An Overview of Sex-Based Differences in the Onset and Progression of DKD in the Well-Known Model, ZSF1 Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Rearing and Handling
2.2. Blood Pressure Measurements
2.3. Urinalysis
2.4. Blood Glucose Measurements
2.5. Statistical Analyses
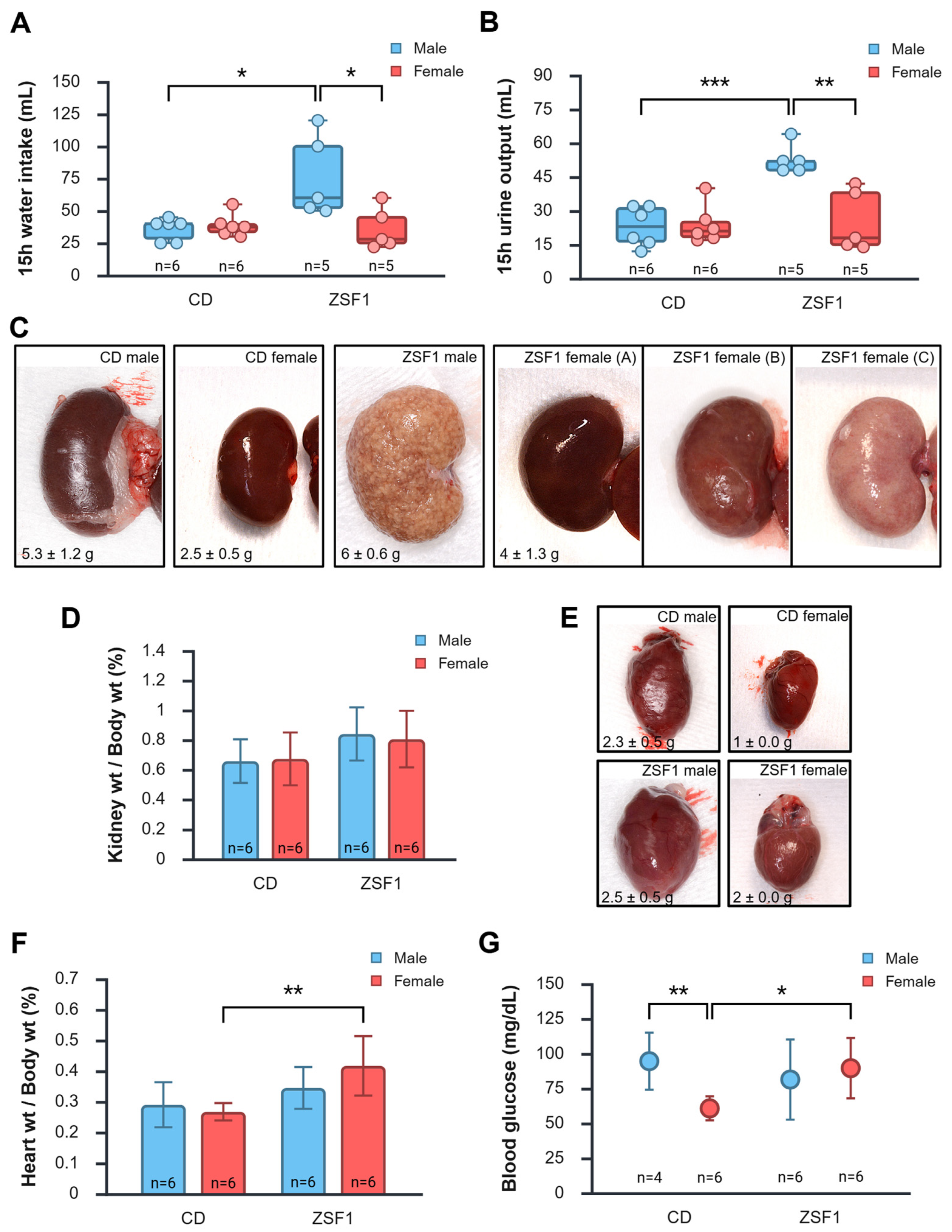
3. Results
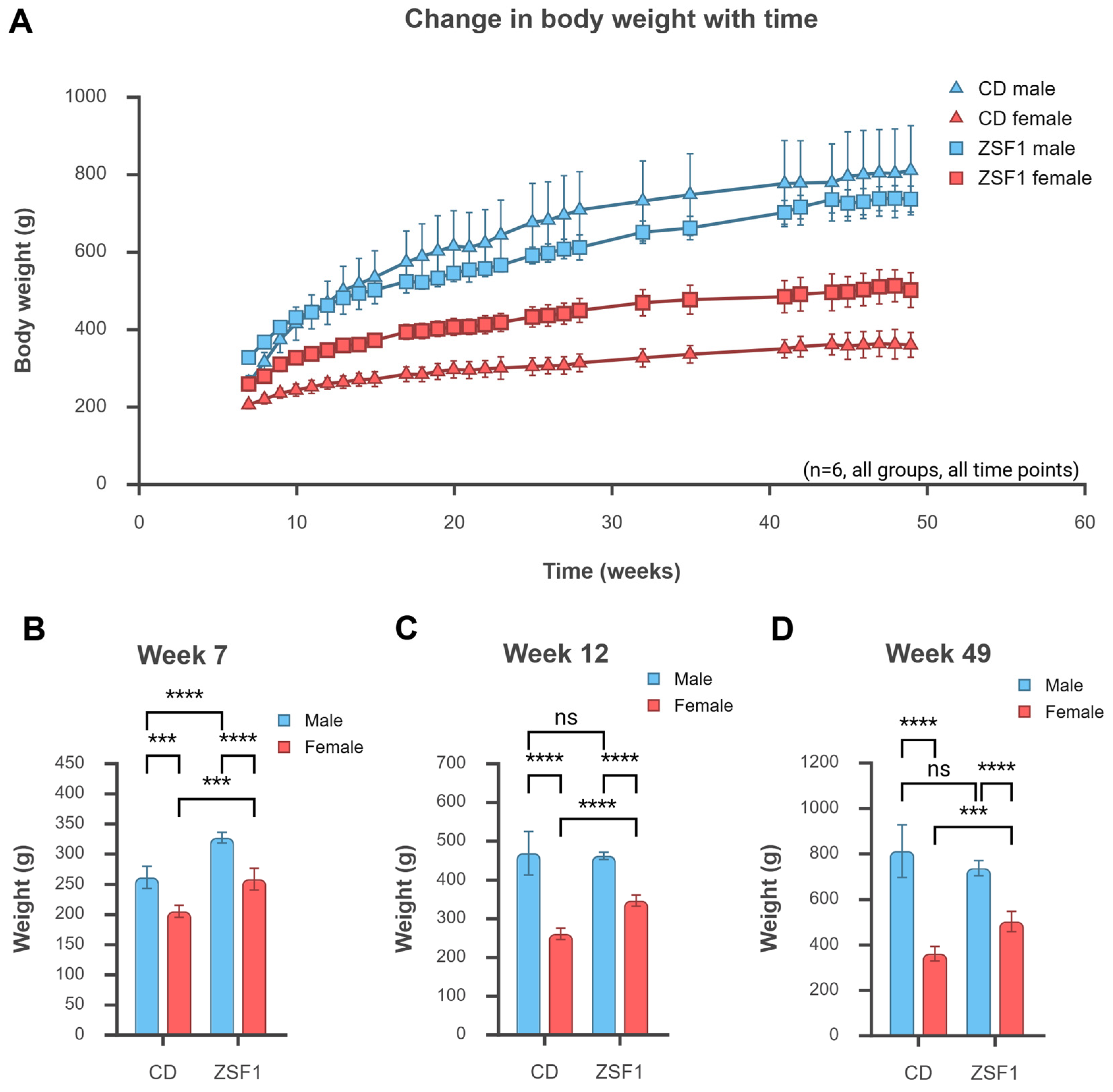
3.1. Growth Parameters of ZSF1 Rats Compared with CD Rats
3.2. Male but Not Female ZSF1 Rats Become Hypertensive over Time
3.2.1. Changes in Blood Pressure
3.2.2. Change in Heart Rate
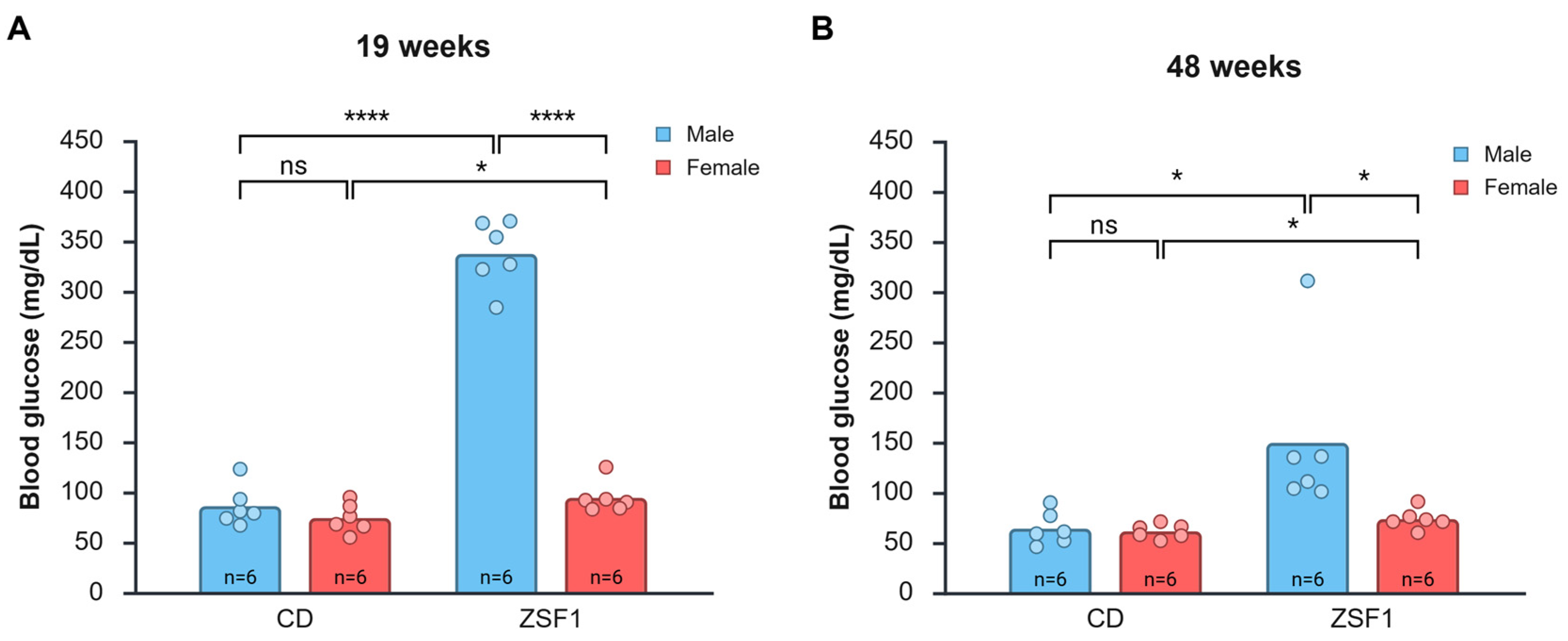
3.3. Hyperglycemia in Male and Female ZSF1 Rats
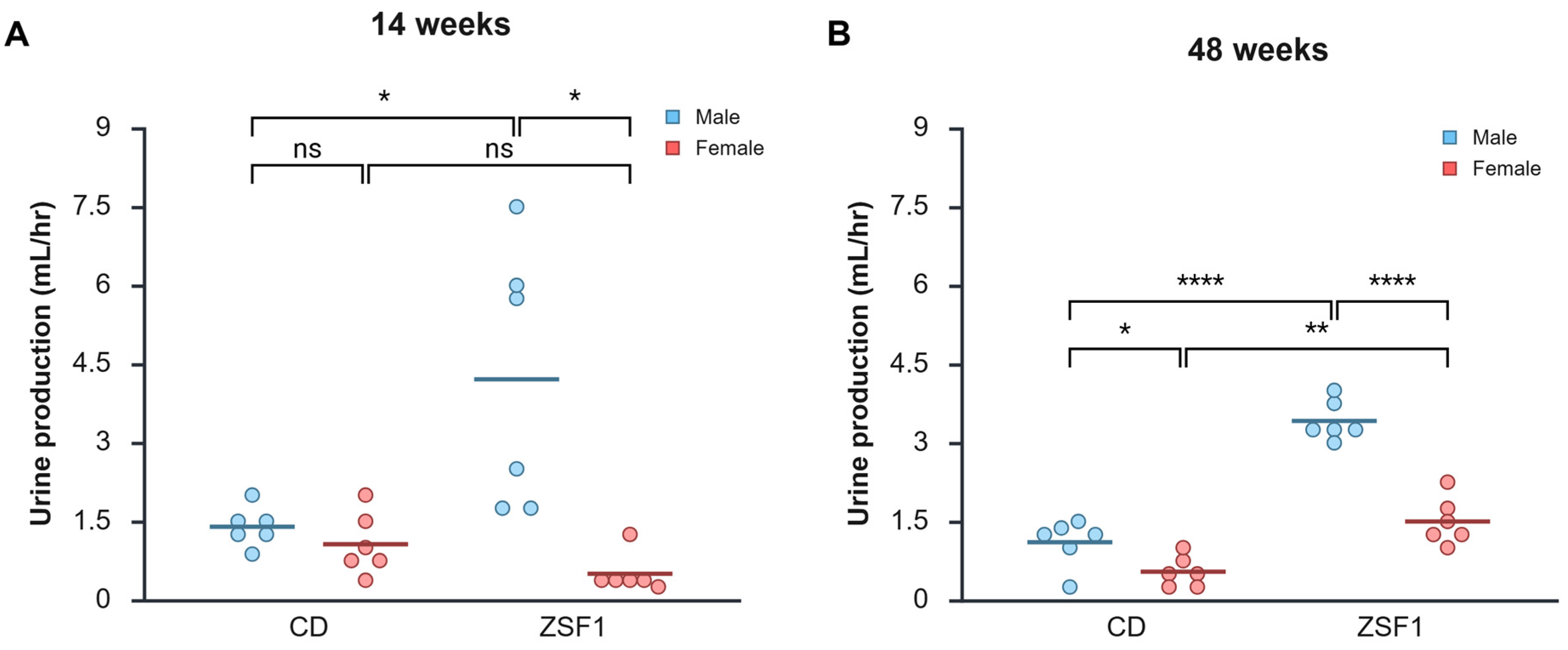
3.4. Urinalysis of Male and Female ZSF1 Rats
3.4.1. Proteinuria
3.4.2. Glucosuria
3.4.3. Indications of Infection
3.5. Peri-Euthanasia Gross Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BP | Blood Pressure |
| CD | caesarean-derived |
| CDC | Centers for disease control and prevention |
| CI | Confidence interval |
| DKD | Diabetic kidney disease |
| DOCA | Deoxycorticosterone acetate |
| eNOS | Endothelial NOS |
| ESRD | End-stage renal disease |
| GFR | Glomerular filtration rate |
| HFpEF | Heart failure with preserved ejection fraction |
| HR | Heart rate |
| IGS | International genetic standardization program |
| iNOS | Inducible NOS |
| IQR | Interquartile range |
| MAP | Mean arterial pressure |
| nNOS | Neuronal NOS |
| NOS | Nitric oxide synthase |
| NT | Not tested |
| RMH | Rats, mice, and hamsters |
| SD | Standard deviation |
| SEM | Standard error of mean |
