High-Resolution Contrast-Enhanced Ultrasound with SRCEUS for Assessing the Intrahepatic Microvasculature and Shunts in Patients with Hereditary Haemorrhagic Teleangiectasia (Osler’s Disease)
Abstract
1. Introduction
2. Materials and Methods
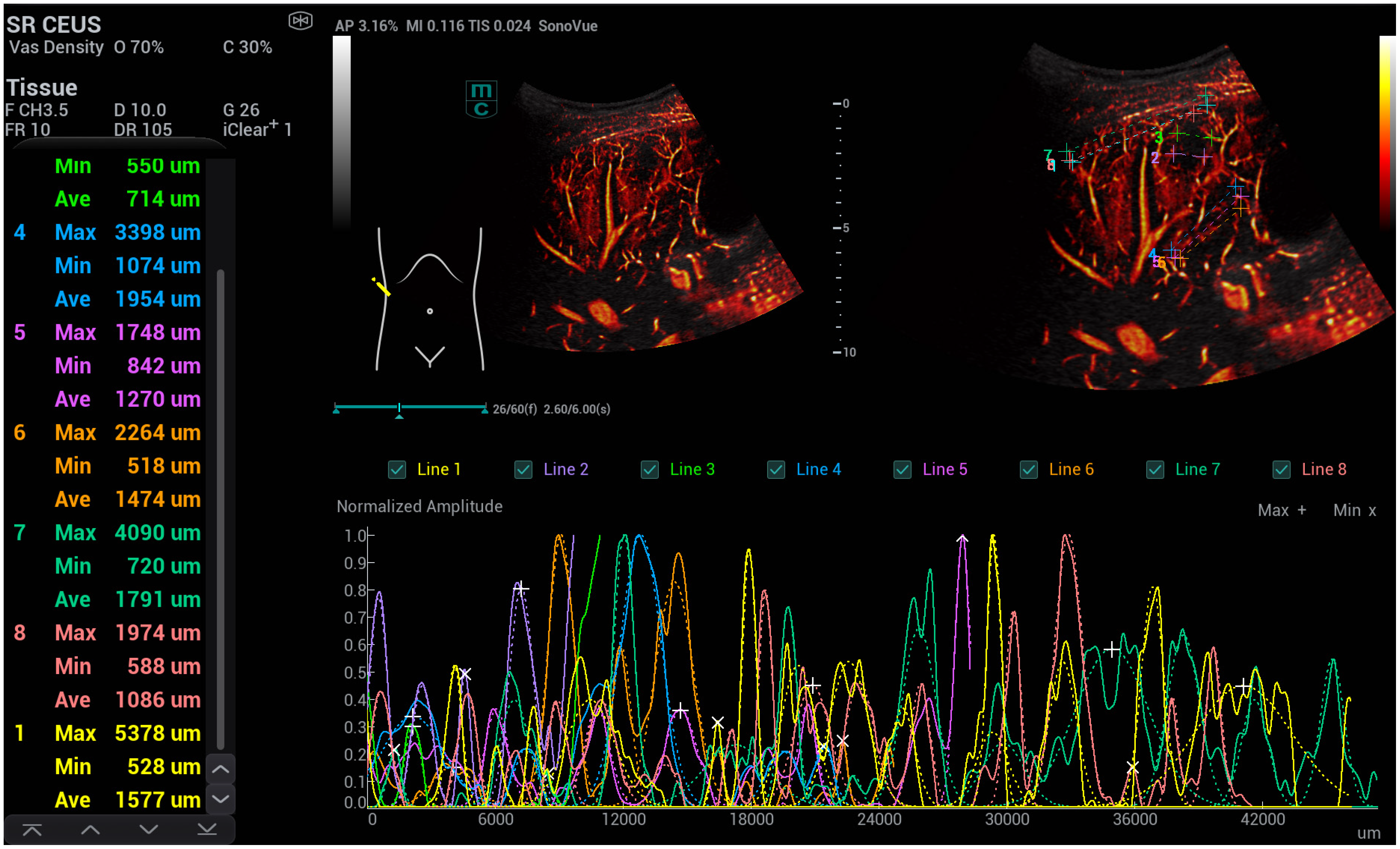
3. Results
- -
- The flow of the hepatic artery was characterised by dilatation > 5 mm.
