Brain Structures, Circuits, and Networks Involved in Immune Regulation, Periodontal Health, and Disease
Abstract
1. Introduction
2. The Dental Microflora-Brain Axis
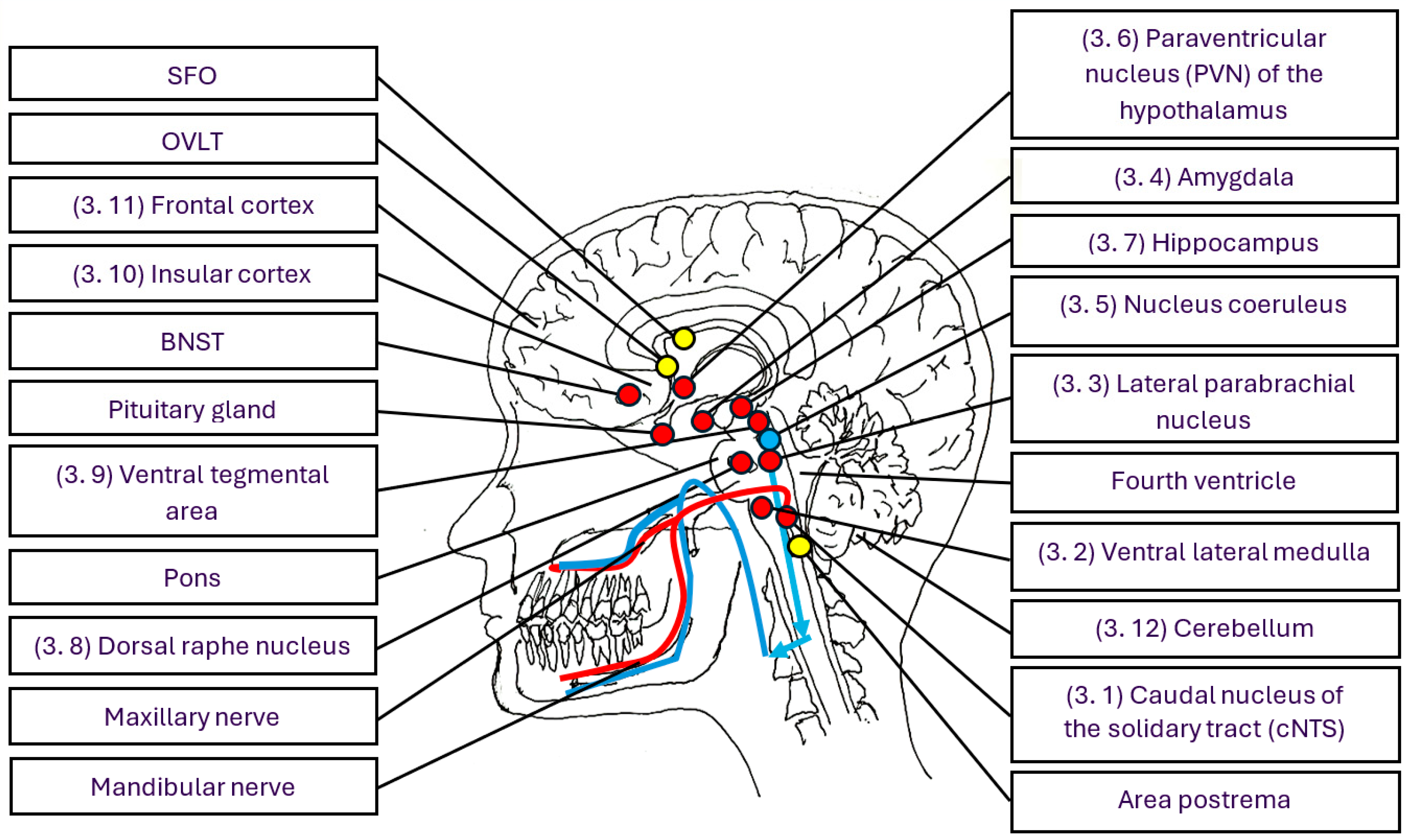
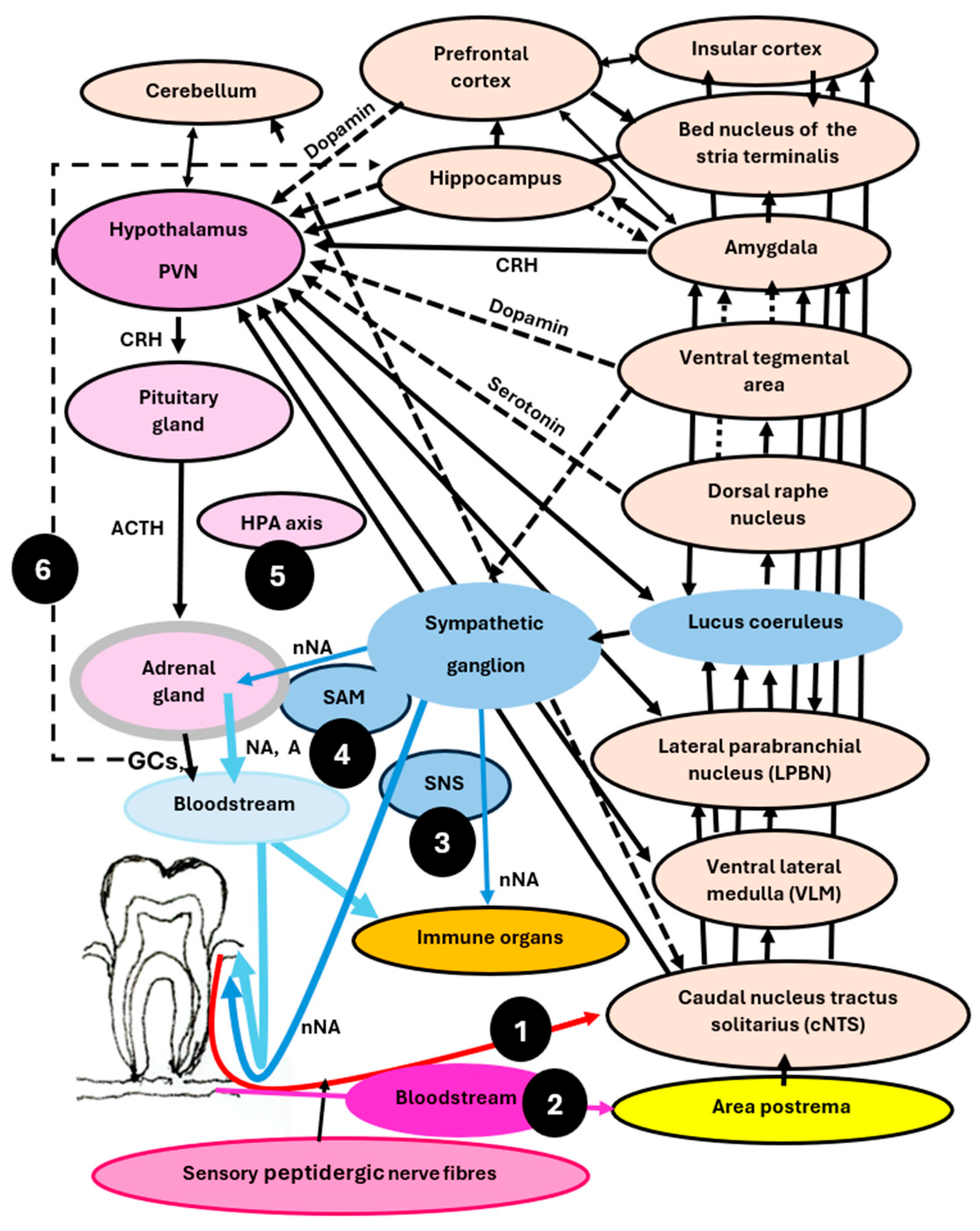
3. Brain Structures, Circuits, Networks, and Their Function in Immune Regulation, Periodontal Health, and Disease
3.1. The Caudal Nucleus of the Nucleus Tractus Solitarius (cNTS)—An Essential Node Processing Information from the Gingiva and Activating the Stress System
3.2. The Ventral Lateral Medulla—Regulating and Coordinating Stress and Immune Responses
3.3. The Lateral Parabrachial Nucleus—Serving as a Hub, Linking Information to Other Brain Regions Involved in Stress and Immune Regulation
3.4. The Amygdala—Alarming the Body When Sensing Pathobionts and Any Other Danger Signals
3.5. The Locus Coeruleus—Driving the Sympathetic Component of the Stress System, Crucial for Brain-Controlled Efferent Immune Regulation
3.6. The Hypothalamus—A Command Centre Regulating Stress and Immune Responses, Playing a Pivotal Role in Periodontal Health and Disease
3.7. Hippocampus—Determining Learning Outcomes, Memory, Navigation, Childhood Experiences, the Strength of Stress and Immune Responses, Periodontal Health and Disease
3.8. The Dorsal Raphe Nucleus—Regulating Stress and Immune Responses Through Serotonin and Is Crucial for the Onset of Depression and Periodontitis
3.9. The Ventral Tegmental Area—Involved in Rewards, Positive Emotions, Stress Responses, and Enhancement of Immune Defence Against Gram-Negative Bacteria and Cancer Cells by Dopamine
3.10. The Insular Cortex—Roles in Decision-Making, Information Storage, Immune Responses and Body Homeostasis
3.11. The Medial Prefrontal Cortex—Controlling Stress and Immune Responses and Playing a Role in Shift Work, Sleep Loss, Sleep Deprivation, and Periodontitis
3.12. The Cerebellum—Regulating Body Balance, Stress and Immune Responses, Emotions, Learning, and Memory
4. Neuroinflammation, Depression, Neurodegenerative Diseases, Oxidative Stress, and Periodontitis
5. Psychological Challenges, Coping Strategies, Periodontal Health and Disease
6. Microbiota–Immune–Brain Interactions and Clinical Consequences
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rey, A.; Besedovsky, H.O. Immune-neuro-endocrine reflexes, circuits, and networks: Physiologic and evolutionary implications. Front. Horm. Res. 2017, 48, 1–18. [Google Scholar]
- Danzer, R. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and functional neuroscience in immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, B.; Yuan, Y.; Zhang, L.; Hu, L.; Jin, S.; Kang, B.; Liao, X.; Sun, W.; Xu, F.; et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 2020, 581, 204–208. [Google Scholar] [CrossRef]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020, 10, 604179. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuroimmune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021, 14, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, J.; Zhang, W.; Liu, G.H. Stress, epigenetics, and ageing: Unraveling the intricate crosstalk. Mol. Cell 2024, 84, 34–54. [Google Scholar] [CrossRef]