| SHHF | Spontaneously hypertensive heart failure |
| T2DM | Type 2 diabetes mellitus |
| UACR | Urine albumin-creatinine ratio |
| UTI | Urinary tract infection |
| VPR | Volume pressure recording |
| WBC | White blood cells |
| ZDF | Zucker diabetic fatty |
| ZSF1 | Zucker fatty/Spontaneously hypertensive heart failure F1 hybrid |
Appendix A
| Measurement | Comparison | Difference Between Means | 95% Confidence Interval | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| Systolic BP (mmHg) at 15 weeks | CD male vs. CD female | 16.0077 | −16.1007 to 48.1161 | 0.293 | 0.6413 |
| ZSF1 male vs. ZSF1 female | 7.8625 | −7.3355 to 23.0605 | 0.276 | 0.6655 | |
| CD male vs. ZSF1 male | 0.5435 | −19.8451 to 20.9321 | 0.954 | 0.03429 | |
| CD female vs. ZSF1 female | −7.6017 | −36.6918 to 21.4884 | 0.573 | −0.3362 | |
| Systolic BP (mmHg) at 45 weeks | CD male vs. CD female | 3.8435 | −19.3548 to 27.0418 | 0.72 | 0.2131 |
| ZSF1 male vs. ZSF1 female | 29.6964 | −7.5212 to 66.9140 | 0.106 | 1.0264 | |
| CD male vs. ZSF1 male | −29.4159 | −54.4443 to −4.3875 | 0.0257 | −1.5119 | |
| CD female vs. ZSF1 female | −3.5630 | −39.5754 to 32.4494 | 0.83 | −0.1273 | |
| Diastolic BP (mmHG) at 15 weeks | CD male vs. CD female | 12.7373 | −15.3886 to 40.8632 | 0.337 | 0.5826 |
| ZSF1 male vs. ZSF1 female | 8.5833 | −5.3765 to 22.5432 | 0.201 | 0.79010 | |
| CD male vs. ZSF1 male | 3.6336 | −9.7688 to 17.0361 | 0.559 | 0.3488 | |
| CD female vs. ZSF1 female | −0.5203 | −28.9161 to 27.8755 | 0.968 | −0.02357 | |
| Diastolic BP (mmHG) at 45 weeks | CD male vs. CD female | 11.5969 | −6.6040 to 29.7978 | 0.186 | 0.8197 |