- -
- The resistance index (RI) was reduced, with a value of <0.55.
- -
- The flow of the portal vein increased proportionally, with a velocity > 30 cm/s.
- -
- The flow of the hepatic veins increased proportionally or was continuous.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, E.M.; Dong, Y.; Jung, F. Current aspects of multimodal ultrasound liver diagnostics using contrast-enhanced ultrasonography (CEUS), fat evaluation, fibrosis assessment, and perfusion analysis—An update. Clin. Hemorheol. Microcirc. 2023, 83, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Kaiser, U.; Herr, W.; Stroszczynski, C.; Jung, F. Novel high-resolution contrast agent ultrasound techniques HiFR CEUS and SR CEUS in combination with shear wave elastography, fat assessment and viscosity of liver parenchymal changes and tumors. Clin. Hemorheol. Microcirc. 2024, 86, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Pausch, A.-M.; Kammerer, S.; Weber, F.; Herr, W.; Stroszczynski, C.; Holler, E.; Edinger, M.; Wolff, D.; Weber, D.; Jung, E.-M.; et al. Parametric Imaging of Contrast-Enhanced Ultrasound (CEUS) for the Evaluation of Acute Gastrointestinal Graft-Versus-Host Disease. Cells 2021, 10, 1092. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Li, J.-W.; Lu, Q.; Luo, Y.; Lin, L.; Shi, Y.-J.; Li, T.; Liu, J.-B.; Lyshchik, A. Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology 2020, 294, 329–339. [Google Scholar] [CrossRef]
- Schellhaas, B.; Bernatik, T.; Bohle, W.; Borowitzka, F.; Chang, J.; Dietrich, C.F.; Dirks, K.; Donoval, R.; Drube, K.; Friedrich-Rust, M.; et al. Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma—A Prospective Multicenter DEGUM Study. Ultraschall Med. 2021, 42, 20. [Google Scholar] [CrossRef]
