Unraveling Stuttering Through a Multi-Omics Lens
Abstract
1. Stuttering Unraveled: Context and the Omics Field
2. Genomic Landscapes: From Linkage Scans to Genome-Wide Associations
3. Targeted Gene Discovery and Pathway Elucidation: Deepening Molecular Insights Within an Omic Context
4. Phenomics: Characterizing the Clinical Landscape for Genetic Discovery
5. A Multi-Omic Future: Integrating Layers for Comprehensive Understanding
- Expanded Genomic Discovery and Validation: Larger, more diverse, and trans-ethnic WES studies are needed to capture the full spectrum of genetic variation, including structural variants, that might contribute to PDS. Functional validation of identified variants in diverse cellular and animal models, including induced pluripotent stem cells (iPSCs) derived from individuals who stutter, is paramount to understand their biological impact.
- Transcriptomic and Epigenomic Profiling: The exemplary observation that a specific variant reduced gene expression [13] or that relevant variant effects manifest post-translationally rather than transcriptionally [21] highlights the importance of going beyond DNA sequence alone. Comprehensive analyses of gene expression (transcriptomics) and epigenetic modifications (epigenomics) in relevant neural regions-such as iPSC-derived neurons, which provide a controllable human cell model for basic and translational research, and strategic tissues, including organoid models—might be relevant for understanding how genetic variants impact gene regulation and cellular function in the context of PDS.
- Proteomics and Metabolomics: Investigating protein profiles (proteomics), including post-translational modifications such as phosphorylation (phosphoproteomics), alongside the comprehensive study of metabolites (metabolomics) through targeted or untargeted approaches, may provide dynamic insights into the functional consequences of genetic and transcriptomic alterations, potentially uncovering novel biomarkers or therapeutic targets. While direct proteomic and metabolomic investigations in individuals with developmental stuttering are currently limited, approaches from related human neurodevelopmental research may be adapted to probe molecular mechanisms and identify candidate biomarkers or relevant molecular and biochemical strategies in future investigations.
- 4.
- Integrated Phenomics and Clinical Data: It is relevant that the meticulous phenotyping efforts, such as those leveraging EHRs and phecode analysis [5], continue to evolve. This includes integrating detailed speech-language phenotyping with neuroimaging, cognitive assessments, and behavioral data to refine PDS sub-types and enable more powerful gene-phenotype correlations. Future research might critically address limitations evident across the literature, including sample sizes, cohort bias, and variability in phenotyping approaches. As illustrated in Table 1, studies ranging from linkage scans in diverse populations and cohorts highlight the importance of larger, multi-site, and trans-ethnic investigations to capture the full genetic heterogeneity of developmental stuttering. To improve reproducibility and discovery power, future work should integrate standardized, high-resolution phenotyping strategies, such as PheRC leveraging EHRs, thereby enabling more robust multi-omics analyses and well-powered GWAS.
- 5.
- Integrating connectomic findings: Benito-Aragón et al. [18] reviewed neuroimaging studies implicating structural and functional alterations across widespread cortical and subcortical networks in developmental stuttering, in addition other relevant aspects regarding neuroanatomical factors and brain connectomic findings. Their synthesis highlighted consistent grey matter differences in regions such as the supplementary motor area, primary motor cortex, inferior frontal gyri, pars opercularis (Brodmann area 44), classical Broca and Wernicke areas, superior temporal gyri, insula, precuneus, basal ganglia-thalamo-cortical loops, and cerebellum, as well as changes in axonal tracts connecting perisylvian, motor, and auditory regions.
- 6.
- Computational Biology: Advanced computational tools and artificial intelligence will be relevant to integrate and interpret the vast, multi-layered datasets generated by omics studies and other related parameters on people who stutter, identifying complex networks, useful tools and approaches, and subtle interactions that traditional or ongoing methods might miss.
- 7.
- Latest Investigations-From Candidate Genes to Polygenicity: Very recently, in 2025, Polikowsky et al. [23] reported a large GWAS study of developmental stuttering, primarily leveraging data from 23andMe, analyzing nearly 100,000 cases and over one million controls. The study confirmed that stuttering has a highly polygenic architecture, identifying 57 genomic loci and validating Polygenic Risk Scores in independent cohorts. Functional analyses showed enrichment in neuronal expression, conserved regions, and enhancer elements, while genetic correlation analyses indicated overlap with autism, depression, and impaired rhythm synchronization, supporting a link between rhythm deficits and stuttering. The study also reinforced previously reported candidate genes [24].
- 8.