| ZSF1 male vs. ZSF1 female | 37.3191 | 1.3423 to 73.2960 | 0.0434 | 1.3344 | |
| CD male vs. ZSF1 male | −24.3935 | −50.9389 to 2.1520 | 0.0678 | −1.1821 | |
| CD female vs. ZSF1 female | 1.3288 | −29.0183 to 31.6758 | 0.924 | 0.05633 | |
| MAP (mmHg) at 15 weeks | CD male vs. CD female | 13.8777 | −14.8004 to 42.5558 | 0.306 | 0.6225 |
| ZSF1 male vs. ZSF1 female | 8.3213 | −5.7224 to 22.3650 | 0.216 | 0.7622 | |
| CD male vs. ZSF1 male | 2.6184 | −11.9249 to 17.1617 | 0.697 | 0.2316 | |
| CD female vs. ZSF1 female | −2.9381 | −31.3661 to 25.4900 | 0.823 | −0.13210 | |
| MAP (mmHg) at 45 weeks | CD male vs. CD female | 8.9702 | −9.9066 to 27.8470 | 0.315 | 0.6113 |
| ZSF1 male vs. ZSF1 female | 34.7522 | −1.4113 to 70.9157 | 0.0579 | 1.2362 | |
| CD male vs. ZSF1 male | −26.0458 | −51.4944 to −0.5972 | 0.0458 | −1.3166 | |
| CD female vs. ZSF1 female | −0.2638 | −32.1464 to 31.6188 | 0.986 | −0.01064 | |
| Heart rate (bpm) at 15 weeks | CD male vs. CD female | −13.6318 | −82.1453 to 54.8817 | 0.667 | −0.25510 |
| ZSF1 male vs. ZSF1 female | −40.3701 | −78.1196 to −2.6205 | 0.0384 | −1.3757 | |
| CD male vs. ZSF1 male | −7.5227 | −65.9253 to 50.8800 | 0.78 | −0.1657 | |
| CD female vs. ZSF1 female | −34.2609 | −86.3019 to 17.7801 | 0.173 | 0.8469 | |
| Heart rate (bpm) at 45 weeks | CD male vs. CD female | −6.6036 | −60.2145 to 47.0073 | 0.789 | −0.1585 |
| ZSF1 male vs. ZSF1 female | 74.0771 | 26.2554 to 121.8988 | 0.00621 | 1.9927 | |
| CD male vs. ZSF1 male | −20.6311 | −48.6419 to 7.3797 | 0.132 | −0.9475 | |
| CD female vs. ZSF1 female | 60.0496 | −6.1050 to 126.2042 | 0.0707 | 1.1677 |