- Strobel, D.; Jung, E.-M.; Ziesch, M.; Praktiknjo, M.; Link, A.; Dietrich, C.F.; Klinger, C.; Schultheiß, M.; Jesper, D.; Schellhaas, B. Real-life assessment of standardized contrast-enhanced ultrasound (CEUS) and CEUS algorithms (CEUS LI-RADS®/ESCULAP) in hepatic nodules in cirrhotic patients—A prospective multicenter study. Eur. Radiol. 2021, 31, 7614–7625. [Google Scholar] [CrossRef]
- Meitner-Schellhaas, B.; Jesper, D.; Goertz, R.S.; Zundler, S.; Strobel, D. Washout appearance of hepatocellular carcinomas using standardized contrast-enhanced ultrasound (CEUS) including an extended late phase observation—Real-world data from the prospective multicentre DEGUM study. Clin. Hemorheol. Microcirc. 2023, 84, 413–424. [Google Scholar] [CrossRef]
- Kallenbach, M.; Qvartskhava, N.; Weigel, C.; Dörffel, Y.; Berger, J.; Kunze, G.; Luedde, T. Contrast-enhanced ultrasound (CEUS) for characterisation of focal liver lesions. Z. Gastroenterol. 2024, 62, 952–970. [Google Scholar]
- Tang, C.; Fang, K.; Guo, Y.; Li, R.; Fan, X.; Chen, P.; Chen, Z.; Liu, Q.; Zou, Y. Safety of Sulfur Hexafluoride Microbubbles in Sonography of Abdominal and Superficial Organs: Retrospective Analysis of 30,222 Cases. J. Ultrasound Med. 2017, 36, 531–538. [Google Scholar] [CrossRef]
- Jung, E.M.; Weber, M.A.; Wiesinger, I. Contrast-enhanced ultrasound perfusion imaging of organs. Radiologe 2021, 61 (Suppl. S1), 19–28. [Google Scholar] [CrossRef]
- Neye, H.; Rauh, P.; Ensberg, D.; Rickes, S. Iron deficiency anaemia over the last decades—Osler’s disease diagnosed by Doppler and contrast-enhanced ultrasound. Z. Gastroenterol. 2013, 51, 216–219. [Google Scholar] [CrossRef]