- Other integrative approaches and strategies: The findings of Jackson et al. [26], demonstrating that adults who stutter largely do not exhibit stuttering during private speech, highlight the central role of social perception and communicative context in the manifestation of stuttering events. This points toward a potential paradigm shift and provides a unique opportunity for multi-omic integration and multidisciplinary strategies, where omic approaches and neuroimaging data could be combined to disentangle the biological underpinnings of context-dependent fluency and PDS. For instance, future studies could leverage omics approaches to investigate molecular signatures associated with neural circuits, social-cognitive networks, and behavioral processes engaged during private versus socially oriented speech, potentially revealing pathways involved in communicative stress regulation and self-monitoring. Moreover, future research might leverage integrative multi-omic frameworks to link behavioral phenotypes with underlying molecular networks, advancing the field from descriptive behavioral observations toward mechanistic models of developmental stuttering. Such approaches could ultimately reveal context-specific biomarkers and therapeutic targets, deepening our understanding of how social and biological dimensions converge to shape speech fluency within the stuttering context.
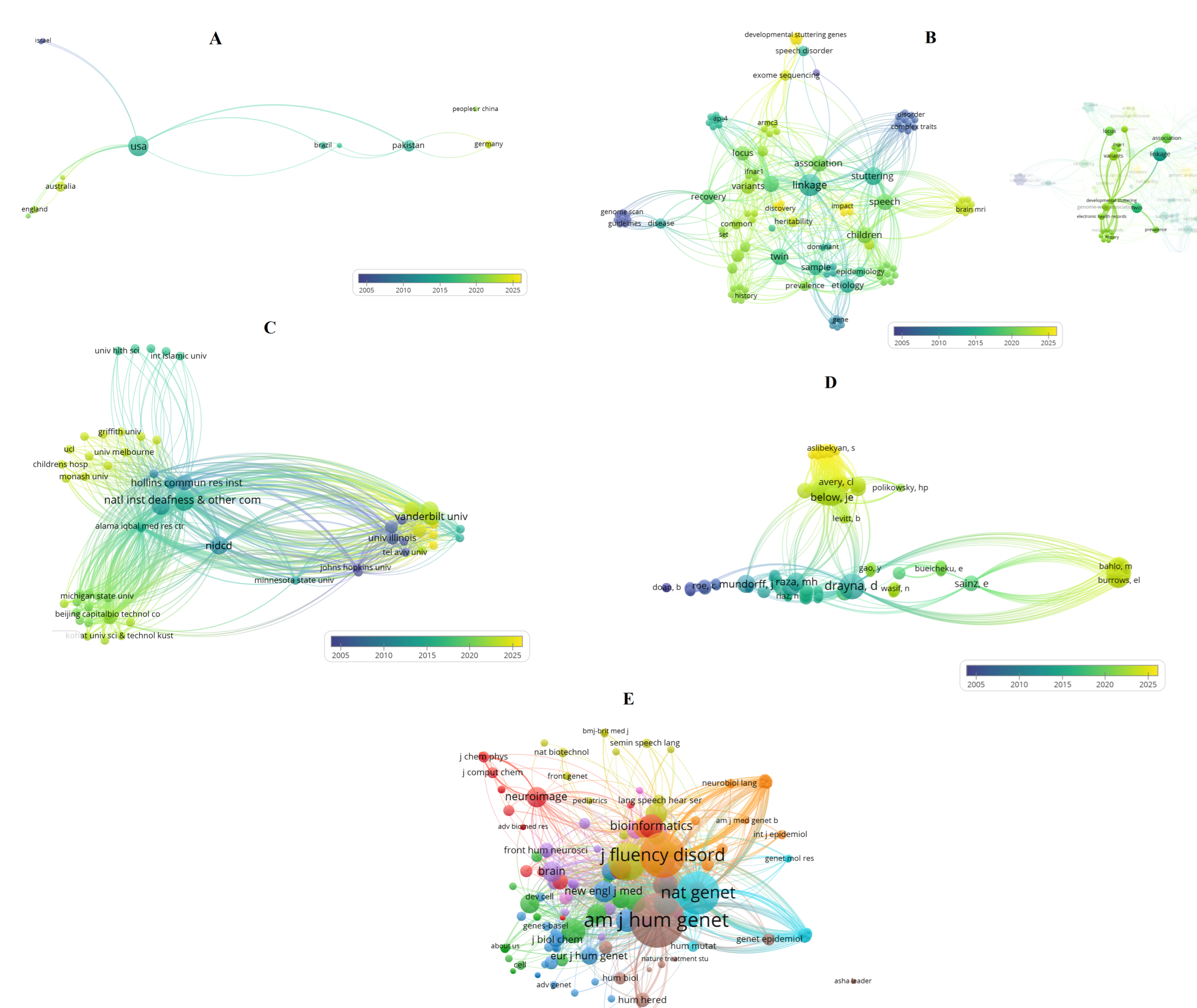
Bibliometric Analysis of Research Trends
6. Impact on Individuals, Policy, and Society
7. Concluding Remarks
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, S.E.; Garnett, E.O.; Etchell, A.; Chow, H.M. Functional and neuroanatomical bases of developmental stuttering: Current insights. Neuroscientist 2019, 25, 566–582. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Zhou, Y.; Zhou, Y.; Zhang, Y.; Wang, D.; Tan, L.-H. IFNAR1 gene mutation may contribute to developmental stuttering in the Chinese population. Hereditas 2021, 158, 46. [Google Scholar] [CrossRef]
- Smith, A.; Weber, C. Childhood stuttering: Where are we and where are we going? Semin. Speech Lang. 2016, 37, 291–297. [Google Scholar] [CrossRef]
- Viswanath, N.; Lee, H.S.; Chakraborty, R. Evidence for a major gene influence on persistent developmental stuttering. Hum. Biol. 2004, 76, 401–412. [Google Scholar] [CrossRef]
- Pruett, D.G.; Shaw, D.M.; Chen, H.-H.; Petty, L.E.; Polikowsky, H.G.; Kraft, S.J.; Jones, R.M.; Below, J.E. Identifying developmental stuttering and associated comorbidities in electronic health records and creating a phenome risk classifier. J. Fluen. Disord. 2021, 68, 105847. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.J.; Yairi, E. Genetic bases of stuttering: The state of the art, 2011. Folia Phoniatr. Logop. 2012, 64, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Shugart, Y.Y.; Mundorff, J.; Kilshaw, J.; Doheny, K.; Doan, B.; Wanyee, J.; Green, E.D.; Drayna, D. Results of a genome-wide linkage scan for stuttering. Am. J. Med. Genet. Part A 2004, 124A, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Ambrose, N.; Roe, C.; Pluzhnikov, A.; Wittke-Thompson, J.K.; Ng, M.C.Y.; Wu, X.; Cook, E.H.; Lundstrom, C.; Garsten, M.; et al. New complexities in the genetics of stuttering: Significant sex-specific linkage signals. Am. J. Hum. Genet. 2006, 78, 554–563. [Google Scholar] [CrossRef]
- Wittke-Thompson, J.K.; Ambrose, N.; Yairi, E.; Roe, C.; Cook, E.H.; Ober, C.; Cox, N.J. Genetic studies of stuttering in a founder population. J. Fluen. Disord. 2007, 32, 33–50. [Google Scholar] [CrossRef]