- Iwamoto, S.; Iwai, S.-I.; Tsujiyama, K.; Kurahashi, C.; Takeshita, K.; Naoe, M.; Masunaga, A.; Ogawa, Y.; Oguchi, K.; Miyazaki, A. TNF-α Drives Human CD14+ Monocytes to Differentiate into CD70+ Dendritic Cells Evoking Th1 and Th17 Responses. J. Immunol. 2007, 179, 1449–1457. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.U. Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med. 2017, 49, e340. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, D.; Gambassi, G.; Piccirillo, C.A.; Cianci, R. The intricate link among gut “immunological niche,” microbiota, and xenobiotics in intestinal pathology. Mediat. Inflamm. 2017, 2017, 8390595. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, S.; Zhang, W.; Cui, H.; Zhang, J.; Yin, X.; Zheng, X.; Shen, T.; Ying, H.; Chen, L.; et al. Cordycepin mitigates dextran sulfate sodium-induced colitis through improving gut microbiota composition and modulating Th1/Th2 and Th17/Treg balance. Biomed. Pharmacother. 2024, 180, 117394. [Google Scholar] [CrossRef]
- Breivik, T.J.; Gjermo, P.; Gundersen, Y.; Opstad, P.K.; Murison, R.; Hugoson, A.; von Hörsten, S.; Fristad, I. Microbiota-immune-brain interactions: A new vision in the understanding of periodontal health and disease. Periodontology 2000 2024, 96, 20–41. [Google Scholar] [CrossRef]
- Breivik, T.; Thrane, P.S.; Gjermo, P.; Opstad, P.K.; Pabst, R.; von Hoersten, S. Hypothalamic-pituiary-adrenal (HPA) axis activation by experimental periodontal disease in rats. J. Periodontal. Res. 2001, 36, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Breivik, T.; Sluyter, F.; Hof, M.; Cools, A. Differential susceptibility to periodontitis in genetically selected Wistar rat lines that differ in their behavioral and endocrinological response to stressors. Behav. Genet. 2000, 30, 123–130. [Google Scholar] [CrossRef]
- Breivik, T.; Opstad, P.K.; Gjermo, P.; Thrane, P.S. Effects of hypothalamic-pituitary-adrenal axis reactivity on periodontal tissue destruction in rats. Eur. J. Oral Sci. 2000, 108, 115–122. [Google Scholar] [CrossRef]
- Breivik, T.; Thrane, P.S.; Gjermo, P.; Fonnum, F. Postnatal glutamate-induced central nervous system lesions alter periodontal disease susceptibility in adult Wistar rats. J. Clin. Periodontol. 2001, 28, 904–909. [Google Scholar] [CrossRef]
- Sluyter, F.; Breivik, T.; Cools, A. Manipulations in maternal environment reverse periodontitis in genetically predisposed rats. Clin. Diagn. Lab. Immunol. 2002, 9, 931–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breivik, T.; Stephan, M.; Brabant, G.E.; Straub, R.H.; Pabst, R.; von Hörsten, S. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav. Immun. 2002, 16, 421–438. [Google Scholar] [CrossRef]
- Breivik, T.; Gundersen, Y.; Osmundsen, H.; Fonnum, F.; Opstad, P.K. Neonatal dexamathasone and chronic tianeptine treatment inhibit ligature-induced periodontitis in adult rats. J. Periodontal. Res. 2006, 41, 23–34. [Google Scholar] [CrossRef]
- Breivik, T.; Gundersen, Y.; Murison, R.; Turner, J.D.; Muller, C.P.; Gjermo, P.; Opstad, K. Maternal Deprivation of Lewis Rat Pups Increases the Severity of Experi-mental Periodontitis in Adulthood. Open Dent. J. 2015, 30, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Breivik, T.; Gundersen, Y.; Gjermo, P.; Opstad, P.K. Chronic treatment with the glucocorticoid receptor antagonist RU486 inhibits diabetes-induced enhancement of experimental periodontitis. J. Periodontal. Res. 2014, 49, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Breivik, T.; Gundersen, Y.; Myhrer, T.; Fonnum, F.; Osmundsen, H.; Murison, R.; Gjermo, P.; von Hörsten, S.; Opstad, P.K. Enhanced susceptibility to periodontitis in an animal model of depression: Reversed by chronic treatment with the anti-depressant tianeptine. J. Clin. Periodontol. 2006, 33, 469–477. [Google Scholar] [CrossRef]
- Breivik, T.; Gundersen, Y.; Gjermo, P.; von Hörsten, S.; Opstad, P.K. Nicotinic acetylcholine receptor activation mediates nicotine-induced enhancement of experimental periodontitis. J. Periodontal. Res. 2009, 44, 110–116. [Google Scholar] [CrossRef]