- Song, P.; Trzasko, J.D.; Manduca, A.; Huang, R.; Kadirvel, R.; Kallmes, D.F.; Chen, S. Improved Super-Resolution Ultrasound Microvessel Imaging With Spatiotemporal Nonlocal Means Filtering and Bipartite Graph-Based Microbubble Tracking. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 149–167. [Google Scholar] [CrossRef]
- Christensen-Jeffries, K.; Couture, O.; Dayton, P.A.; Eldar, Y.C.; Hynynen, K.; Kiessling, F.; O’Reilly, M.; Pinton, G.F.; Schmitz, G.; Tang, M.X. Super-resolution Ultrasound Imaging. Ultrasound Med. Biol. 2020, 46, 865–891. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Gong, P.; Lok, U.-W.; Tang, S.; Yin, T.; Zhang, X.; Zhu, L.; Sang, M.; Song, P.; et al. Super-resolution ultrasound localization microscopy based on a high frame-rate clinical ultrasound scanner: An in-human feasibility study. Phys. Med. Biol. 2021, 66, 08NT01. [Google Scholar] [CrossRef]
- Wiesinger, I.; Wiggermann, P.; Zausig, N.; Beyer, L.P.; Salzberger, B.; Stroszczynski, C.; Jung, E.M. Percutaneous Treatment of Malignant Liver Lesions: Evaluation of Success Using Contrast- Enhanced Ultrasound (CEUS) and Perfusion Software. Ultraschall Med. 2018, 39, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e48. [Google Scholar] [PubMed]
- Apfelbeck, M.; Loupas, T.; Chaloupka, M.; Clevert, D.A. Improved diagnostic confidence using Super Resolution CEUS imaging in testicular lesions. Clin. Hemorheol. Microcirc. 2024, 88 (Suppl. S1), S113–S125. [Google Scholar] [CrossRef]