- Raza, M.H.; Riazuddin, S.; Drayna, D. Identification of an autosomal recessive stuttering locus on chromosome 3q13.2–3q13.33. Hum. Genet. 2010, 128, 461–463. [Google Scholar] [CrossRef]
- Raza, M.H.; Gertz, E.M.; Mundorff, J.; Lukong, J.; Kuster, J.; Schäffer, A.A.; Drayna, D. Linkage analysis of a large African family segregating stuttering suggests polygenic inheritance. Hum. Genet. 2013, 132, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.E.F.; Olivera, C.M.C.; Oliveira, B.V.; Juste, F.S.; Andrade, C.R.F.; Giacheti, C.M.; Moretti-Ferreira, D.; Drayna, D. A genetic linkage study in Brazil identifies a new locus for persistent developmental stuttering on chromosome 10. Genet. Mol. Res. 2014, 13, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Polikowsky, H.G.; Shaw, D.M.; Petty, L.E.; Chen, H.-H.; Pruett, D.G.; Linklater, J.P.; Viljoen, K.Z.; Beilby, J.M.; Highland, H.M.; Levitt, B.; et al. Population-based genetic effects for developmental stuttering. Hum. Genet. Genom. Adv. 2022, 3, 100073. [Google Scholar] [CrossRef]
- Shaw, D.M.; Polikowsky, H.P.; Pruett, D.G.; Chen, H.-H.; Petty, L.E.; Viljoen, K.Z.; Beilby, J.M.; Jones, R.M.; Kraft, S.J.; Below, J.E. Phenome risk classification enables phenotypic imputation and gene discovery in developmental stuttering. Am. J. Hum. Genet. 2021, 108, 2271–2283. [Google Scholar] [CrossRef]
- Kang, C.; Riazuddin, S.; Mundorff, J.; Krasnewich, D.; Friedman, P.; Mullikin, J.C.; Drayna, D. Mutations in the lysosomal enzyme–targeting pathway and persistent stuttering. N. Engl. J. Med. 2010, 362, 677–685. [Google Scholar] [CrossRef]
- Raza, M.H.; Domingues, C.E.F.; Webster, R.; Sainz, E.; Paris, E.; Rahn, R.; Gutierrez, J.; Chow, H.M.; Mundorff, J.; Kang, C.S.; et al. Mucolipidosis types II and III and non-syndromic stuttering are associated with different variants in the same genes. Eur. J. Hum. Genet. 2016, 24, 529–534. [Google Scholar] [CrossRef]
- Raza, M.H.; Mattera, R.; Morell, R.; Sainz, E.; Rahn, R.; Gutierrez, J.; Paris, E.; Root, J.; Solomon, B.; Brewer, C.; et al. Association between rare variants in AP4E1, a component of intracellular trafficking, and persistent stuttering. Am. J. Hum. Genet. 2015, 97, 715–725. [Google Scholar] [CrossRef]
- Benito-Aragón, C.; Gonzalez-Sarmiento, R.; Liddell, T.; Diez, I.; d’Oleire Uquillas, F.; Ortiz-Terán, L.; Bueichekú, E.; Chow, H.M.; Chang, S.-E.; Sepulcre, J. Neurofilament-lysosomal genetic intersections in the cortical network of stuttering. Prog. Neurobiol. 2020, 184, 101718. [Google Scholar] [CrossRef]
- Nandhini Devi, G.; Yadav, N.; Jayashankaran, C.; Margret, J.J.; Krishnamoorthy, M.; Lakshmi A, S.; Sundaram, C.M.; Karthikeyan, N.P.; Thelma, B.K.; Srisailapathy, C.R.S. Genetic analyses of a large consanguineous south Indian family reveal novel variants in NAGPA and four hitherto unreported genes in developmental stuttering. Ann. Hum. Genet. 2025, 89, 31–46. [Google Scholar] [CrossRef]
- Rehman, A.U.; Hamid, M.; Khan, S.A.; Eisa, M.; Ullah, W.; Rehman, Z.U.; Khan, M.A.; Basit, S.; Muhammad, N.; Khan, S.; et al. The expansion of the spectrum in stuttering disorders to a novel ARMC gene family (ARMC3). Genes 2022, 13, 2299. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.T.; Scerri, T.S.; Vogel, A.P.; Reid, C.A.; Quach, M.; Jackson, V.E.; McKenzie, C.; Burrows, E.L.; Bennett, M.F.; Turner, S.J.; et al. Stuttering associated with a pathogenic variant in the chaperone protein cyclophilin 40. Brain 2023, 146, 5086–5097. [Google Scholar] [CrossRef]