References
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://www.diabetesatlas.org (accessed on 15 August 2025).
- The International Diabetes Federation (IDF) and the International Society of Nephrology (ISN). Policy Brief “Renewing the Fight: A Call to Action on Diabetes and Chronic Kidney Disease”; The International Diabetes Federation (IDF) and the International Society of Nephrology (ISN): Brussels, Belgium, 2023; Available online: https://idf.org/what-we-do/advocacy/resources/ (accessed on 15 August 2025).
- Ma, X.; Liu, R.; Xi, X.; Zhuo, H.; Gu, Y. Global burden of chronic kidney disease due to diabetes mellitus, 1990–2021, and projections to 2050. Front. Endocrinol. 2025, 16, 1513008. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, H.; Liu, H.; Wu, H.; Gao, W. Global, Regional, and National Temporal Trends in Incidence for Type 2 Diabetes Mellitus Related Chronic Kidney Disease from 1992 to 2021. Diabetes Metab. J. 2025, 49, 848–861. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.; Li, L.; Liu, M.; Wu, Y.; Chen, R.; He, J.; Mai, W.; Li, X. Global, Regional, and National Prevalence of Chronic Type 2 Diabetic Kidney Disease From 1990 to 2021: A Trend and Health Inequality Analyses Based on the Global Burden of Disease Study 2021. J. Diabetes 2025, 17, e70098. [Google Scholar] [CrossRef]
- U.S. Centers for Disease Control and Prevention (CDC). $413 Billion in Diabetes Costs Social Media Graphic. Available online: https://www.cdc.gov/diabetes/communication-resources/413-billion-diabetes-costs.html (accessed on 15 August 2025).