- Putz, F.J.; Verloh, N.; Erlmeier, A.; Schelker, R.C.; Schreyer, A.G.; Hautmann, M.G.; Stroszczynski, C.; Banas, B.; Jung, E.M. Influence of limited examination conditions on contrast-enhanced sonography for characterising liver lesions. Clin. Hemorheol. Microcirc. 2019, 71, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Bastati-Huber, N.; Pötter-Lang, S.; Ba-Ssalamah, A. Fokale noduläre Hyperplasie und hepatozelluläres Adenom. Der Radiologe. 2015, 55, 18–26. [Google Scholar] [CrossRef]
- Eusebi, L.; Masino, F.; Bertolotto, M.; Montatore, M.; Sortino, G.; Pitoni, L.; Santarelli, S.; Galosi, A.B.; Guglielmi, G. Contrast-enhanced ultrasound in the evaluation and management of solid renal lesions based on EFSUMB guidelines. J. Med. Ultrason. 2025, 52, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Correas, J.-M.; Cui, X.-W.; Dong, Y.; Havre, R.F.; Jenssen, C.; Jung, E.M.; Krix, M.; Lim, A.; Lassau, N.; et al. EFSUMB Technical Review—Update 2023: Dynamic Contrast-Enhanced Ultrasound (DCE-CEUS) for the Quantification of Tumor Perfusion. Ultraschall Med. 2024, 45, 36–46. [Google Scholar] [CrossRef]
- Barr, R.G.; Huang, P.; Luo, Y.; Xie, X.; Zheng, R.; Yan, K.; Jing, X.; Luo, Y.; Xu, H.; Fei, X.; et al. Contrast-enhanced ultrasound imaging of the liver: A review of the clinical evidence for SonoVue and Sonazoid. Abdom. Radiol. 2020, 45, 3779–3788. [Google Scholar] [CrossRef]
- da Silva, N.P.B.; Beyer, L.; Hottenrott, M.; Hackl, C.; Schlitt, H.; Stroszczynski, C.; Wiggermann, P.; Jung, E. Efficiency of contrast enhanced ultrasound for immediate assessment of ablation status after intraoperative radiofrequency ablation of hepatic malignancies. Clin. Hemorheol. Microcirc. 2017, 66, 357–368. [Google Scholar] [CrossRef]