- Eising, E.; Dzinovic, I.; Vino, A.; Stipdonk, L.; Pavlov, M.; Winkelmann, J.; Sommer, M.; Franken, M.J.P.; Oexle, K.; Fisher, S.E. De novo protein-coding gene variants in developmental stuttering. Mol. Psychiatry. 2025. [Google Scholar] [CrossRef] [PubMed]
- Polikowsky, H.G.; Scartozzi, A.C.; Shaw, D.M.; Pruett, D.G.; Chen, H.H.; Petty, L.E.; Petty, A.S.; Lowther, E.J.; Cho, S.H.; Yu, Y.; et al. Large-scale genome-wide analyses of stuttering. Nat. Genet. 2025, 57, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhnyy, A.; Eyers, P.A.; Eyers, C.E.; Bowler-Barnett, E.; Martin, M.J.; Sun, Z.; Deutsch, E.W.; Jones, A.R. Profiling the human phosphoproteome to estimate the true extent of protein phosphorylation. J. Proteome Res. 2022, 21, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, D.; Jarnuczak, A.F.; Viéitez, C.; Gehre, M.; Soucheray, M.; Mateus, A.; Kleefeldt, A.A.; Hill, A.; Garcia-Alonso, L.; Stein, F.; et al. The functional landscape of the human phosphoproteome. Nat. Biotechnol. 2020, 38, 365–373. [Google Scholar] [CrossRef]
- Jackson, E.S.; Miller, L.R.; Warner, H.J.; Yaruss, J.S. Adults who stutter do not stutter during private speech. J. Fluen. Disord. 2021, 70, 105878. [Google Scholar] [CrossRef]
- Bale, T.L. The placenta and neurodevelopment: Sex differences in prenatal vulnerability. Dialogues Clin. Neurosci. 2016, 18, 459–464. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
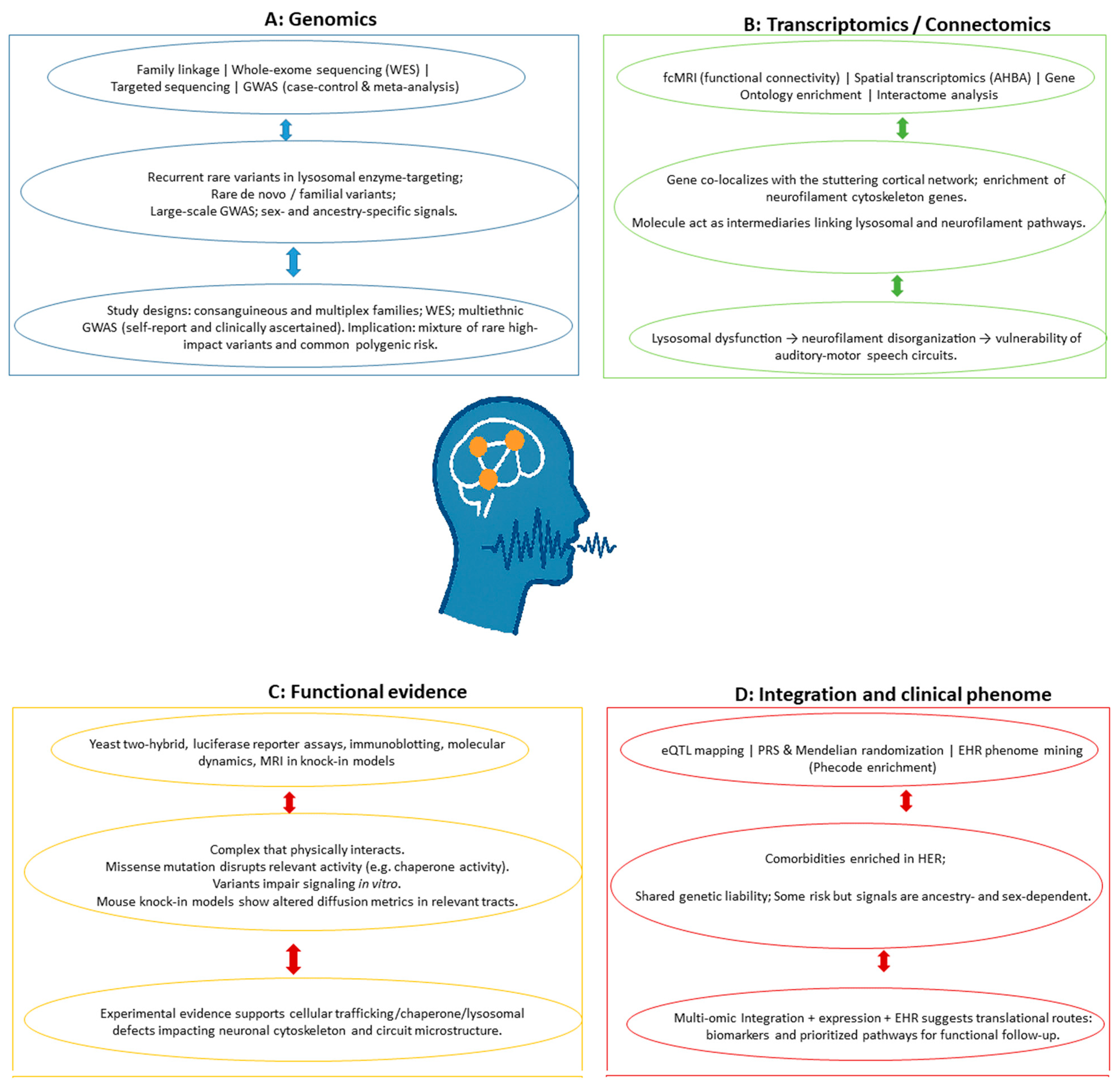
| Omics Layer and Method/Technology | Key Findings | Biological/Clinical Relevance for Human Stuttering | Sample/Population/Reference |
|---|---|---|---|
| Transcriptomics/Connectomics: Functional connectivity MRI (fcMRI)/Graph theory; Transcriptome spatial similarity analysis (AHBA); GO/Interactome analysis. | Gene GNPTG (mannose-6-phosphate lysosomal targeting pathway) co-localized significantly with the stuttering cortical network. Genes related to neurofilament cytoskeleton organization (NEFH, NEFL, INA) were highly enriched. Intermediary genes CDK5 and SNCA act as functional links between lysosomal and neurofilament pathways. | Supports that stuttering is linked to lysosomal dysfunction which has deleterious effects on the neurofilament organization of speech neuronal circuits. The auditory-motor integration network is highly vulnerable to GNPTG-lysosomal malfunctioning. | Adult Homo sapiens (AHBA transcriptome data); Adults and CWS and NFC (Homo sapiens) (fcMRI data)/[18] |
| Genomics/Exome Sequencing. | A novel heterozygous exonic variant (c.322G > A) in NAGPA segregated with the phenotype. Other heterozygous exonic variants were found in RIMS2 and XYLT1 in severely affected members, and ATP13A2 (PARK9) was found via pathway analysis. Context: These variants suggest contributions from the lysosomal pathway (NAGPA, ATP13A2) and potentially dopamine signaling (RIMS2, ATP13A2). | Stuttering likely arises from the combined effect of gene variants at multiple loci (genetic heterogeneity). The findings suggest a role for lysosomal dysfunction and, for the first time, a likely role of dopamine signaling in stuttering. | A multiplex Homo sapiens family pedigree (n = 27 samples for sequencing; n = 21 additional family members for validation/[19] |
