Can Improved Biosecurity Measures Reduce the Presence of the Most Common ESBL-Producing Enterobacteriaceae? A Study from Greek Pig Farms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Farm Selection
2.2. Sample Collection and ESBL Detection
2.2.1. Sample Collection
2.2.2. ESBL Detection and Characterization
2.3. Statistical Analysis
2.3.1. Data Transformation
2.3.2. Normality Testing
2.3.3. Association Analyses
3. Results
3.1. Farm Operational Characteristics
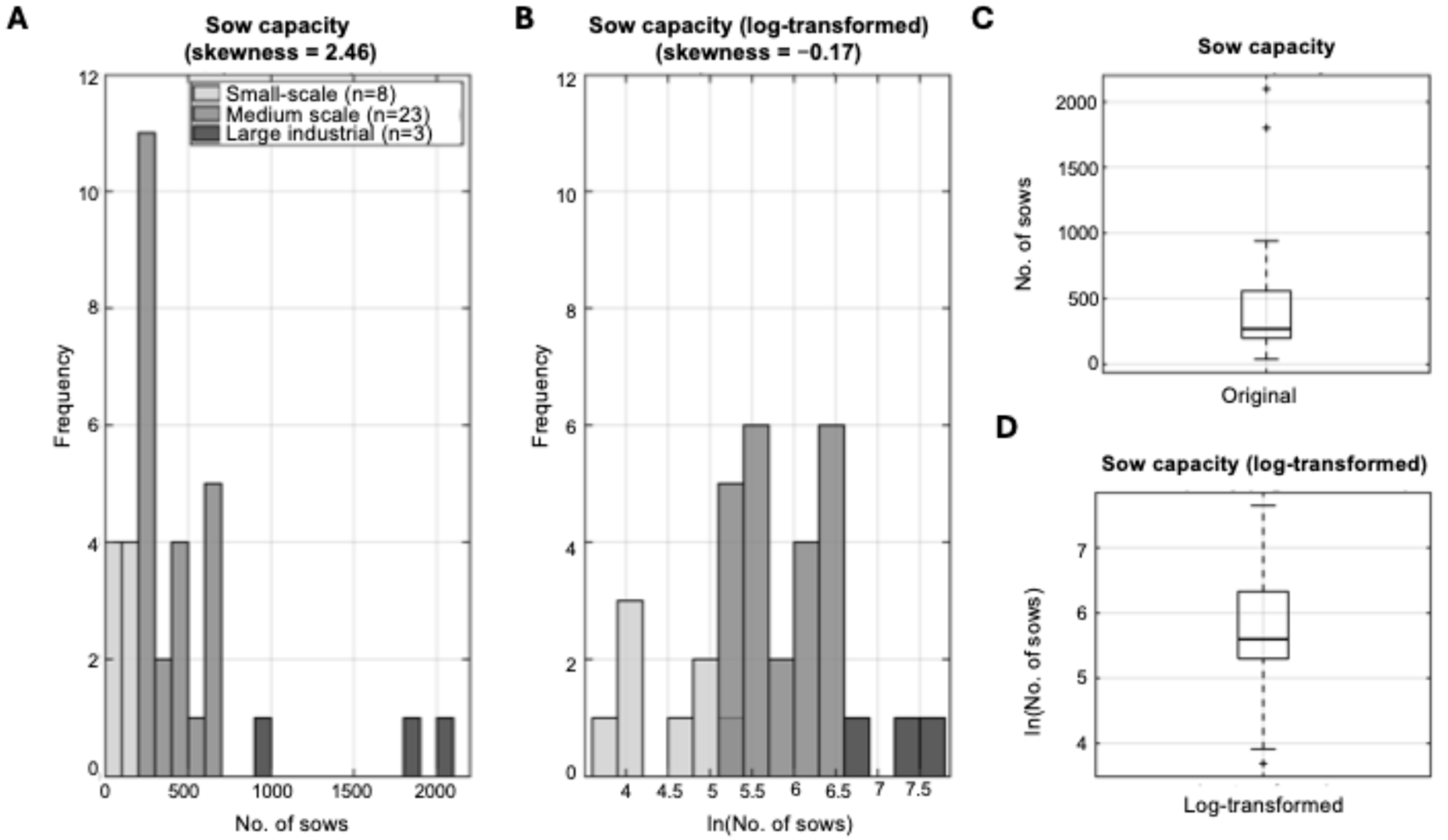
3.1.1. Farm Size Distribution
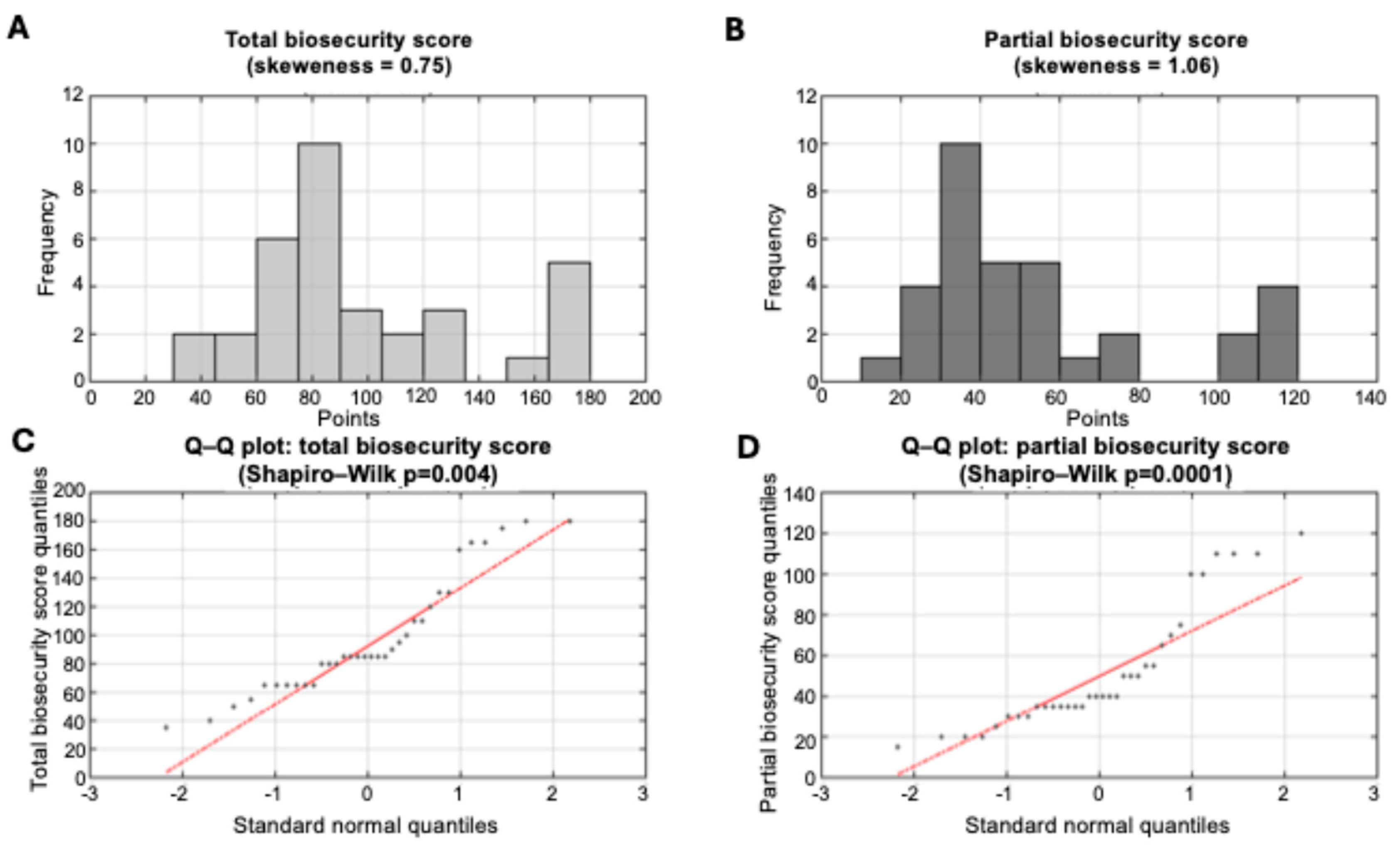
3.1.2. Biosecurity Implementation Levels
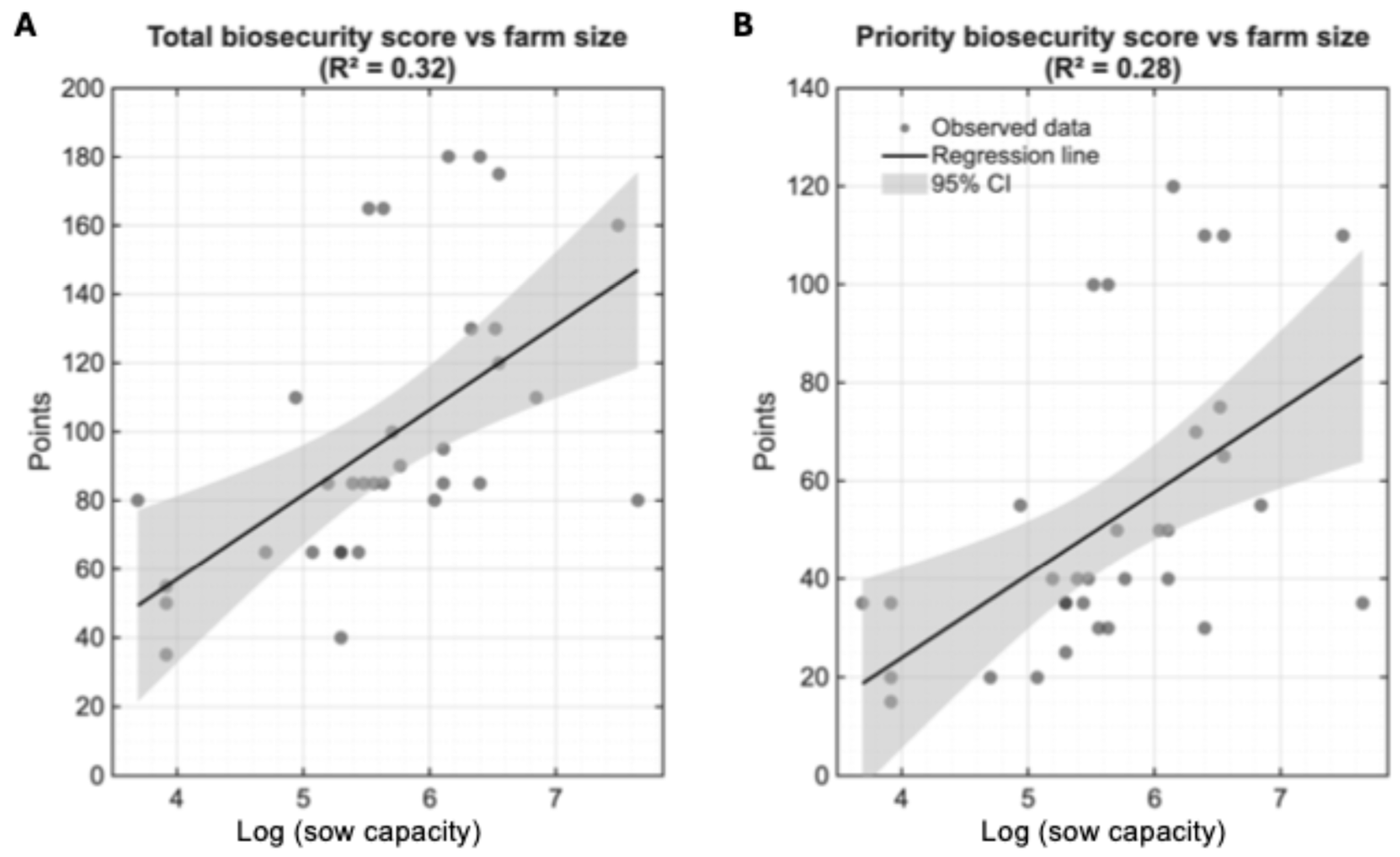
3.1.3. Impact of Farm Size on Biosecurity Implementation Practices
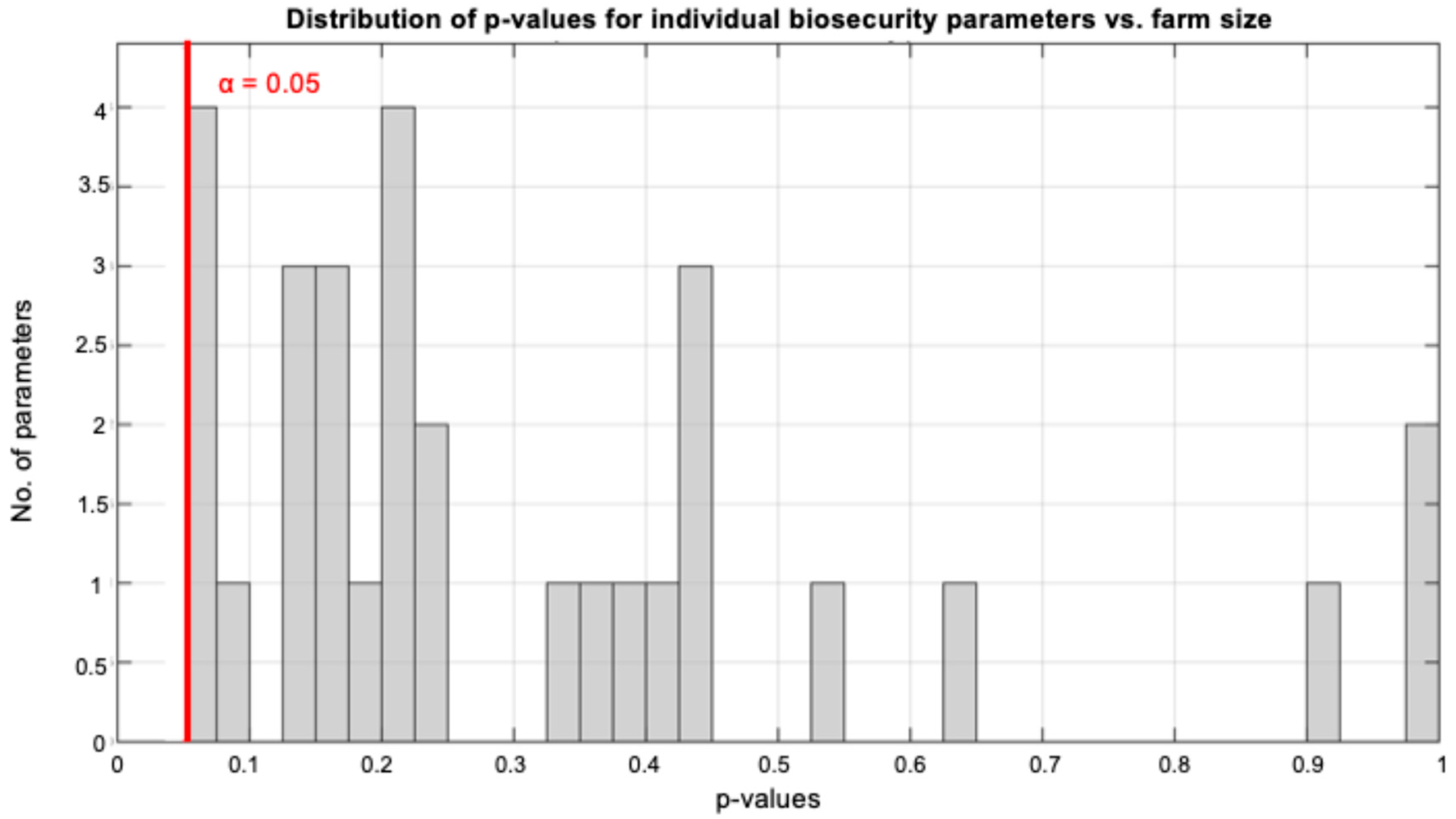
3.1.4. Farm Size Associations with Individual Biosecurity Parameters
3.2. ESBL Prevalence and Species Distribution