- Dropco, I.; Kaiser, U.; Wagner, L.; Brunner, S.M.; Schlitt, H.J.; Stroszcynski, C.; Jung, F.; Yi, D.; Herr, W.; Jung, E.M. Color Mapping using Ultrasound System-integrated Perfusion Software for Evaluation of Focal Liver Lesions: A Possible First Step for More Independent Reading. J. Gastrointestin. Liver Dis. 2023, 32, 479–486. [Google Scholar] [CrossRef]
- Kaiser, U.; Vehling-Kaiser, U.; Kück, F.; Gilanschah, M.; Jung, F.; Jung, E.M. Super-Resolution contrast-enhanced ultrasound examination down to the microvasculature enables quantitative analysis of liver lesions: First Results. Life 2025, 15, 991. [Google Scholar] [CrossRef]
- Schelker, R.C.; Andorfer, K.; Putz, F.; Herr, W.; Jung, E.M. Identification of two distinct hereditary hemorrhagic telangiectasia patient subsets with different hepatic perfusion properties by combination of contrast-enhanced ultrasound (CEUS) with perfusion imaging quantification. PLoS ONE 2019, 14, e0215178. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B. Application of contrast-enhanced ultrasound in hemothorax of hereditary hemorrhagic telangiectasia: A case report and literature review. Clin. Hemorheol. Microcirc. 2023, 83, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Schelker, R.C.; Barreiros, A.P.; Hart, C.; Herr, W.; Jung, E.M. Macro- and microcirculation patterns of intrahepatic blood flow changes in patients with hereditary hemorrhagic telangiectasia. World J. Gastroenterol. 2017, 23, 486–495. [Google Scholar] [CrossRef]
- Yanna-Schulze, A.; Schneider, G.; Maßmann, A.; Gräber, S.; Geisthoff, U.W. Carotid ultrasound for pulmonary arteriovenous malformation screening. Open Med. 2015, 10, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Thimm, M.A.; Rhee, D.; Takemoto, C.M.; Karnsakul, W.; Cuffari, C.; Guerrerio, A.L.; Garcia, A.; Gearhart, J.; Huisman, T.A.G.M.; Hwang, M. Diagnosis of congenital and acquired focal lesions in the neck, abdomen, and pelvis with contrast-enhanced ultrasound: A pictorial essay. Eur. J. Pediatr. 