| Genomics/Genome-wide linkage scan; SNP genotyping; Microsatellite fine mapping. | A novel linkage locus for PDS was identified at chromosome 10q21. Significant evidence of linkage was found under an autosomal dominant model of inheritance, generating a combined maximum multipoint LOD score of 4.28 (at marker D10S1790). | Identification of a novel genetic mechanism and locus (10q21) in an admixed population, consistent with the high genetic heterogeneity of PDS. The mode of inheritance observed was dominant, contrasting with recessive models often found in consanguineous populations. | 43 unrelated Brazilian families (Homo sapiens) recruited in São Paulo State, Brazil (312 total individuals); linkage signal was driven by two multiplex families (BRPD47 and BRPD50)/[12] |
| Genomics/WES; De novo variant identification (Trio design). | A pathogenic de novo stop-gain variant was found in SPTBN1. Likely pathogenic de novo missense variants were identified in PRPF8, TRIO, and ZBTB7A. Context: These are rare variants in genes previously linked to various neurodevelopmental disorders. | Provides the first direct genetic link between stuttering (including both persistent and transient forms) and other neurodevelopmental disorders (e.g., speech delay and aphasia). Results support etiological heterogeneity, as the associated genes do not converge onto a shared biological pathway or expression pattern. | 85 independent parent–child trios (Homo sapiens) (children with transient or persistent stuttering; unaffected parents)/[22] |
| Genomics/Linkage Analysis; DNA Sequencing (Exons, Exon-Intron Boundaries, Promoter regions). | Pathogenic mutations (missense, stop-gain, deletion, duplication) were identified in three genes: GNPTAB, GNPTG, and NAGPA. These genes encode enzymes responsible for generating the M6P signal, central to the lysosomal enzyme–targeting pathway. | Susceptibility to nonsyndromic, PDS is associated with genetic variations in the lysosomal metabolism pathway. This suggests that deficits in lysosomal enzyme targeting may specifically affect the neural structures and motor functions required for fluent speech. | Consanguineous Pakistani families and unrelated affected subjects from Pakistan, North America, and Britain (Homo sapiens adults with persistent stuttering)/[15] |
| Genomics/Exome Sequencing; Molecular Dynamics Simulation; MRI ( DWI, QSM). | An ultra-rare missense variant (c.808C>T, p.Pro270Ser) in PPID, encoding the chaperone protein CYP40, segregated with stuttering. Context: The mutation disrupts the TPR2 domain and is predicted to inhibit Cyp40 binding to Hsp90. | Implicates a chaperone protein in PDS pathogenesis. Findings support disruption of the CSTC network. DWI of the knock-in model revealed significant microstructural changes (increased ADC, AD, RD) in the left corticospinal tract. | Four-generation Australian Caucasian family (Homo sapiens); Humanized knock-in Ppid p.Pro270Ser mice (Mus musculus)/[21] |
| Genomics/ GWAS; Meta-analyses (sex and ancestry-specific/combined); Genetic correlation; MR. | Identification of 57 unique loci associated with stuttering, mapping to 48 unique genes. Sentinel hits included VRK2, CAMTA1 (European ancestry [EUR] males), and SLC39A8, DCC (EUR females). Context: VRK2 is implicated in musical beat synchronization, providing genetic support for the Atypical Rhythm Risk Hypothesis. | Stuttering risk is highly polygenic and genetically complex, exhibiting sexual dimorphism. Revealed shared molecular underpinnings (genetic correlation) between stuttering and impaired beat synchronization, ASD, and depression. Enrichment analysis showed variants are active in neurons and brain regions (frontal cortex, basal ganglia) related to speech motor planning. | Over 1.1 million individuals (Homo sapiens) (99,776 cases/1,023,243 controls) from 23andMe (self-reported stuttering), stratified by sex and ancestry (EUR, African ancestry [AFR], East Asian Ancestry [EAS], American/Admixed Americana Ancestry [AMR])/[23] |