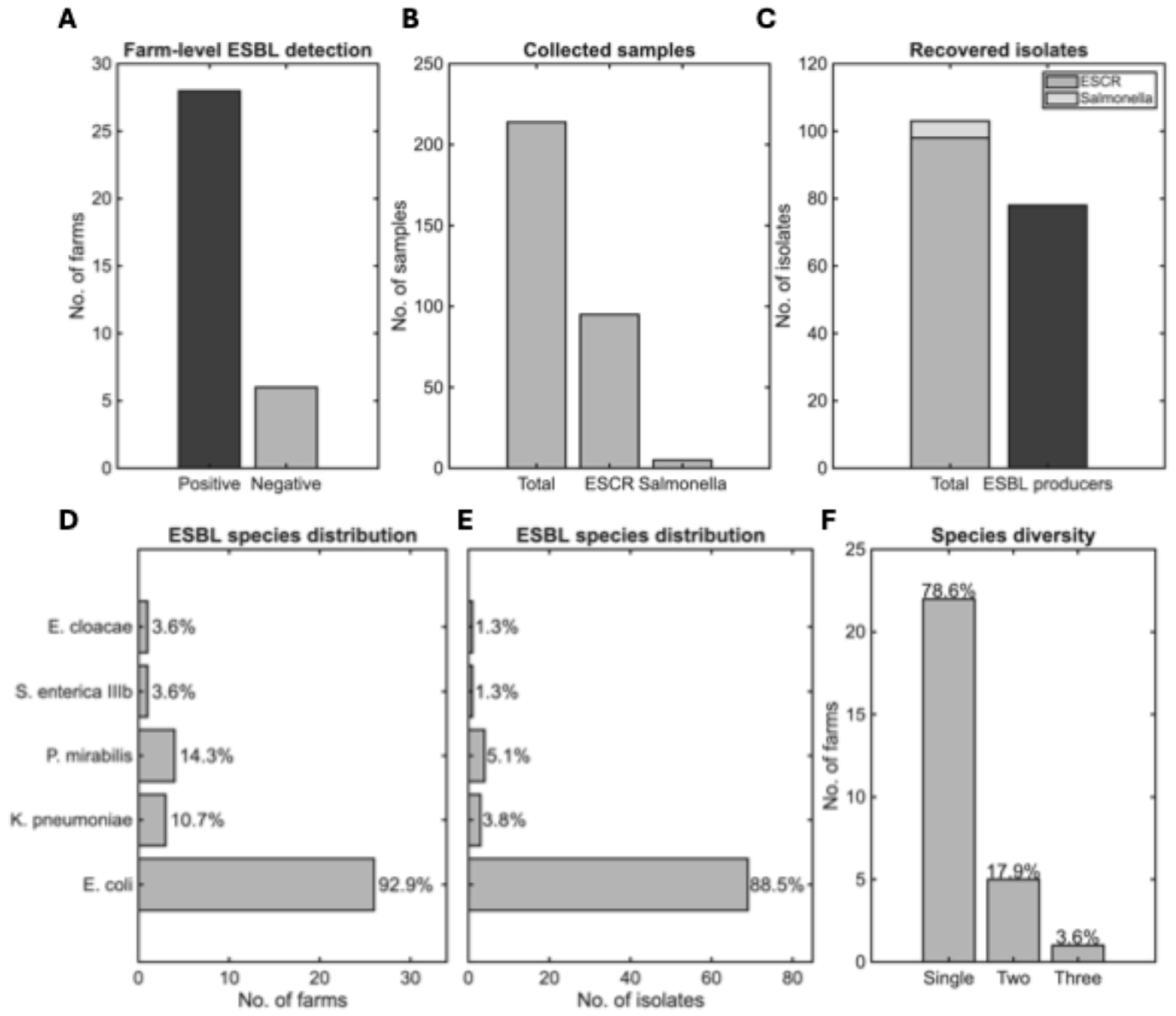
3.3. Biosecurity Implementation as a Predictor of ESBL Detection
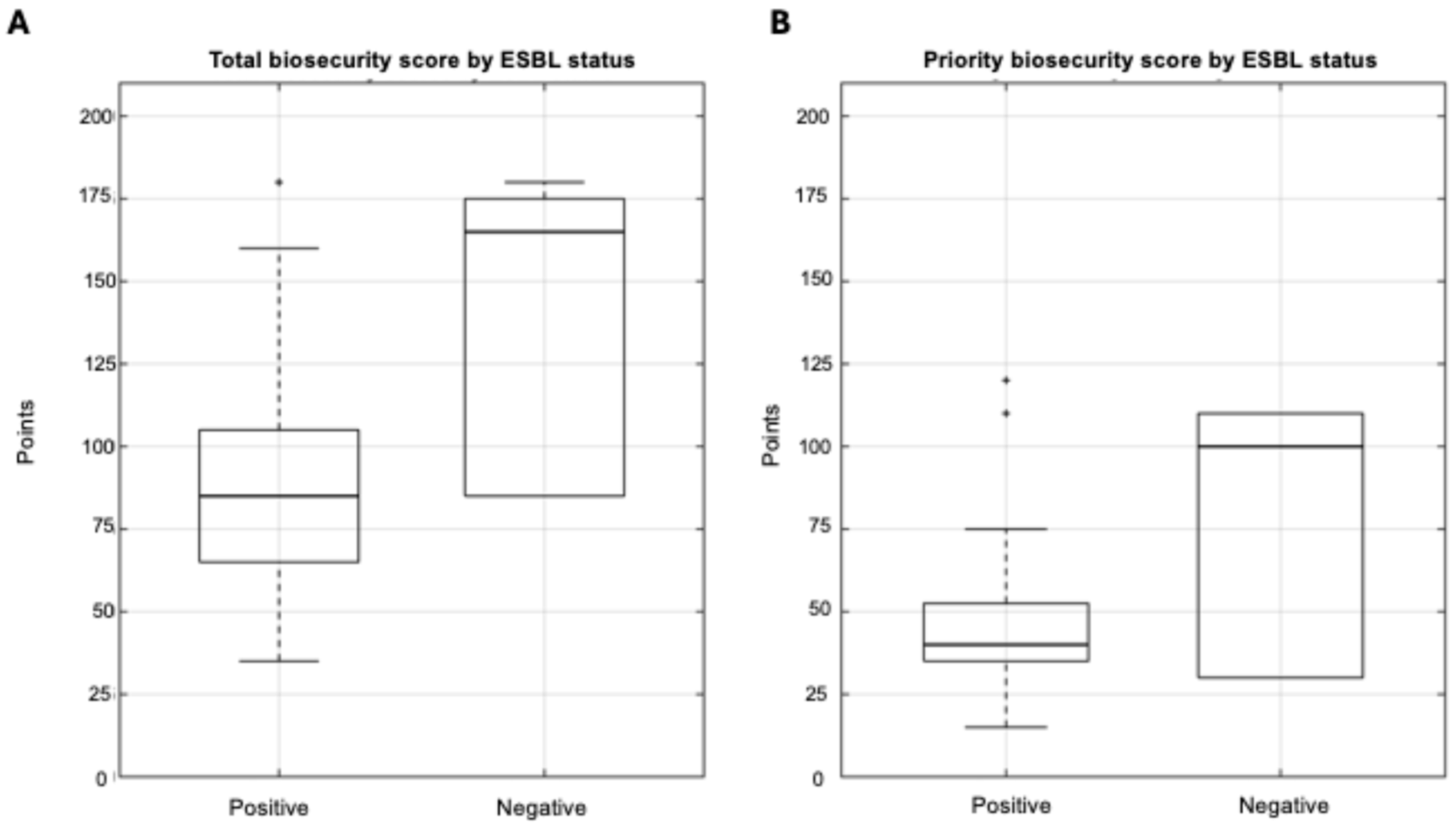
3.3.1. Biosecurity Scores and ESBL Status
3.3.2. Species-Specific Biosecurity Associations
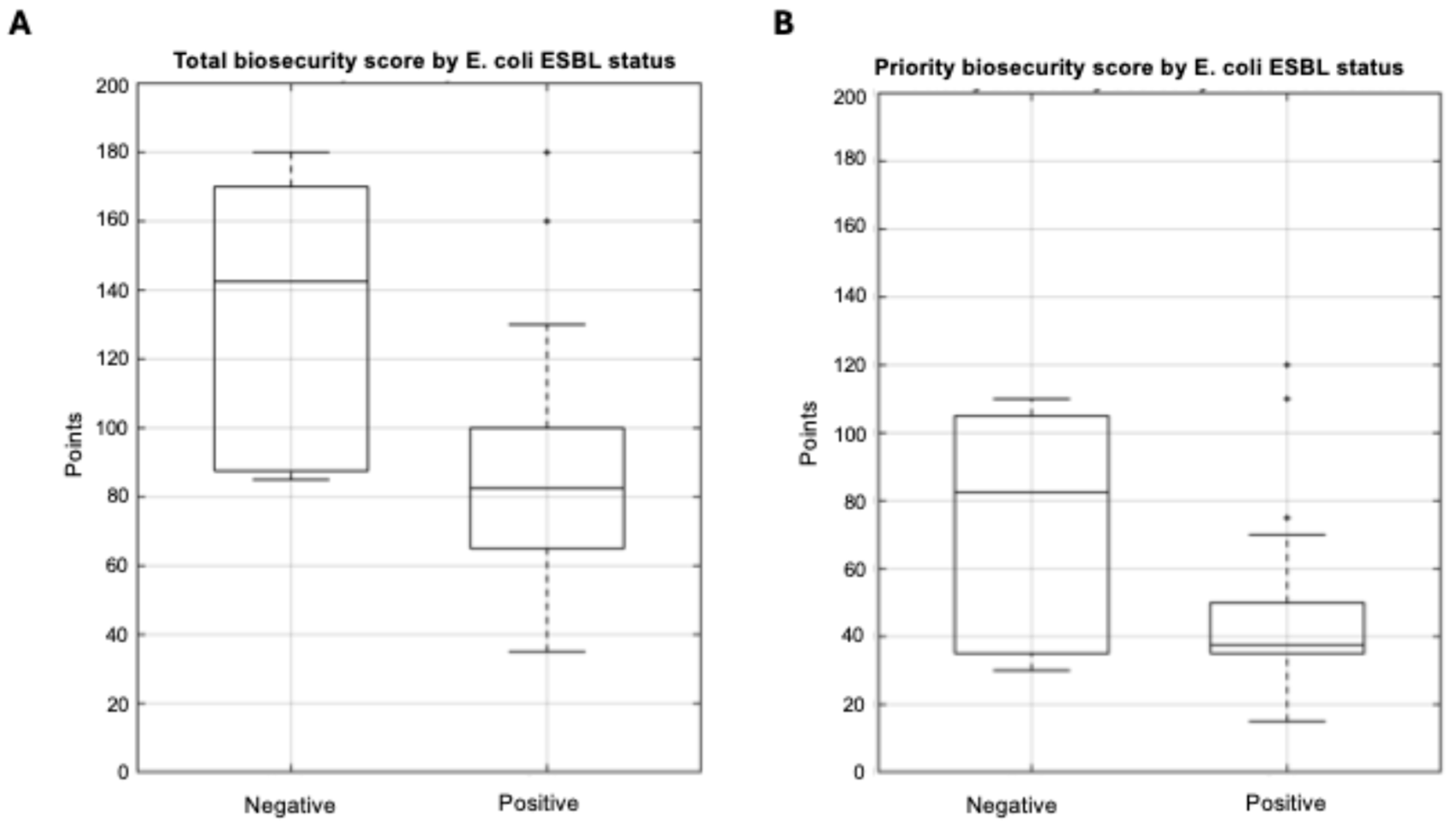
3.3.3. E. coli ESBL and Biosecurity Implementation
3.3.4. Biosecurity Patterns for Other ESBL Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESBLs | Extended-spectrum β-lactamases |
| WHO | World Health Organization |
| AMR | Antimicrobial resistance |
| ASF | African Swine Fever |
| PRRSV | Porcine Reproductive and Respiratory Syndrome Virus |
| AMU | Antimicrobial use |
| EU | European Union |
| FDR | False discovery rate |
| ARGs | Antimicrobial resistance genes |
References
- Bud, R. Antibiotics: The epitome of a wonder drug. BMJ 2007, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Gwynn, M.N.; Portnoy, A.; Rittenhouse, S.F.; Payne, D.J. Challenges of antibacterial discovery revisited. Ann. N. Y. Acad. Sci. 2010, 1213, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Center for Veterinary Medicine. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. US Food and Drug Administration. Available online: https://www.fda.gov/media/163739/download?attachment (accessed on 17 February 2025).