- Kim, K.; Crook, J.; Lu, C.C.; Nyman, H.; Sarker, J.; Nelson, R.; LaFleur, J. Healthcare Costs Across Diabetic Kidney Disease Stages: A Veterans Affairs Study. Kidney Med. 2024, 6, 100873. [Google Scholar] [CrossRef]
- Tofovic, S.P.; Kusaka, H.; Kost, C.K., Jr.; Bastacky, S. Renal function and structure in diabetic, hypertensive, obese ZDFxSHHF-hybrid rats. Ren. Fail. 2000, 22, 387–406. [Google Scholar] [CrossRef]
- Prabhakar, S.; Starnes, J.; Shi, S.; Lonis, B.; Tran, R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J. Am. Soc. Nephrol. 2007, 18, 2945–2952. [Google Scholar] [CrossRef]
- Bilan, V.P.; Salah, E.M.; Bastacky, S.; Jones, H.B.; Mayers, R.M.; Zinker, B.; Poucher, S.M.; Tofovic, S.P. Diabetic nephropathy and long-term treatment effects of rosiglitazone and enalapril in obese ZSF1 rats. J. Endocrinol. 2011, 210, 293–308. [Google Scholar] [CrossRef]
- Dower, K.; Zhao, S.; Schlerman, F.J.; Savary, L.; Campanholle, G.; Johnson, B.G.; Xi, L.; Nguyen, V.; Zhan, Y.; Lech, M.P.; et al. High resolution molecular and histological analysis of renal disease progression in ZSF1 fa/faCP rats, a model of type 2 diabetic nephropathy. PLoS ONE 2017, 12, e0181861. [Google Scholar] [CrossRef]
- Nguyen, I.; Samuelsson, A.M.; Joles, J.; Verhaar, M. In Obese Zsf1 Rats, Females Show Increased Salt-Sensitivity Compared to Males. Nephrol. Dial. Transplant. 2018, 33, i370. [Google Scholar] [CrossRef]
- Nguyen, I.T.N.; Cramer, M.J.; Joles, J.A.; Verhaar, M. #5907 Renal Injury in Relation to Obesity and the Additive Effect of Hypertension in Female and Male Obese and Lean Zsf1 Rats. Nephrol. Dial. Transplant. 2023, 38, I985. [Google Scholar] [CrossRef]
- Nguyen, I.T.; Joles, J.A.; Verhaar, M.C. Contributions of Obesity and Hypertension to Progression of Cardiorenal Syndrome in Non-Diabetic Obese Female ZSF1 Rats. J. Am. Soc. Nephrol. 2021, 32, 564. [Google Scholar] [CrossRef]
- Babelova, A.; Burckhardt, B.C.; Wegner, W.; Burckhardt, G.; Henjakovic, M. Sex-differences in renal expression of selected transporters and transcription factors in lean and obese Zucker spontaneously hypertensive fatty rats. J. Diabetes Res. 2015, 2015, 483238. [Google Scholar] [CrossRef]
- Nguyen, I.T.N.; Cramer, M.J.; Joles, J.A.; Verhaar, M.C. Renal injury in relation to obesity and the additive effect of hypertension in female and male obese and lean ZSF1 rats. Am. J. Physiol. Renal. Physiol. 2023, 325, F73–F86. [Google Scholar] [CrossRef]
- Su, Z.; Widomski, D.; Ma, J.; Namovic, M.; Nikkel, A.; Leys, L.; Olson, L.; Salte, K.; Donnelly-Roberts, D.; Esbenshade, T.; et al. Longitudinal Changes in Measured Glomerular Filtration Rate, Renal Fibrosis and Biomarkers in a Rat Model of Type 2 Diabetic Nephropathy. Am. J. Nephrol. 2016, 44, 339–353. [Google Scholar] [CrossRef]
- Pollitzer, E. Biology: Cell sex matters. Nature 2013, 500, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.Y.; Mansukhani, N.A.; Stubbs, V.C.; Helenowski, I.B.; Woodruff, T.K.; Kibbe, M.R. Sex bias exists in basic science and translational surgical research. Surgery 2014, 156, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Allegra, S.; Chiara, F.; Di Grazia, D.; Gaspari, M.; De Francia, S. Evaluation of Sex Differences in Preclinical Pharmacology Research: How Far Is Left to Go? Pharmaceuticals 2023, 16, 786. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Loeffler, I.; Ziller, N. Sex-Related Aspects in Diabetic Kidney Disease-An Update. J. Clin. Med. 2023, 12, 2834. [Google Scholar] [CrossRef]
- Karp, N.A.; Reavey, N. Sex bias in preclinical research and an exploration of how to change the status quo. Br. J. Pharmacol. 2019, 176, 4107–4118. [Google Scholar] [CrossRef]
- Handling and Training of Mice and Rats for Low Stress Procedures. Available online: https://nc3rs.org.uk/3rs-resource-library/handling-and-training-mice-and-rats-low-stress-procedures (accessed on 5 April 2023).
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J. Vis. Exp. 2009, 27, 1291. [Google Scholar] [CrossRef]
- Blood Sampling: Rat > Tail Vein (Non-Surgical). Available online: https://nc3rs.org.uk/3rs-resources/blood-sampling/blood-sampling-rat#tail-vein-(non-surgical) (accessed on 15 August 2022).