| Genomics/GWAS; Meta-analysis; Expression Quantitative Trait Locus (eQTL) mapping. | Identification of one GWS protective variant (rs113284510) in an intronic/genic upstream region of SSUH2. Context: The protective allele (T) acts as an eQTL, reducing SSUH2 expression in esophagus-muscularis tissue, and increasing CAV3 expression in tibial artery tissue. | Stuttering risk is highly polygenic and complex. Findings suggest involvement of genes related to structural organization, nervous system development, and neurogenesis. Preliminary support for shared genetic liability with ASD. | 16,461 total individuals (Homo sapiens) (2130 cases: International Stuttering Project [ISP] clinically ascertained cases + Add Health self-reported cases; 14,331 controls). Trans-ancestry/Multiethnic cohort/[13] |
| Clinical Phenome/(EHR data mining; Phenotype Code (Phecode) enrichment analysis; Machine Learning ([PheRC]/Classification and Regression Tree). | Identification of 38 significantly enriched phecodes (comorbidities). Key enriched phecodes included developmental Delays (315), speech and language disorder (315.2), sleep disorders, hearing loss, atopic triad (allergies/dermatitis), neurological deficits (including aphasia/speech disturbance), and atypical weight regulation. | Established a valid, multi-step method (keyword search, text-mining, manual review) for identifying developmental stuttering cases in EHRs. Comorbidity analysis confirmed known associations and suggested novel shared etiology (e.g., with infections, body weight issues). The resultant PheRC facilitates future high-powered genetic etiology studies (GWAS). | 1143 confirmed developmental stuttering cases (Homo sapiens); EHR data from Vanderbilt University Medical Center Synthetic Derivative (VUMC SD, ~2.8 million records)/[5] |
| Genomics/WES; Dideoxy sequencing; Yeast two-hybrid (Y2H) assay; Functional assembly assay (Immunoblotting). | Rare coding variants, including two specific heterozygous variants (c.1549G>A, p.Val517Ile and c.2401G>A, p.Glu801Lys), were identified and co-segregated with stuttering in AP4E1. Context: AP4E1 encodes the epsilon subunit of the Adaptor protein complex 4 (AP-4) complex, which is involved in protein sorting at the Trans-Golgi Network (TGN). The AP-4 complex (specifically its m4 subunit) was shown to physically interact with NAGPA (a previously associated stuttering gene). | Implicates deficits in intracellular trafficking and the endosomal transport system in PDS. Establishes a direct functional link between the newly identified gene (AP4E1) and the previously established lysosomal pathway genes (NAGPA, GNPTAB, GNPTG). Stuttering in heterozygotes is a non-syndromic presentation, lacking the severe symptoms of homozygous AP4E1 deficiency. | Large Cameroonian family (CAMST01); Unrelated individuals with persistent stuttering from Cameroon, Pakistan, and North America (Homo sapiens). Human embryonic kidney 293 cells (HEK-293 cells) (for biochemical assays)/[17] |
| Genomics/Targeted Sequencing (Exons and Intronic flanks). | Rare non-synonymous coding variants in GNPTAB, GNPTG, and NAGPA were significantly enriched in cases (16% incidence vs. 7% background rate). Context: The variants found in stuttering subjects (92.6% missense substitutions) suggest a less severe defect in the lysosomal enzyme-targeting pathway compared to the severe LoF mutations found in Mucolipidosis (ML II/III). | Confirms that genetic variants in the lysosomal enzyme-targeting pathway contribute significantly to non-syndromic PDS (estimated 16% of cases). The difference in mutation type (missense vs. frameshift/stop-gain) explains why stuttering patients do not exhibit ML II/III symptoms. | 1013 unrelated individuals with non-syndromic PDS (Homo sapiens) from worldwide cohorts (NAF, England, PKST, STCR, BRCS)/[16] |