- Breivik, T.; Thrane, P.S.; Gjermo, P.; Opstad, P.K. Glucocorticoid receptor antagonist RU 486 treatment reduces periodontitis in Fischer 344 rats. J. Periodontal. Res. 2000, 35, 285–290. [Google Scholar] [CrossRef]
- Breivik, T.; Bogen, I.L.; Haug, K.H.; Fonnum, F.; Opstad, P.K.; Eide, D.M.; Myhre, O. Effects of long-term exposure of 3,4-methylenedioxymethamphetamine (MDMA.; “ecstasy”) on neuronal transmitter transport, brain immuno-regulatory systems and progression of experimental periodontitis in rats. Neurochem. Int. 2014, 72, 30–36. [Google Scholar] [CrossRef]
- Breivik, T.; Rook, G.A.W. Prevaccination with SRL 172 (heat-killed Mycobacterium vaccae) inhibits experimental periodontal disease in Wistar rats. Clin. Exp. Immunol. 2000, 120, 463–467. [Google Scholar] [CrossRef]
- Breivik, T.; Rook, G.A.W. Oral treatment with SRP299 (killed Mycobacterium vaccae) inhibits experimental periodontal disease in Wistar rats. J. Clin. Periodontol. 2003, 30, 931–936. [Google Scholar] [CrossRef]
- Breivik, T.; Opstad, P.K.; Engstad, R.; Gundersen, G.; Gjermo, P.; Preus, H. Soluble beta-1,3/1,6-glucan from yeast inhibits experimental periodontal disease in Wistar rats. J. Clin. Periodontol. 2005, 32, 347–352. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L., 4th; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mazmanian, S.K. Microbiota-brain axis: Context and causality. Science 2022, 376, 938–939. [Google Scholar] [CrossRef]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef] [PubMed]
- Barrio, C.; Arias-Sánchez, S.; Martín-Monzón, I. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Dobrin, N.; Costea, D.; Glavan, L.A.; Covache-Busuioc, R.A.; Dumitrascu, D.I.; Bratu, B.G.; Costin, H.P.; Ciurea, A.V. Mind, Mood and Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 3340. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; O’Riordan, K.J.; Clarke, G.; Cryan, J.F. Feeding gut microbes to nourish the brain: Unravelling the diet-microbiota-gut-brain axis. Nat. Metab. 2024, 6, 1454–1478. [Google Scholar] [CrossRef]
- Hosang, L.; Flügel, A.; Odoardi, F. Body-brain axis: Orchestrating immune responses. Cell Res. 2024, 34, 757–758. [Google Scholar] [CrossRef]
- Herman, J.P.; Ostrander, M.M.; Mueller, N.K.; Figueiredo, H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1201–1213. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Stanojevic, S.; Kustrimovic, N.; Leposavic, G. Endpoint effector stress mediators in neuroimmune interactions: Their role in immune system homeostasis and autoimmune pathology. Immunol. Res. 2012, 52, 64–80. [Google Scholar] [CrossRef]
- Takahashi, A.; Flanigan, M.E.; McEwen, B.S.; Russo, S.J. Aggression, Social Stress, and the Immune System in Humans and Animal Models. Front. Behav. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2021, 11, e01960. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.M.; Ciotu, C.I.; Szallasi, A. The Mysteries of Capsaicin-Sensitive Afferents. Front. Physiol. 2020, 11, 554195. [Google Scholar] [CrossRef] [PubMed]
- Szőke, É.; Helyes, Z. Molecular Links between Sensory Nerves, Inflammation, and Pain 2.0. Int. J. Mol. Sci. 2023, 24, 12243. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Chen, O.; Ji, R.R. How Do Sensory Neurons Sense Danger Signals? Trends Neurosci. 2020, 43, 822–838. [Google Scholar] [CrossRef]