- Maslikowska, J.A.; Walker, S.A.N.; Elligsen, M.; Mittmann, N.; Palmay, L.; Daneman, N.; Simor, A. Impact of infection with extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella species on outcome and hospitalization costs. J. Hosp. Infect. 2016, 92, 33–41. [Google Scholar] [CrossRef]
- Tseng, C.H.; Liu, C.W.; Liu, P.Y. Extended-Spectrum β-Lactamases (ESBL) Producing Bacteria in Animals. Antibiotics 2023, 12, 661. [Google Scholar] [CrossRef]
- Filioussis, G.; Kachrimanidou, M.; Christodoulopoulos, G.; Kyritsi, M.; Hadjichristodoulou, C.; Adamopoulou, M.; Tzivara, A.; Kritas, S.K.; Grinberg, A. Short communication: Bovine mastitis caused by a multidrug-resistant, mcr-1-positive (colistin-resistant), extended-spectrum β-lactamase-producing Escherichia coli clone on a Greek dairy farm. J. Dairy. Sci. 2020, 103, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial Resistance Genes in ESBL-Producing Escherichia coli Isolates from Animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Tsekouras, N.; Athanasakopoulou, Z.; Diezel, C.; Kostoulas, P.; Braun, S.D.; Sofia, M.; Monecke, S.; Ehricht, R.; Chatzopoulos, D.C.; Gary, D.; et al. Cross-Sectional Survey of Antibiotic Resistance in Extended Spectrum β-Lactamase-Producing Enterobacteriaceae Isolated from Pigs in Greece. Animals 2022, 12, 1560. [Google Scholar] [CrossRef]
- Xi, M.; Wu, Q.; Wang, X.; Yang, B.; Xia, X.; Li, D. Characterization of extended-spectrum beta-lactamase-producing Escherichia coli strains isolated from retail foods in Shaanxi Province, China. J. Food Prot. 2015, 78, 1018–1023. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, X.; Fu, K.; Liu, B.; Xu, D.; Ji, S.; Zhou, L. Detection of extended-spectrum b-lactamase and plasmid-mediated quinolone resistance determinants in Escherichia coli isolates from retail meat in China. J. Food Prot. 2013, 76, 2040–2044. [Google Scholar] [CrossRef]
- Bergšpica, I.; Kaprou, G.; Alexa, E.A.; Prieto, M.; Alvarez-Ordóñez, A. Extended Spectrum β-Lactamase (ESBL) Producing Escherichia coli in Pigs and Pork Meat in the European Union. Antibiotics 2020, 9, 678. [Google Scholar] [CrossRef]
- Laanen, M.; Persoons, D.; Ribbens, S.; de Jong, E.; Callens, B.; Strubbe, M.; Maes, D.; Dewulf, J. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 2013, 198, 508–512. [Google Scholar] [CrossRef]
- Sahlström, L.; Virtanen, T.; Kyyrö, J.; Lyytikäinen, T. Biosecurity on Finnish cattle, pig and sheep farms—Results from a questionnaire. Prev. Vet. Med. 2014, 117, 59–67. [Google Scholar] [CrossRef]
- Papatsiros, V.G. Biosecurity management practices for the prevention and control of PRRS. Porc. Res. 2013, 3, 1. [Google Scholar]
- Alarcón, L.V.; Allepuz, A.; Mateu, E. Biosecurity in pig farms: A review. Porc. Health Manag. 2021, 7, 5. [Google Scholar] [CrossRef]
- Otake, S.; Yoshida, M.; Dee, S.A. Review of Swine Breeding Herd Biosecurity in the United States to Prevent Virus Entry Using Porcine Reproductive and Respiratory Syndrome Virus as a Model Pathogen. Animals 2024, 14, 2694. [Google Scholar] [CrossRef]
- Stygar, A.H.; Chantziaras, I.; Toppari, I.; Maes, D.; Niemi, J.K. High biosecurity and welfare standards in fattening pig farms are associated with reduced antimicrobial use. Animal 2020, 14, 2178–2186. [Google Scholar] [CrossRef]
- Postma, M.; Backhans, A.; Collineau, L.; Loesken, S.; Sjölund, M.; Belloc, C.; Emanuelson, U.; Grosse Beilage, E.; Stärk, K.D.C.; Dewulf, J. MINAPIG consortium. The biosecurity status and its associations with production and management characteristics in farrow-to-finish pig herds. Animal 2016, 10, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Mallioris, P.; Teunis, G.; Lagerweij, G.; Joosten, P.; Dewulf, J.; Wagenaar, J.A.; Stegeman, A.; Mughini-Gras, L. Biosecurity, and antimicrobial use in broiler farms across nine European countries: Towards identifying farm-specific options for reducing antimicrobial usage. Epidemiol. Infect. 2022, 151, e13. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.D. Disease Control, Prevention and On-Farm Biosecurity: The Role of Veterinary Epidemiology. Engineering 2020, 6, 20–25. [Google Scholar] [CrossRef]
- Postma, M.; Vanderhaeghen, W.; Sarrazin, S.; Maes, D.; Dewulf, J. Reducing antimicrobial usage in pig production without jeopardizing production parameters. Zoonoses Public Health 2017, 64, 63–74. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chem. 2013, 69, 827–834. [Google Scholar] [CrossRef]
- Dohmen, W.; Liakopoulos, A.; Bonten, M.J.M.; Mevius, D.J.; Heederik, D.J.J. Longitudinal Study of Dynamic Epidemiology of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Pigs and Humans Living and/or Working on Pig Farms. Microbiol. Spectr. 2023, 11, e0294722. [Google Scholar] [CrossRef]