| Genomics/Genome-wide linkage scan; SNP genotyping; Microsatellite fine mapping. | Identification of an autosomal recessive stuttering locus on chromosome 3q13.2–3q13.33. Significant linkage was confirmed with a maximum two-point LOD score of 4.23. Context: The region spans 3.24 Mb and contains 46 known/predicted genes, including DRD3 (which showed no variation upon sequencing). | Identification of a highly significant genetic locus for PDS, supporting the substantial heritability of the disorder and demonstrating the power of using highly consanguineous families for complex trait linkage analysis. | A newly ascertained consanguineous Pakistani family (PKST77) with persistent stuttering (Homo sapiens)/[10] |
| Genomics/Genome-Wide Linkage Scan; Microsatellite and SNP genotyping; Two-locus analysis (Superlink). | Identification of multiple linkage loci (2p, 3p, 3q, 14q, and two on 15q). Context: The strongest combined evidence came from the 2p + 15q two-locus model, achieving LOD scores up to 6.57, supporting a complex, polygenic model. | Provides strong evidence for polygenic inheritance and locus heterogeneity in PDS. This mechanism explains the large number of affected individuals in a family without clear consanguinity. The affected individuals are typically multilingual stutterers. | Large Cameroonian family pedigree (CAMST01, 71 individuals, at least 33 affected) (Homo sapiens)/[11] |
| Genomics/WES; Sanger Sequencing. | A homozygous splice site variant (c.916 + 1G > A) in ARMC3 was identified. Context: This LoF variant causes skipping of exon-8, predicted to lead to NMD, and significantly alters the protein’s folding and its interaction with MYCBPAP. | Implicates the novel Armadillo repeat (ARM) gene family and potential dysfunction in the Wnt signaling pathway in non-syndromic PDS. ARMC3 is highly expressed in brain regions critical for motor function and emotion, such as the basal ganglia and cerebellum. | A consanguineous Pashtun family of Pakistani origin (Homo sapiens) with autosomal recessive PDS (two affected individuals sequenced) [20] |
| Genomics/GWAS; Phenome Risk Classification (Phenome Machine Learning [PheML])/Classification and Regression Tree Model. | Identification of genome-wide significant variants: rs12613255 (near CYRIA) in EUR ancestry, and rs7837758 (intronic in ZMAT4) in AFR ancestry. Context: Both genes are highly expressed in CNS regions, including the cerebral cortex and basal ganglia. | Successful population-based GWAS for developmental stuttering, confirming the risk is highly polygenic and genetically complex. The findings support a genetic etiology related to CNS function and neural circuits controlling speech motor planning. Validation through PRS confirms the model captures the genetic liability of clinically ascertained stuttering cases. | 9239 PheML-imputed affected individuals (Homo sapiens) from the BioVU EHR-linked biorepository, analyzed in ancestry-stratified cohorts [14] |