- Ferraz, C.C.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J. Endod. 2011, 37, 45–48. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Breivik, T.; Gundersen, Y.; Gjermo, P.; Fristad, I.; Opstad, P.K. Systemic chemical desensitization of peptidergic sensory neurons with resiniferatoxin inhibits experimental periodontitis. Open Dent. J. 2011, 5, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salvi, G.E.; Lang, N.P. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. Curr. Pharm. Des. 2005, 11, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Southall, M.D.; Vasko, M.R. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitisation of sensory neurons. J. Biol. Chem. 2001, 276, 16083–16091. [Google Scholar] [CrossRef]
- Brusentsova, A.E.; Lyashev, Y.D.; Tsygan, N.V.; Serikov, V.S. Disorders of the Biochemical Composition of the Periodontium in Rats with Periodontitis and Chronic Pain Syndrome. Bull. Exp. Biol. Med. 2022, 173, 14–16. [Google Scholar] [CrossRef]
- Miyata, S. Glial functions in the blood-brain communication at the circumventricular organs. Front. Neurosci. 2022, 16, 991779. [Google Scholar] [CrossRef]
- Jeong, J.K.; Dow, S.A.; Young, C.N. Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation. Metabolites 2021, 11, 494. [Google Scholar] [CrossRef]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Fawley, J.A.; Hegarty, D.M.; Aicher, S.A.; Beaumont, E.; Andresen, M.C. Dedicated C-fiber vagal sensory afferent pathways to the paraventricular nucleus of the hypothalamus. Brain Res. 2021, 1769, 147625. [Google Scholar] [CrossRef]
- Fukuwada, N.; Kanno, M.; Yoshida, S.; Seki, K. Gαq protein signaling in the bed nucleus of the stria terminalis regulate the lipopolysaccharide-induced despair-like behavior in mice. AIMS Neurosci. 2020, 7, 438–458. [Google Scholar] [CrossRef] [PubMed]
- Fristad, I.; Heyeraas, K.J.; Kvinnsland, I.H. Neuropeptide Y expression in the trigeminal ganglion and mandibular division of the trigeminal nerve after inferior alveolar nerve axotomy in young rats. Exp. Neurol. 1996, 142, 276–286. [Google Scholar] [CrossRef]
- Parker, T.L.; Kesse, W.K.; Mohamed, A.A.; Afework, M. The innervation of the mammalian adrenal gland. J. Anat. 1993, 183 Pt 2, 265–276. [Google Scholar]
- Wolff, L.F.; Aeppli, D.M.; Pihlstrom, B.; Anderson, L.; Stoltenberg, J.; Osborn, J.; Hardie, N.; Shelburne, C.; Fischer, G. Natural distribution of 5 bacteria associated with periodontal disease. J. Clin. Periodontol. 1993, 20, 699–706. [Google Scholar] [CrossRef]
- Lalla, R.V.; Patton, L.L.; Dongari-Bagtzoglou, A. Oral candidiasis: Pathogenesis, clinical presentation, diagnosis and treatment strategies. J. Calif. Dent. Assoc. 2013, 41, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community development between Porphyromonas gingivalis and Candida albicans mediated by InlJ and Als3. mBio 2018, 9, e00202-18. [Google Scholar] [CrossRef]
- Slots, J. Periodontal herpesviruses: Prevalence, pathogenicity, systemic risk. Periodontology 2000 2015, 69, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Mazmanian, S.K. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 2010, 7, 265–276. [Google Scholar] [CrossRef]
- Hornef, M. Pathogens, commensal symbionts, and pathobionts: Discovery and functional effects on the host. ILAR J. 2015, 56, 159–162. [Google Scholar] [CrossRef]
- Jiao, Y.; Hasegawa, M.; Inohara, N. The role of oral pathobionts in dysbiosis during periodontitis development. J. Dent. Res. 2014, 93, 539–546. [Google Scholar] [CrossRef]
- Gomez-Bris, R.; Saez, A.; Herrero-Fernandez, B.; Rius, C.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 2696. [Google Scholar] [CrossRef]