2018, 177, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Grosso, M.; Groppo Marchisio, F.; Testa, F.; Gallarato, G.; Balderi, A.; Lingua, G.; Mondino, I.; Pedrazzini, F.; Danesino, C.; Buscarini, E. Pulmonary arteriovenous malformations: Percutaneous treatment preserving parenchyma in high-flow fistulae. Radiol. Med. 2008, 113, 395–413. [Google Scholar] [CrossRef]
- Möller, K.; Tscheu, T.; De Molo, C.; Serra, C.; Cui, X.W.; Dong, Y.; Hocke, M.; Lim, A.; Zadeh, E.S.; Görg, C.; et al. Comments and illustrations of the WFUMB CEUS liver guidelines: Rare congenital vascular pathology. Med. Ultrason. 2022, 24, 461–472. [Google Scholar] [CrossRef]
- Jung, E.M.; Ocaña Moran, V.; Engel, M.; Krüger-Genge, A.; Stroszczynski, C.; Jung, F. Modified contrast-enhanced ultrasonography with the new high-resolution examination technique of high frame rate contrast-enhanced ultrasound (HiFR-CEUS) for characterization of liver lesions: First results. Clin. Hemorheol. Microcirc. 2023, 83, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Peltec, A.; Sporea, I. Multiparametric ultrasound as a new concept of assessment of liver tissue damage. World J. Gastroenterol. 2024, 30, 1663–1669. [Google Scholar] [CrossRef] [PubMed]

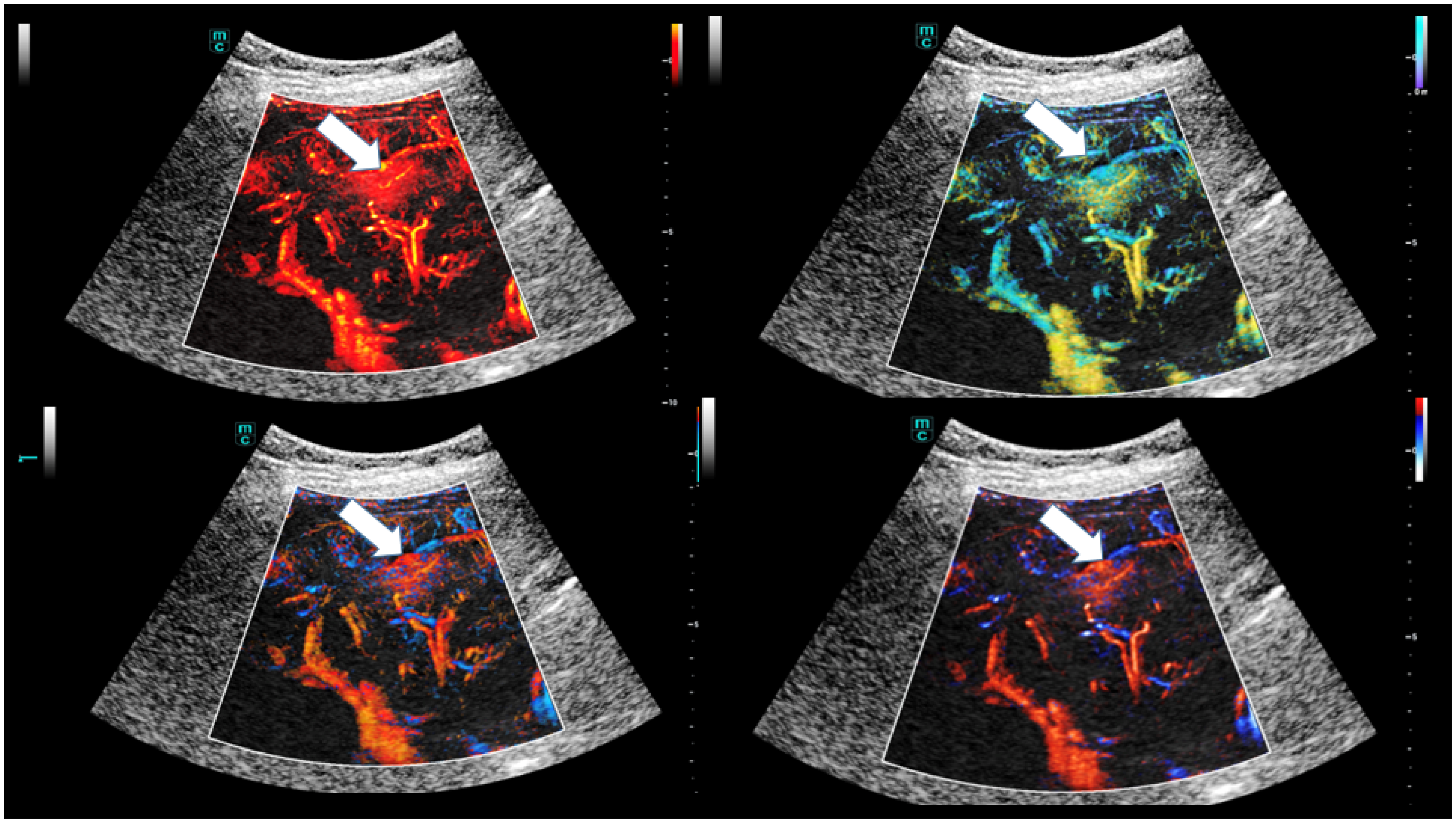
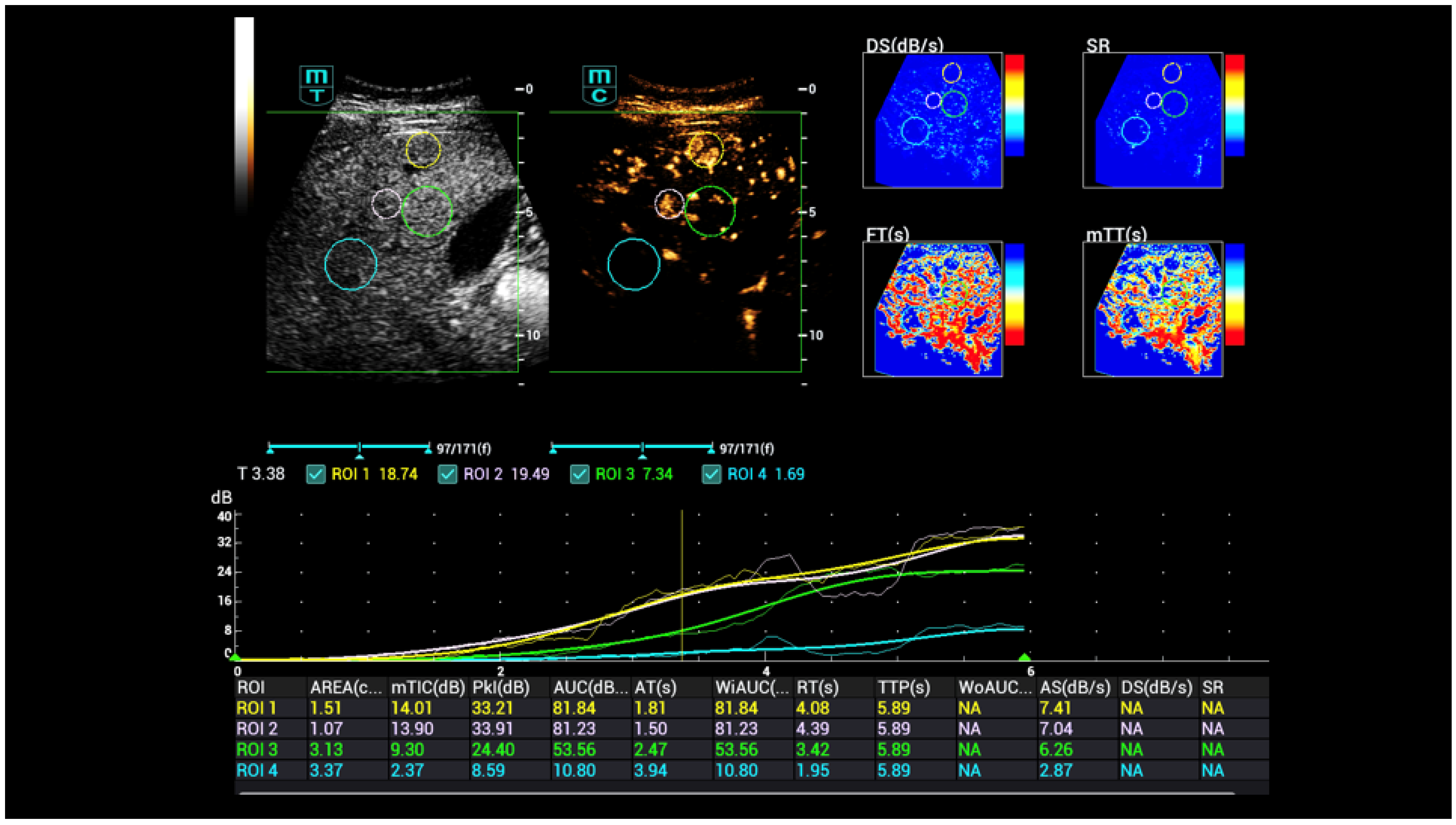
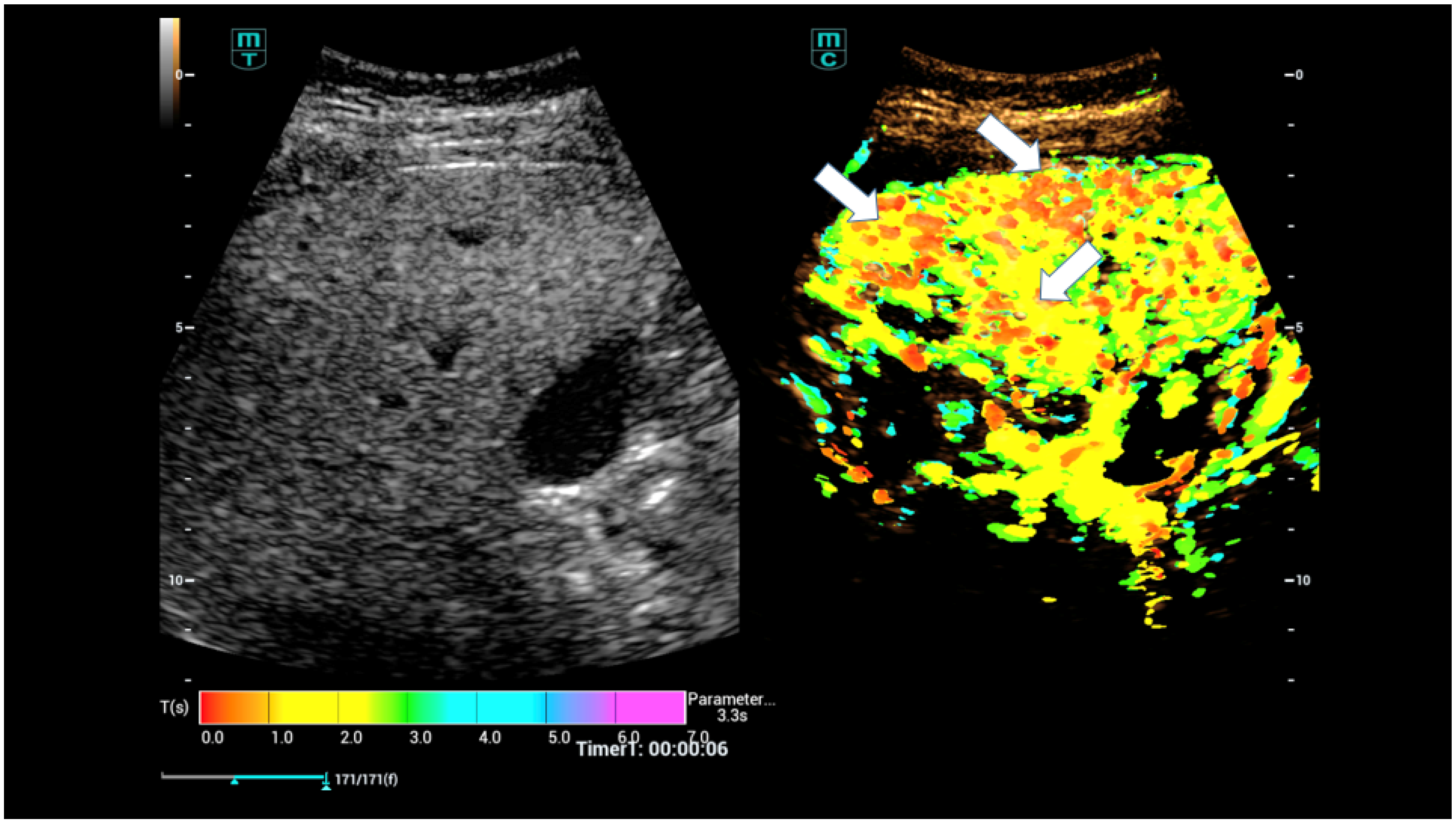

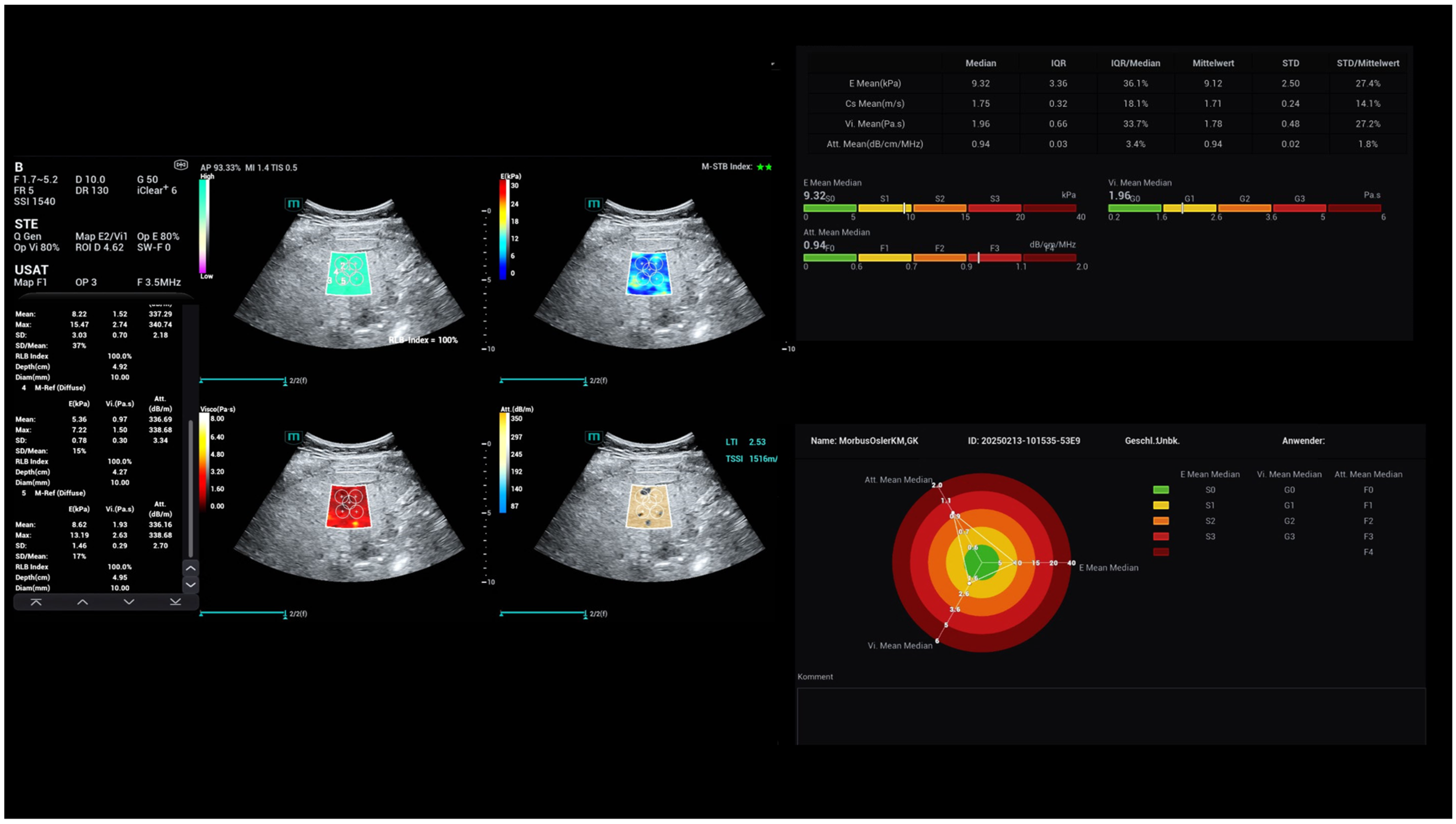




| Osler’s Disease | No Osler’s Disease | p-Value | |
|---|---|---|---|