| Genomics/Genome-Wide Linkage Scan; Non-Parametric Linkage Analysis; Fine mapping. | Evidence for a predisposing locus on chromosome 18 (18p and proximal 18q). Best overall Non-Parametric Linkage statistic (NPL) (Z-log odds ratio statistic [Zlr]) score was 5.143 at marker D18S78. Context: Candidate genes include the CDH2 gene and the desmoglein/desmoc olin family, suggesting cell adhesion/intercellular communication deficits. | Suggests chromosome 18 harbors a key locus contributing to PDS. The implicated pathways (cell adhesion) may affect neurons involved in speech production. Data supports locus heterogeneity and an incomplete dominant inheritance pattern. | 68 outbred families (226 individuals, 188 affected) from North America and Great Britain (Homo sapiens); subjects displayed persistent stuttering (>=4% dysfluencies) [7] |
| Genomics/WES; Sanger sequencing; Luciferase reporter assay (Functional validation). | Rare coding variants in IFNAR1 (including Leu552Pro, Lys428Gln, Gly301Glu, and Pro335del) cosegregated with stuttering. Functional studies showed that three of these variants significantly impaired Type IIFN signaling (Janus kinase-signal transducer and activator of transcription [JAK-STAT] pathway activation). | Suggests IFNAR1 is a novel pathogenic gene for PDS, particularly in the Chinese population. Impaired Type I IFN signaling links stuttering to underlying neurodegenerative events, abnormal autophagy, and lysosomal dysfunction. | 10 independent PDS families and 84 sporadic PDS cases (DNA samples) from the Chinese population (Homo sapiens); HEK-293 cells (for in vitro functional assays) [2] |
| Genomics/Genome-wide Linkage Scan; High-density SNP genotyping (110K SNP array). | Genomewide-significant sex-specific linkage signals: Chromosome 21 (LOD 4.5) in female-only data; Chromosome 7 (LOD 2.99) in male-only data. Context: Conditional analysis supported the established Chromosome 12 locus and implicated Chromosome 2 (193 cM) through interactions. | Strong support for sexual dimorphism in the genetic architecture of stuttering. Suggests overlapping genetic liability with Autism and SLI, particularly in the Chromosome 7 and 2 regions. | 100 families of European descent (Homo sapiens) (US, Sweden, Israel), including 252 individuals with persistent stuttering and 45 recovered cases [8] |
| Genomics/Linkage Analysis; Association Analysis (Transmission Disequilibrium Test [TDT], Family-Based Association Test [FBAT]); Meta-analysis (GSMA-related analysis). | Nominal linkage peaks found on Chromosomes 3, 13, and 15 (Hutterites). Highest NPL all peak was on Chromosome 13 at 52.6 cM (p = 0.012), which overlapped with suggestive TDT and FBAT signals. Meta-analysis highlighted Chromosomes 2 and 5. | Supports that stuttering is a polygenic disorder. The Chromosome 13 locus suggests shared genetic susceptibility with other neurodevelopmental phenotypes, including SLI, autism, and Tourette’s syndrome. | 40 genotyped individuals (Homo sapiens) who had ever stuttered (persistent and recovered cases) from a large Hutterite founder population; meta-analysis included outbred Caucasian families [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novaes Marques, D. Unraveling Stuttering Through a Multi-Omics Lens. Life 2025, 15, 1630. https://doi.org/10.3390/life15101630
Novaes Marques D. Unraveling Stuttering Through a Multi-Omics Lens. Life. 2025; 15(10):1630. https://doi.org/10.3390/life15101630
Chicago/Turabian StyleNovaes Marques, Deyvid. 2025. "Unraveling Stuttering Through a Multi-Omics Lens" Life 15, no. 10: 1630. https://doi.org/10.3390/life15101630
APA StyleNovaes Marques, D. (2025). Unraveling Stuttering Through a Multi-Omics Lens. Life, 15(10), 1630. https://doi.org/10.3390/life15101630







