Fear of falling (FES-I) Functional balance (BBS) Dynamic balance/mobility (TUG) |
| Zhao, R et al., 2023 (China) [64] Nom-blinded RCT Setting: Elderly Healthcare Institution Funding: Yes. | 50 women diagnosed with osteoporosis Mean age: 72.7 ± 0.8 years | 25 women performed non-immersive virtual reality exercises with the VR rehabilitation training device, 50 min per session, 3 days a week for 12 months. | 25 women performed an exercise routine to prevent falls, including aerobic and machine exercises, 50 min per session, 3 days a week for 12 months. | Functional balance (BBS) Dynamic balance/mobility (TUG) Bone mineral density (dual energy X-ray absorptiometry) |
| Study | Items | Total | Quality | Biases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i1 | i2 | i3 | i4 | i5 | i6 | i7 | i8 | i9 | i10 | i11 | ||||
| Gilani, M et al., 2023 * [60] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 | Good | Performance |
| Morone, G et al., 2016 [61] | Y | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7/10 | Good | Performance |
| Nambi, G et al., 2020 [65] | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | 7/10 | Good | Performance |
| Riaz, S et al., 2024 * [64] | Y | Y | Y | Y | N | N | Y | N | N | Y | Y | 6/10 | Good | Performance |
| Riaz, S et al., 2024b [66] | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | 7/10 | Good | Performance |
| Yilmaz, N et al., 2024 * [63] | N | Y | N | Y | N | N | N | Y | Y | Y | Y | 6/10 | Good | Selection, performance and detection |
| Zhao, R et al., 2023 * [64] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6/10 | Good | Selection, performance and detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Pérez, I.; Díaz-Fernández, Á.; Osuna-Pérez, M.C.; García-López, H.; Romero-Del-Rey, R.; Obrero-Gaitán, E. Virtual Reality-Based Therapy Improves Balance, Quality of Life, and Mitigates Pain and Fear of Falling in Women with Bone Mineral Density Loss: A Meta-Analysis of Randomized Controlled Trials. Life 2025, 15, 1654. https://doi.org/10.3390/life15111654
Cortés-Pérez I, Díaz-Fernández Á, Osuna-Pérez MC, García-López H, Romero-Del-Rey R, Obrero-Gaitán E. Virtual Reality-Based Therapy Improves Balance, Quality of Life, and Mitigates Pain and Fear of Falling in Women with Bone Mineral Density Loss: A Meta-Analysis of Randomized Controlled Trials. Life. 2025; 15(11):1654. https://doi.org/10.3390/life15111654
Chicago/Turabian StyleCortés-Pérez, Irene, Ángeles Díaz-Fernández, María Catalina Osuna-Pérez, Héctor García-López, Raúl Romero-Del-Rey, and Esteban Obrero-Gaitán. 2025. "Virtual Reality-Based Therapy Improves Balance, Quality of Life, and Mitigates Pain and Fear of Falling in Women with Bone Mineral Density Loss: A Meta-Analysis of Randomized Controlled Trials" Life 15, no. 11: 1654. https://doi.org/10.3390/life15111654
APA StyleCortés-Pérez, I., Díaz-Fernández, Á., Osuna-Pérez, M. C., García-López, H., Romero-Del-Rey, R., & Obrero-Gaitán, E. (2025). Virtual Reality-Based Therapy Improves Balance, Quality of Life, and Mitigates Pain and Fear of Falling in Women with Bone Mineral Density Loss: A Meta-Analysis of Randomized Controlled Trials. Life, 15(11), 1654. https://doi.org/10.3390/life15111654