- Wei, D.; Ma, P.; Fan, Q.; Yu, H.; Peng, Y.; Li, X. Yanning Syrup ameliorates the lipopolysaccharide-induced inflammation: Adjusting the gut microbiota, short-chain fatty acids, and the CD4+ T cell balance. J. Ethnopharmacol. 2022, 283, 114729. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, J.; Xie, Y.; Xu, W.; Zhu, W.; Xia, L.; Fang, J.; Yu, D.; Liu, J.; Zheng, Z.; et al. Gegen Qinlian decoction ameliorates TNBS-induced ulcerative colitis by regulating Th2/Th1 and Tregs/Th17 cells balance, inhibiting NLRP3 inflammasome activation and reshaping gut microbiota. J. Ethnopharmacol. 2024, 328, 117956. [Google Scholar] [CrossRef]
- Sagar, S.M.; Price, K.J.; Kasting, N.W.; Sharp, F.R. Anatomic patterns of Fos immunostaining in rat brain following systemic endotoxin administration. Brain Res. Bull. 1995, 36, 381–392. [Google Scholar] [CrossRef]
- Kim, J.; Sullivan, O.; Lee, K.; Jao, J.; Tamayo, J.; Madany, A.M.; Wong, B.; Ashwood, P.; Ciernia, A.V. Repeated LPS induces training and tolerance of microglial responses across brain regions. J. Neuroinflamm. 2024, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef] [PubMed]
- Han, K.M.; Ham, B.J. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J. Clin. Neurol. 2021, 17, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Owoyele, P.V.; Malekzadeh, S. Porphyromonas gingivalis, neuroinflammation and Alzheimer’s disease. Niger. J. Physiol. Sci. 2022, 37, 157–164. [Google Scholar] [CrossRef]
- Ganchrow, D.; Ganchrow, J.R.; Cicchini, V.; Bartel, D.L.; Kaufman, D.; Girard, D.; Whitehead, M.C. Nucleus of the solitary tract in the C57BL/6J mouse: Subnuclear parcellation, chorda tympani nerve projections, and brainstem connections. J. Comp. Neurol. 2014, 522, 1565–1596. [Google Scholar] [CrossRef]
- Reyes, E.P.; Abarzúa, S.; Martin, A.; Rodríguez, J.; Cortés, P.P.; Fernández, R. LPS-induced c-Fos activation in NTS neurons and plasmatic cortisol increases in septic rats are suppressed by bilateral carotid chemodenervation. Adv. Exp. Med. Biol. 2012, 758, 185–190. [Google Scholar]
- Holt, M.K. The ins and outs of the caudal nucleus of the solitary tract: An overview of cellular populations and anatomical connections. J. Neuroendocrinol. 2022, 34, e13132. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Caraveo, A.; Sayd, A.; Robledo-Montaña, J.; Caso, J.R.; Madrigal, J.L.M.; García-Bueno, B.; Leza, J.C. Toll-like receptor 4 agonist and antagonist lipopolysaccharides modify the innate immune response in rat brain circumventricular organs. J. Neuroinflamm. 2020, 17, 6. [Google Scholar] [CrossRef]
- Jin, H.; Li, M.; Jeong, E.; Castro-Martinez, F.; Zuker, C.S. A body-brain circuit that regulates body inflammatory responses. Nature 2024, 630, 695–703. [Google Scholar] [CrossRef]
- Henry, C.J.; Huang, Y.; Wynne, A.M.; Godbout, J.P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 2009, 23, 309–317. [Google Scholar] [CrossRef]
- Ilanges, A.; Shiao, R.; Shaked, J.; Luo, J.D.; Yu, X.; Friedman, J.M. Brainstem ADCYAP1+ neurons control multiple aspects of sickness behaviour. Nature 2022, 609, 761–771. [Google Scholar] [CrossRef]
- Spooner, C.E.; Markowitz, N.P.; Saravolatz, L.D. The role of tumour necrosis factor in sepsis. Clin. Immunol. Immunopathol. 1992, 62 Pt 2, S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Jagot, F.; Gaston-Breton, R.; Choi, A.J.; Pascal, M.; Bourhy, L.; Dorado-Doncel, R.; Conzelmann, K.K.; Lledo, P.M.; Lepousez, G.; Eberl, G. The parabrachial nucleus elicits a vigorous corticosterone feedback response to the pro-inflammatory cytokine IL-1β. Neuron 2023, 111, 2367–2382.e6. [Google Scholar] [CrossRef]
- Du, J.; Wang, P.; Gou, Q.; Jin, S.; Xue, H.; Li, D.; Tian, D.; Sun, J.; Zhang, X.; Teng, X.; et al. Hydrogen sulfide ameliorated preeclampsia via suppression of toll-like receptor 4-activated inflammation in the rostral ventrolateral medulla of rats. Biomed. Pharmacother. 2022, 150, 113018. [Google Scholar] [CrossRef]