- Lee, G.; Goosens, K.A. Sampling blood from the lateral tail vein of the rat. J. Vis. Exp. 2015, 99, 52766. [Google Scholar] [CrossRef]
- Zou, W.; Yang, Y.; Gu, Y.; Zhu, P.; Zhang, M.; Cheng, Z.; Liu, X.; Yu, Y.; Peng, X. Repeated Blood Collection from Tail Vein of Non-Anesthetized Rats with a Vacuum Blood Collection System. J. Vis. Exp. 2017, 130, 55852. [Google Scholar] [CrossRef]
- Islam, S.; Mir, A.R.; Arfat, M.Y.; Alam, K.; Ali, A. Studies on glycoxidatively modified human IgG: Implications in immuno-pathology of type 2 diabetes mellitus. Int. J. Biol. Macromol. 2017, 104, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Franssen, C.; Lourenco, A.; Falcao-Pires, I.; Fontoura, D.; Leite, S.; Plettig, L.; Lopez, B.; Ottenheijm, C.A.; Becher, P.M.; et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ. Heart Fail. 2013, 6, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.; Cerqueira, R.J.; Ibarrola, J.; Fontoura, D.; Fernandez-Celis, A.; Zannad, F.; Falcao-Pires, I.; Paulus, W.J.; Leite-Moreira, A.F.; Rossignol, P.; et al. Arterial Remodeling and Dysfunction in the ZSF1 Rat Model of Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e005596. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Babelova, A.; Burckhardt, B.C.; Salinas-Riester, G.; Pommerenke, C.; Burckhardt, G.; Henjakovic, M. Next generation sequencing of sex-specific genes in the livers of obese ZSF1 rats. Genomics 2015, 106, 204–213. [Google Scholar] [CrossRef]
- Rahhal, M.N.; Gharaibeh, N.E.; Rahimi, L.; Ismail-Beigi, F. Disturbances in Insulin-Glucose Metabolism in Patients With Advanced Renal Disease With and Without Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4949–4966. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Glucose Homeostasis, Hypoglycemia, and the Burnt-Out Diabetes Phenomenon in Kidney Disease. Semin. Nephrol. 2021, 41, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Derose, S.F.; Nicholas, S.; Benner, D.; Sharma, K.; Kovesdy, C.P. Burnt-out diabetes: Impact of chronic kidney disease progression on the natural course of diabetes mellitus. J. Ren. Nutr. 2009, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.J.; Holycross, B.J.; Hoepf, T.M.; McCune, S.A. Increased salt sensitivity secondary to leptin resistance in SHHF rats is mediated by endothelin. Mol. Cell. Biochem. 2003, 242, 57–63. [Google Scholar] [CrossRef]
- Khadour, F.H.; Kao, R.H.; Park, S.; Armstrong, P.W.; Holycross, B.J.; Schulz, R. Age-dependent augmentation of cardiac endothelial NOS in a genetic rat model of heart failure. Am. J. Physiol. 1997, 273, H1223–H1230. [Google Scholar] [CrossRef]
- Cittadini, A.; Napoli, R.; Monti, M.G.; Rea, D.; Longobardi, S.; Netti, P.A.; Walser, M.; Sama, M.; Aimaretti, G.; Isgaard, J.; et al. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes 2012, 61, 944–953. [Google Scholar] [CrossRef]
- Koser, F.; Hobbach, A.J.; Abdellatif, M.; Herbst, V.; Turk, C.; Reinecke, H.; Kruger, M.; Sedej, S.; Linke, W.A. Acetylation and phosphorylation changes to cardiac proteins in experimental HFpEF due to metabolic risk reveal targets for treatment. Life Sci. 2022, 309, 120998. [Google Scholar] [CrossRef]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschope, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Nguyen, I.T.N.; Brandt, M.M.; van de Wouw, J.; van Drie, R.W.A.; Wesseling, M.; Cramer, M.J.; de Jager, S.C.A.; Merkus, D.; Duncker, D.J.; Cheng, C.; et al. Both male and female obese ZSF1 rats develop cardiac dysfunction in obesity-induced heart failure with preserved ejection fraction. PLoS ONE 2020, 15, e0232399. [Google Scholar] [CrossRef]
- Cook, R.F.; Bussey, C.T.; Mellor, K.M.; Cragg, P.A.; Lamberts, R.R. beta(1)-Adrenoceptor, but not beta(2)-adrenoceptor, subtype regulates heart rate in type 2 diabetic rats in vivo. Exp. Physiol. 2017, 102, 911–923. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Griffin, K.A.; Bidani, A.K.; Davisson, R.L.; Hall, J.E. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: Blood pressure measurement in experimental animals: A statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension 2005, 45, 299–310. [Google Scholar] [CrossRef]