- Fischer, J.; Hille, K.; Ruddat, I.; Mellmann, A.; Köck, R.; Kreienbrock, L. Simultaneous occurrence of MRSA and ESBL-producing Enterobacteriaceae on pig farms and in nasal and stool samples from farmers. Vet. Microbiol. 2017, 200, 107–113. [Google Scholar] [CrossRef]
- Raasch, S.; Postma, M.; Dewulf, J.; Stärk, K.D.C.; Grosse Beilage, E. Association between antimicrobial usage, biosecurity measures as well as farm performance in German farrow-to-finish farms. Porc. Health Manag. 2018, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Caekebeke, N.; Jonquiere, F.J.; Ringenier, M.; Tobias, T.J.; Postma, M.; van den Hoogen, A.; Houben, M.A.M.; Velkers, F.C.; Sleeckx, N.; Stegeman, J.A.; et al. Comparing Farm Biosecurity and Antimicrobial Use in High-Antimicrobial-Consuming Broiler and Pig Farms in the Belgian-Dutch Border Region. Front. Vet. Sci. 2020, 7, 558455. [Google Scholar] [CrossRef] [PubMed]
- National Academies Press. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Silva, G.S.; Leotti, V.B.; Castro, S.M.J.; Medeiros, A.A.R.; Silva, A.P.S.P.; Linhares, D.C.L.; Corbellini, L.G. Assessment of biosecurity practices and development of a scoring system in swine farms using item response theory. Prev. Vet. Med. 2019, 167, 128–136. [Google Scholar] [CrossRef]
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:6579:-1:ed-1:v1:en (accessed on 17 February 2025).
- Dhaka, P.; Chantziaras, I.; Vijay, D.; Bedi, J.S.; Makovska, I.; Biebaut, E.; Dewulf, J. Can Improved Farm Biosecurity Reduce the Need for Antimicrobials in Food Animals? A Scoping Review. Antibiotics 2023, 12, 893. [Google Scholar] [CrossRef]
- Jaleta, M.; Junker, V.; Kolte, B.; Börger, M.; Werner, D.; Dolsdorf, C.; Schwenker, J.; Hölzel, C.; Zentek, J.; Amon, T.; et al. Improvements of weaned pigs barn hygiene to reduce the spread of antimicrobial resistance. Front. Microbiol. 2024, 15, 1393923. [Google Scholar] [CrossRef]
- Salgado-Caxito, M.; Léon, D.; Bardales, O.; Jara, L.M.; Medrano, P.; Murga, C.; Pérez, V.; Aylas-Jurado, B.; Su-Tello, R.; Najarro, J.; et al. Unexplained High Prevalence of ESBL-Escherichia coli Among Cattle and Pigs in Peru. Antibiotics 2025, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Z.; Hu, X. Status, evaluation, and influencing factors of biosecurity levels in pig farms in China. BMC Vet. Res. 2023, 19, 272. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Q. The impact of epidemic experiences on biosecurity behavior of pig farmers: An analysis based on protection motivation theory. One Health 2024, 19, 100936. [Google Scholar] [CrossRef]
- Mandujano-Hernández, A.; Martínez-Vázquez, A.V.; Paz-González, A.D.; Herrera-Mayorga, V.; Sánchez-Sánchez, M.; Lara-Ramírez, E.E.; Vázquez, K.; de Jesús de Luna-Santillana, E.; Bocanegra-García, V.; Rivera, G. The Global Rise of ESBL-Producing Escherichia coli in the Livestock Sector: A Five-Year Overview. Animals 2024, 14, 2490. [Google Scholar] [CrossRef]
- da Silva, S.K.S.M.; Fuentes-Castillo, D.A.; Ewbank, A.C.; Sacristán, C.; Catão-Dias, J.L.; Sevá, A.P.; Lincopan, N.; Deem, S.L.; Feitosa, L.C.S.; Catenacci, L.S. ESBL-Producing Enterobacterales at the Human–Domestic Animal–Wildlife Interface: A One Health Approach to Antimicrobial Resistance in Piauí, Northeastern Brazil. Vet. Sci. 2024, 11, 195. [Google Scholar] [CrossRef]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic Resistance Is the Quintessential One Health Issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Gulhan, T. Determination of Antibiotic Resistance Patterns and Genotypes of Escherichia coli Isolated from Wild Birds. Microbiome 2024, 12, 8. [Google Scholar] [CrossRef]
- Kim, J.-I.; Moon, B.-Y.; Ali, M.S.; Kang, H.-S.; Choi, J.-H.; Kim, J.-M.; Park, S.-C.; Lim, S.-K. High prevalence of blaCTX-M-55-carrying Escherichia coli in both ceftiofur-use and non-use pig farms. Appl. Environ. Microbiol. 2025, 91, e0252524. [Google Scholar] [CrossRef]
- Ventura, N.K.O.; Freitas, L.R.; Sousa, F.A.; Cossi, M.V.C.; Nero, L.A.; Yamatogi, R.S. Colistin and β-lactam resistance in Escherichia coli isolates from bovines, swine, and humans. J. Infect. Dev. Ctries. 2025, 19, 49–57. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Scollo, A.; Perrucci, A.; Stella, M.C.; Ferrari, P.; Robino, P.; Nebbia, P. Biosecurity and Hygiene Procedures in Pig Farms: Effects of a Tailor-Made Approach as Monitored by Environmental Samples. Animals 2023, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Scollo, A.; Levallois, P.; Fourichon, C.; Motta, A.; Mannelli, A.; Lombardo, F.; Ferrari, P. Monitoring Means and Results of Biosecurity in Pig Fattening Farms: Systematic Assessment of Measures in Place and Exploration of Biomarkers of Interest. Animals 2022, 12, 2655. [Google Scholar] [CrossRef]