- Silva, T.M.; Takakura, A.C.; Moreira, T.S. Acute hypoxia activates hypothalamic paraventricular nucleus-projecting catecholaminergic neurons in the C1 region. Exp. Neurol. 2016, 285 Pt A, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci. 2018, 41, 280–293. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, J.; Zeng, R.; Zhao, X.; Gao, W.; Quan, J.; Hu, X.; Shen, Z.; Zhang, J. The Role of Prostaglandin E2 Synthesized in Rat Lateral Parabrachial Nucleus in LPS-Induced Fever. Neuroendocrinology 2022, 112, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Darmohra, D.; Yao, Y.; Sima, J.; Hao Chen, C.H.; Silverman, D.; Chen, C.; Dan, Y. Brainstem circuit for sickness-induced sleep. bioRxiv 2025. [Google Scholar] [CrossRef]
- Ma, H.; Li, C.; Wang, J.; Zhang, X.; Li, M.; Zhang, R.; Huang, Z.; Zhang, Y. Amygdala-hippocampal innervation modulates stress-induced depressive-like behaviors through AMPA receptors. Proc. Natl. Acad. Sci. USA 2021, 118, e2019409118. [Google Scholar] [CrossRef]
- Kang, S.J.; Liu, S.; Ye, M.; Kim, D.I.; Pao, G.M.; Copits, B.A.; Roberts, B.Z.; Lee, K.F.; Bruchas, M.R.; Han, S. A central alarm system that gates multi-sensory innate threat cues to the amygdala. Cell Rep. 2022, 40, 111222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.H.; Tu, J.L.; Li, X.H.; Hua, Q.; Liu, W.Z.; Liu, Y. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav. Immun. 2021, 91, 505–518. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, J.Y.; Holmes, A.; Pan, B.X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol. Psychiatry 2021, 89, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kim, J.; Tonegawa, S. Amygdala Reward Neurons Form and Store Fear Extinction Memory. Neuron 2020, 105, 1077–1093.e7. [Google Scholar] [CrossRef]
- Hu, R.K.; Zuo, Y.; Ly, T.; Wang, J.; Meera, P.; Wu, Y.E.; Hong, W. An amygdala-to-hypothalamus circuit for social reward. Nat. Neurosci. 2021, 24, 831–842. [Google Scholar] [CrossRef]
- Robson, M.J.; Quinlan, M.A.; Blakely, R.D. Immune system activation and depression: Roles of serotonin in the central nervous system and periphery. ACS Chem. Neurosci. 2017, 8, 932–942. [Google Scholar] [CrossRef]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Slavova, D.; Ortiz, V.; Blaise, M.; Bairachnaya, M.; Giros, B.; Isingrini, E. Role of the locus coeruleus-noradrenergic system in stress-related psychopathology and resilience: Clinical and pre-clinical evidences. Neurosci. Biobehav. Rev. 2024, 167, 105925. [Google Scholar] [CrossRef]
- Calcagni, E.; Elenkov, I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann. N. Y. Acad. Sci. 2006, 1069, 62–76. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2014, 182, 15–41. [Google Scholar] [CrossRef]
- Sharma, D.; Farrar, J.D. Adrenergic regulation of immune cell function and inflammation. Semin. Immunopathol. 2020, 42, 709–717. [Google Scholar] [CrossRef]
- Chhatar, S.; Lal, G. Role of adrenergic receptor signalling in neuroimmune communication. Curr. Res. Immunol. 2021, 2, 202–217. [Google Scholar] [CrossRef]
- McAlees, J.W.; Smith, L.T.; Erbe, R.S.; Jarjoura, D.; Ponzio, N.M.; Sanders, V.M. Epigenetic regulation of beta2-adrenergic receptor expression in T(H)1 and T(H)2 cells. Brain Behav. Immun. 2011, 25, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rui, X.X.; Shi, H.; Qiu, Y.H.; Peng, Y.P. Norepinephrine inhibits Th17 cells via beta2-adrenergic receptor (beta2-AR) signaling in a mouse model of rheumatoid arthritis. Med. Sci. Monit. 2018, 24, 1196–1204. [Google Scholar] [CrossRef]