| N = patient | n = 10 | n= 20 | |
| N = number of measurements | n = 30 | n = 60 | |
| diameter: close to the capsule | 5133.7 ± 1456.6 | 1972.3 + 399.2 | 6.18989 × 10−13 |
| diameter: at the level of the portal vein | 2175.3 ± 417.5 | 2890.7 + 606.9 | 3.00779 × 10−11 |
| area: close to the capsule | 68.9 ± 8.8 | 8.9 + 1.99 | 1.4051 × 10−26 |
| area: at the level of the portal vein | 35.8 ± 7.73 | 16.8 + 3.66 | 8.29298 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieber, I.M.; Jung, F.; Jung, E.M. High-Resolution Contrast-Enhanced Ultrasound with SRCEUS for Assessing the Intrahepatic Microvasculature and Shunts in Patients with Hereditary Haemorrhagic Teleangiectasia (Osler’s Disease). Life 2025, 15, 1631. https://doi.org/10.3390/life15101631
Sieber IM, Jung F, Jung EM. High-Resolution Contrast-Enhanced Ultrasound with SRCEUS for Assessing the Intrahepatic Microvasculature and Shunts in Patients with Hereditary Haemorrhagic Teleangiectasia (Osler’s Disease). Life. 2025; 15(10):1631. https://doi.org/10.3390/life15101631
Chicago/Turabian StyleSieber, Irmgard Maria, Friedrich Jung, and Ernst Michael Jung. 2025. "High-Resolution Contrast-Enhanced Ultrasound with SRCEUS for Assessing the Intrahepatic Microvasculature and Shunts in Patients with Hereditary Haemorrhagic Teleangiectasia (Osler’s Disease)" Life 15, no. 10: 1631. https://doi.org/10.3390/life15101631
APA StyleSieber, I. M., Jung, F., & Jung, E. M. (2025). High-Resolution Contrast-Enhanced Ultrasound with SRCEUS for Assessing the Intrahepatic Microvasculature and Shunts in Patients with Hereditary Haemorrhagic Teleangiectasia (Osler’s Disease). Life, 15(10), 1631. https://doi.org/10.3390/life15101631