- Casal, J.; De Manuel, A.; Mateu, E.; Martín, M. Biosecurity measures on swine farms in Spain: Perceptions by farmers and their relationship to current on-farm measures. Prev. Vet. Med. 2007, 82, 138–150. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Scoring Criteria | Points |
|---|---|---|
| Presence of a sign to declare prohibited access to the farm | Absence/Presence | 0/10 |
| Unique entrance in the farm | Multiple entrances/Unique access | 0/10 |
| Stable and perimetral fence covering the territory of the farm | Absence of perimetral fence/Disabled fence/Presence of perimetral fence | 0/5/10 |
| Presence of bird-proof nets in the barns | Absence/Presence | 0/10 |
| External feed loading | Absence/Presence | 0/10 |
| Shower for all visitors at the entrance | Absence/Presence | 0/10 |
| Presence of dressing room for visitors | Absence/Presence | 0/10 |
| Clean and dirty zones at visitors and personnel dressing room | Absence/Presence | 0/10 |
| Application of disinfection for all vehicles | No implementation/Implementation | 0/10 |
| Presence of visitors parking | Absence/Presence | 0/10 |
| Prohibited access for trucks inside the farm | No implementation/Implementation | 0/10 |
| Equipment for visitors provided by the farm | Absence/Presence | 0/10 |
| Equipment for personnel provided by the farm | Absence/Presence | 0/10 |
| Truck cleaning at farm’s entrance | No implementation/Implementation | 0/10 |
| Parameter | Scoring Criteria | Points |
|---|---|---|
| Pig breeding by neighbors | Breeding/Lack of neighbors breeding pigs | 0/5 |
| Cover of land around the farm | Cover/Absence of any activity | 0/5 |
| Biosecurity protocol for visitors | Absence/Presence | 0/5 |
| Visitors entrance | Frequent/Rare | 0/5 |
| Entrance of trucks transporting living animals | Entry/Restricted access | 0/5 |
| Vehicles dedicated to specific production site | No specific trucks/Specific truck for each farm’s site | 0/5 |
| No truck driver access in the farm | Access/No access | 0/5 |
| Presence of disinfection equipment room | Absence/Presence | 0/5 |
| Chemical water treatment | No application/Application | 0/5 |
| Water chemical examination | No application/Application | 0/5 |
| Equipment disinfection during entrance | No application/Application | 0/5 |
| Other animal species breeding in the farm | Presence/Absence | 0/5 |
| Restricted staff contact with other farm animals | Contact/No contact | 0/5 |
| Unique source for animal replacement | Multiple sources/Unique sources | 0/5 |
| All in–all out system | Absence/Presence | 0/5 |
| Special equipment per pen | Absence/Presence | 0/5 |
| Maintenance of sick animal pen | Absence/Presence | 0/5 |
| Application of rodent control | No implementation/Implementation | 0/5 |
| Application of flies control | No implementation/Implementation | 0/5 |
| Implementation of biosecurity training for farm personnel | No implementation/Implementation | 0/5 |
| Use of disposable gloves to handle dead animals | No use/Use | 0/5 |
| ESBL Species | Farms | Total Biosecurity Score | Priority Biosecurity Score | ||
|---|---|---|---|---|---|
| No | Median (IQR) | Range | Median (IQR) | Range | |
| E. coli | 26 | 82 (65–100) | 35–180 | 38 (35–50) | 15–120 |
| P. mirabilis | 4 | 92 (85–128) | 80–160 | 45 (38–80) | 35–110 |
| K. pneumoniae | 3 | 80 (69–110) | 62–110 | 50 (39–60) | 35–65 |
| No ESBL | 6 | 165 (85–175) | 85–110 | 100 (30–110) | 30–110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsekouras, N.; Antoniadis, S.; Athanasakopoulou, Z.; Chatzopoulos, D.C.; Kantas, D.; Spyrou, V.; Christodoulopoulos, G.; Billinis, C.; Papatsiros, V.G. Can Improved Biosecurity Measures Reduce the Presence of the Most Common ESBL-Producing Enterobacteriaceae? A Study from Greek Pig Farms. Life 2025, 15, 1629. https://doi.org/10.3390/life15101629
Tsekouras N, Antoniadis S, Athanasakopoulou Z, Chatzopoulos DC, Kantas D, Spyrou V, Christodoulopoulos G, Billinis C, Papatsiros VG. Can Improved Biosecurity Measures Reduce the Presence of the Most Common ESBL-Producing Enterobacteriaceae? A Study from Greek Pig Farms. Life. 2025; 15(10):1629. https://doi.org/10.3390/life15101629
Chicago/Turabian StyleTsekouras, Nikolaos, Spyridon Antoniadis, Zoi Athanasakopoulou, Dimitris C. Chatzopoulos, Dimitrios Kantas, Vassiliki Spyrou, Georgios Christodoulopoulos, Charalambos Billinis, and Vasileios G. Papatsiros. 2025. "Can Improved Biosecurity Measures Reduce the Presence of the Most Common ESBL-Producing Enterobacteriaceae? A Study from Greek Pig Farms" Life 15, no. 10: 1629. https://doi.org/10.3390/life15101629
APA StyleTsekouras, N., Antoniadis, S., Athanasakopoulou, Z., Chatzopoulos, D. C., Kantas, D., Spyrou, V., Christodoulopoulos, G., Billinis, C., & Papatsiros, V. G. (2025). Can Improved Biosecurity Measures Reduce the Presence of the Most Common ESBL-Producing Enterobacteriaceae? A Study from Greek Pig Farms. Life, 15(10), 1629. https://doi.org/10.3390/life15101629