- Lu, J.H.; Rui, X.X.; Wang, T.T.; Wang, X.Q.; Peng, Y.P.; Qiu, Y.H. Activation of β2-adrenergic Receptor Alleviates Collagen-induced Arthritis by Ameliorating Th17/Treg Imbalance. Iran. J. Immunol. 2023, 20, 16–25. [Google Scholar]
- Torrillas-de la Cal, A.; Torres-Sanchez, S.; Bravo, L.; Llorca-Torralba, M.; Garcia-Partida, J.A.; Arroba, A.I.; Berrocoso, E. Chemogenetic activation of locus coeruleus neurons ameliorates the severity of multiple sclerosis. J. Neuroinflamm. 2023, 20, 198. [Google Scholar] [CrossRef]
- Okada, Y.; Hamada, N.; Kim, Y.; Takahashi, Y.; Sasaguri, K.; Ozono, S.; Sato, S. Blockade of sympathetic β-receptors inhibits Porphyromonas gingivalis-induced alveolar bone loss in an experimental rat periodontitis model. Arch. Oral Biol. 2010, 55, 502–508. [Google Scholar] [CrossRef]
- Breivik, T.; Gundersen, Y.; Opstad, P.K.; Fonnum, F. Chemical sympathectomy inhibits periodontal disease in Fischer 344 rats. J. Periodontal. Res. 2005, 40, 325–330. [Google Scholar] [CrossRef]
- Safar, H.A.; Mustafa, A.S.; Amoudy, H.A.; El-Hashim, A. The effect of adjuvants and delivery systems on Th1, Th2, Th17 and Treg cytokine responses in mice immunized with Mycobacterium tuberculosis-specific proteins. PLoS ONE 2020, 15, e0228381. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, R.; Sato, T.; Hamamura, K.; Miyazawa, K.; Takeguchi, A.; Tabuchi, M.; Togari, A.; Goto, S. Guanabenz inhibits alveolar bone resorption in a rat model of periodontitis. J. Pharmacol. Sci. 2021, 147, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Han, H.J.; Choi, W.K.; Yoo, S.; Baek, S.; Lee, J. Immunomodulatory effects of intraoperative dexmedetomidine on T helper 1, T helper 2, T helper 17 and regulatory T cells cytokine levels and their balance: A prospective, randomised, double-blind, dose-response clinical study. BMC Anesthesiol. 2018, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Loudon, A.; Chawla, A. Immunity around the clock. Science 2016, 354, 999–1003. [Google Scholar] [CrossRef]
- Romanov, R.A.; Tretiakov, E.O.; Kastriti, M.E.; Zupancic, M.; Häring, M.; Korchynska, S.; Popadin, K.; Benevento, M.; Rebernik, P.; Lallemend, F.; et al. Molecular design of hypothalamus development. Nature 2020, 582, 246–252. [Google Scholar] [CrossRef]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Konsman, J.P.; Veeneman, J.; Combe, C.; Poole, S.; Luheshi, G.N.; Dantzer, R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur. J. Neurosci. 2008, 28, 2499–2510. [Google Scholar] [CrossRef]
- Jiang, Z.; Rajamanickam, S.; Justice, N.J. CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiol. Stress 2019, 11, 100192. [Google Scholar] [CrossRef]
- Bao, A.M.; Meynen, G.; Swaab, D.F. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brain Res. Rev. 2008, 57, 531–553. [Google Scholar] [CrossRef]
- Cavagni, J.; Soletti, A.C.; Gaio, E.J.; Rösing, C.K. The effect of dexamethasone in the pathogenesis of ligature-induced periodontal disease in Wistar rats. Braz. Oral Res. 2005, 19, 290–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Almeida, J.M.; Matheus, H.R.; Fiorin, L.G.; Furquim, E.M.A.; Gusman, D.J.R. Influence of immunosuppression on the progression of experimental periodontitis and on healthy periodontal tissue: A rat in vivo study. J. Dent. Res. Dent. Clin. Dent. Prospects 2021, 15, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zăgrean, A.M.; Zăgrean, L. The association between Diabetes mellitus and Depression. J. Med. Life 2016, 9, 120–125. [Google Scholar]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. N. Am. 2003, 32, 805–822. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontology 2000 2020, 83, 40–45. [Google Scholar] [CrossRef]