- Harrison, D.G.; Bader, M.; Lerman, L.O.; Fink, G.; Karumanchi, S.A.; Reckelhoff, J.F.; Sequeira-Lopez, M.L.S.; Touyz, R.M. Tail-Cuff Versus Radiotelemetry to Measure Blood Pressure in Mice and Rats. Hypertension 2024, 81, 3–5. [Google Scholar] [CrossRef]
- Kapsdorferova, V.; Gresova, S.; Svorc, P. Measurement of blood pressure in rats: Invasive or noninvasive methods? Physiol. Rep. 2024, 12, e70041. [Google Scholar] [CrossRef]
- Schipper, L.; Harvey, L.; van der Beek, E.M.; van Dijk, G. Home alone: A systematic review and meta-analysis on the effects of individual housing on body weight, food intake and visceral fat mass in rodents. Obes. Rev. 2018, 19, 614–637. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Hagaman, J.R.; Smithies, O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 1995, 25, 1111–1115. [Google Scholar] [CrossRef]
- Feng, M.; Whitesall, S.; Zhang, Y.; Beibel, M.; D’Alecy, L.; DiPetrillo, K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am. J. Hypertens. 2008, 21, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.; Aubdool, A.A.; Thakore, P.; Baldissera, L., Jr.; Alawi, K.M.; Keeble, J.; Nandi, M.; Brain, S.D. Tail-Cuff Technique and Its Influence on Central Blood Pressure in the Mouse. J. Am. Heart Assoc. 2017, 6, e005204. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Nara, Y.; Yamori, Y. Indirect systolic and mean blood pressure determination by a new tail cuff method in spontaneously hypertensive rats. Lab. Anim. 1991, 25, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.B.; Turner, S.W.; Mangos, G.J.; Ludbrook, J.; Whitworth, J.A. Comparison of telemetric and tail-cuff blood pressure monitoring in adrenocorticotrophic hormone-treated rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 831–835. [Google Scholar] [CrossRef]
- Ibrahim, J.; Berk, B.C.; Hughes, A.D. Comparison of simultaneous measurements of blood pressure by tail-cuff and carotid arterial methods in conscious spontaneously hypertensive and Wistar-Kyoto rats. Clin. Exp. Hypertens. 2006, 28, 57–72. [Google Scholar] [CrossRef]
- da Palma Valério, M.; Martini, S.C.; da Silva Boschi, S.R.M.; Scardovelli, T.A.; da Silva, A.P. Non-invasive versus invasive method to measure blood pressure in rodents: An integrative literature review. Res. Soc. Dev. 2022, 11, e4911830789. [Google Scholar] [CrossRef]
- Russo, G.T.; De Cosmo, S.; Viazzi, F.; Mirijello, A.; Ceriello, A.; Guida, P.; Giorda, C.; Cucinotta, D.; Pontremoli, R.; Fioretto, P.; et al. Diabetic kidney disease in the elderly: Prevalence and clinical correlates. BMC Geriatr. 2018, 18, 38. [Google Scholar] [CrossRef]
- Chen, Y.; Kanwar, Y.S.; Chen, X.; Zhan, M. Aging and Diabetic Kidney Disease: Emerging Pathogenetic Mechanisms and Clinical Implications. Curr. Med. Chem. 2024, 31, 697–725. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, J.; Zhao, Y.; Du, D. Aging and chronic kidney disease: Epidemiology, therapy, management and the role of immunity. Clin. Kidney J. 2024, 17, sfae235. [Google Scholar] [CrossRef]
- Huxley, R.; Barzi, F.; Woodward, M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ 2006, 332, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Arnetz, L.; Ekberg, N.R.; Alvarsson, M. Sex differences in type 2 diabetes: Focus on disease course and outcomes. Diabetes Metab. Syndr. Obes. 2014, 7, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Raile, K.; Galler, A.; Hofer, S.; Herbst, A.; Dunstheimer, D.; Busch, P.; Holl, R.W. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: Effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007, 30, 2523–2528. [Google Scholar] [CrossRef]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R.; Group, U.S. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef]
- O’Seaghdha, C.M.; Hwang, S.J.; Upadhyay, A.; Meigs, J.B.; Fox, C.S. Predictors of incident albuminuria in the Framingham Offspring cohort. Am. J. Kidney Dis. 2010, 56, 852–860. [Google Scholar] [CrossRef]
- Schultz, C.J.; Konopelska-Bahu, T.; Dalton, R.N.; Carroll, T.A.; Stratton, I.; Gale, E.A.; Neil, A.; Dunger, D.B. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes Care 1999, 22, 495–502. [Google Scholar] [CrossRef]
- Yu, M.K.; Katon, W.; Young, B.A. Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology 2015, 20, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Krzentowski, G.; Albert, A.; Lefebvre, P.J. Factors predictive of nephropathy in DCCT Type 1 diabetic patients with good or poor metabolic control. Diabet. Med. 2003, 20, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Finne, P.; Reunanen, A.; Stenman, S.; Groop, P.H.; Gronhagen-Riska, C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005, 294, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Yanai, M.; Takeuchi, K.; Matsuyama, K.; Nitta, K.; Hayashi, K.; Takahashi, S. Sex differences in the prevalence, progression, and improvement of chronic kidney disease. Kidney Blood Press. Res. 2014, 39, 279–288. [Google Scholar] [CrossRef]
- Tofovic, S.P.; Dubey, R.K.; Jackson, E.K. 2-Hydroxyestradiol attenuates the development of obesity, the metabolic syndrome, and vascular and renal dysfunction in obese ZSF1 rats. J. Pharmacol. Exp. Ther. 2001, 299, 973–977. [Google Scholar] [CrossRef]
- Bergeron, R.; Mentor, J.S.; Cote, I.; Ngo Sock, E.T.; Rabasa-Lhoret, R.; Lavoie, J.M. Loss of ovarian estrogens causes only mild deterioration of glucose homeostasis in female ZDF rats preventable by voluntary running exercise. Horm. Metab. Res. 2014, 46, 774–781. [Google Scholar] [CrossRef]
- Gades, M.D.; Stern, J.S.; van Goor, H.; Nguyen, D.; Johnson, P.R.; Kaysen, G.A. Estrogen accelerates the development of renal disease in female obese Zucker rats. Kidney Int. 1998, 53, 130–135. [Google Scholar] [CrossRef][Green Version]
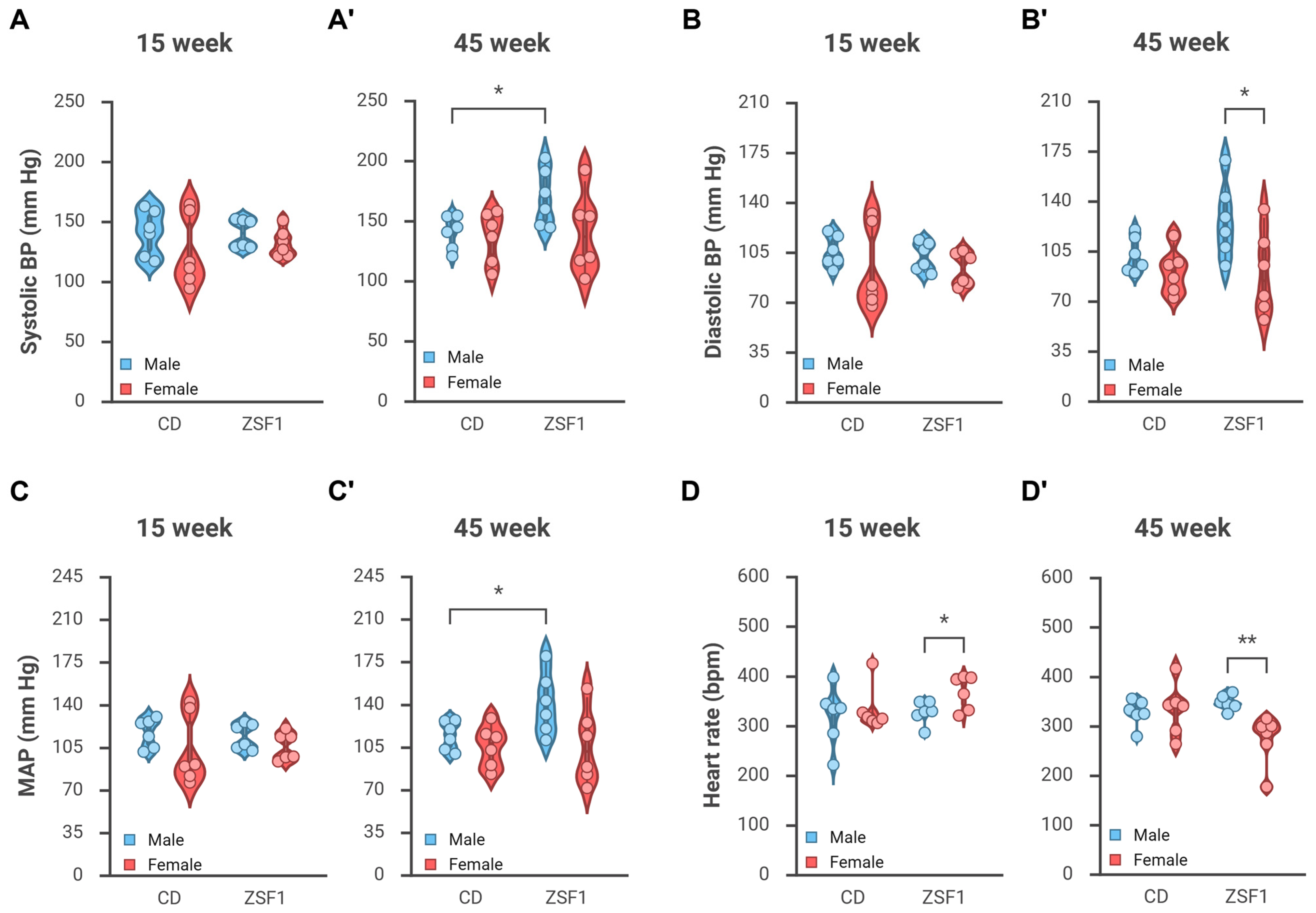
| Groups | 15 Weeks | 45 Weeks | p-Value * | |
|---|---|---|---|---|
| Systolic BP (mmHg) | CD male | 140.51 ± 7.72 | 139.98 ± 5.67 | 0.9524 |
| CD female | 124.50 ± 12.17 | 136.13 ± 8.73 | 0.4030 | |
| ZSF1 male | 139.96 ± 4.91 | 169.39 ± 9.69 | 0.0463 | |
| ZSF1 female | 132.10 ± 4.74 | 139.69 ± 13.60 | 0.6704 | |
| Diastolic BP (mmHg) | CD male | 105.42 ± 4.43 | 102.28 ± 5.11 | 0.2887 |
| CD female | 92.68 ± 11.82 | 90.68 ± 6.37 | 0.8846 | |
| ZSF1 male | 101.78 ± 4.07 | 126.67 ± 10.76 | 0.0749 | |
| ZSF1 female | 93.20 ± 4.76 | 89.35 ± 12.04 | 0.8070 | |
| MAP (mmHg) | CD male | 116.78 ± 4.91 | 114.49 ± 4.82 | 0.6137 |
| CD female | 102.90 ± 11.90 | 105.52 ± 6.97 | 0.8456 | |
| ZSF1 male | 114.16 ± 4.30 | 140.54 ± 10.36 | 0.0620 | |
| ZSF1 female | 105.84 ± 4.61 | 105.79 ± 12.50 | 0.9973 | |
| Heart rate (bpm) | CD male | 319.11 ± 24.48 | 326.50 ± 10.93 | 0.6728 |
| CD female | 332.74 ± 18.61 | 333.10 ± 21.44 | 0.9898 | |
| ZSF1 male | 326.63 ± 9.36 | 347.13 ± 6.21 | 0.1550 | |
| ZSF1 female | 367.00 ± 14.12 | 273.05 ± 20.54 | 0.0226 |
| Groups | 19 Weeks | 48 Weeks | p-Value * | |
|---|---|---|---|---|
| Average blood glucose levels (mg/dL) | CD male | 86.17 ± 19.98 | 64.17 ± 16.41 | 0.0356 |
| CD female | 74.33 ± 14.49 | 61.50 ± 7.01 | 0.1251 | |
| ZSF1 male | 337.5 ± 33.08 | 149.67 ± 80.47 | 0.0083 | |
| ZSF1 female | 94.50 ± 15.50 | 73.67 ± 10.07 | 0.0508 |
| Groups | 14 Weeks | 32 Weeks | 48 Weeks | |
|---|---|---|---|---|
| Proteinuria | CD male | ± | ± | ± |
| CD female | ± | ± | ± | |
| ZSF1 male | ++ | ++++ | ++++ | |
| ZSF1 female | ++ | ++++ | +++ | |
| Glucosuria | CD male | − | − | − |
| CD female | − | − | − | |
| ZSF1 male | +++ | ++++ | + | |
| ZSF1 female | + | + | + | |
| Leukocytes | CD male | − | NT | − |
| CD female | − | NT | − | |
| ZSF1 male | + | NT | +++ | |
| ZSF1 female | − | NT | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, A.; Prabhakar, S.S. An Overview of Sex-Based Differences in the Onset and Progression of DKD in the Well-Known Model, ZSF1 Rats. Life 2025, 15, 1627. https://doi.org/10.3390/life15101627
Chatterjee A, Prabhakar SS. An Overview of Sex-Based Differences in the Onset and Progression of DKD in the Well-Known Model, ZSF1 Rats. Life. 2025; 15(10):1627. https://doi.org/10.3390/life15101627
Chicago/Turabian StyleChatterjee, Arunita, and Sharma S. Prabhakar. 2025. "An Overview of Sex-Based Differences in the Onset and Progression of DKD in the Well-Known Model, ZSF1 Rats" Life 15, no. 10: 1627. https://doi.org/10.3390/life15101627
APA StyleChatterjee, A., & Prabhakar, S. S. (2025). An Overview of Sex-Based Differences in the Onset and Progression of DKD in the Well-Known Model, ZSF1 Rats. Life, 15(10), 1627. https://doi.org/10.3390/life15101627