- Ingrosso, D.M.F.; Primavera, M.; Samvelyan, S.; Tagi, V.M.; Chiarelli, F. Stress and Diabetes Mellitus: Pathogenetic Mechanisms and Clinical Outcome. Horm. Res. Paediatr. 2023, 96, 34–43. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G. Chronic Stress and Diabetes Mellitus: Interwoven Pathologies. Curr. Diabetes Rev. 2020, 16, 546–556. [Google Scholar] [PubMed]
- Jiang, L.; Huang, Y.; Fang, M.; Chen, X.; Feng, D.; Liu, J.; Jiang, Q.; Tao, R. Dynamic changes of Th1/Th2/Th17 cytokines and hBD-2/3 in erosive oral lichen planus patients’ saliva before and after prednisone acetate treatment. Heliyon 2024, 10, e24043. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Esmaeili, M.H.; Bahari, B.; Salari, A.A. ATP-sensitive potassium channel inhibitor glibenclamide attenuates HPA axis hyperactivity and depression- and anxiety-related symptoms in a rat model of Alzheimer’s disease. Brain Res. Bull. 2018, 137, 265–276. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef]
- Madison, F.N.; Bingman, V.P.; Smulders, T.V.; Lattin, C.R. A bird’s eye view of the hippocampus beyond space: Behavioral, neuroanatomical, and neuroendocrine perspectives. Horm. Behav. 2024, 157, 105451. [Google Scholar] [CrossRef]
- Hashioka, S.; Inoue, K.; Miyaoka, T.; Hayashida, M.; Wake, R.; Oh-Nishi, A.; Inagaki, M. The Possible Causal Link of Periodontitis to Neuropsychiatric Disorders: More Than Psychosocial Mechanisms. Int. J. Mol. Sci. 2019, 20, 3723. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Qiu, Y.; Liu, Z. Periodontal disease and the risk of Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Psychogeriatrics 2021, 21, 813–825. [Google Scholar] [CrossRef]
- Breivik, T.; Thrane, P.S.; Gjermo, P.; Cools, A.; Myhrer, T. Effects of hippocampal lesioning on experimental periodontitis in Wistar rats. J. Periodontal. Res. 2002, 37, 360–365. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.J.; D’Errico, N.C.; Stees, J.; Hughes, D.A. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics 2012, 7, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Chalfun, G.; Reis, M.M.; de Oliveira, M.B.G.; de Araújo Brasil, A.; Dos Santos Salú, M.; da Cunha, A.J.L.A.; Prata-Barbosa, A.; de Magalhães-Barbosa, M.C. Perinatal stress and methylation of the NR3C1 gene in newborns: Systematic review. Epigenetics 2022, 17, 1003–1019. [Google Scholar] [CrossRef]
- Khader, Y.S.; Ta’ani, Q. Periodontal diseases and the risk of preterm birth and low birth weight: A meta-analysis. J. Periodontol. 2005, 76, 161–165. [Google Scholar] [CrossRef]
- Konopka, T.; Paradowska-Stolarz, A. Periodontitis and risk of preterm birth and low birth weight—A meta-analysis. Ginekol. Pol. 2012, 83, 446–453. [Google Scholar]
- Uwambaye, P.; Munyanshongore, C.; Rulisa, S.; Shiau, H.; Nuhu, A.; Kerr, M.S. Assessing the association between periodontitis and premature birth: A case-control study. BMC Pregnancy Childbirth 2021, 21, 204. [Google Scholar] [CrossRef]
- Sosnowski, D.W.; Booth, C.; York, T.P.; Amstadter, A.B.; Kliewer, W. Maternal prenatal stress and infant DNA methylation: A systematic review. Dev. Psychobiol. 2018, 60, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.; Jones, A.; Goulden, P.A. Birth weight, stress, and the metabolic syndrome in adult life. Ann. N. Y. Acad. Sci. 2006, 1083, 28–36. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in health and disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [PubMed]
- Palma-Gudiel, H.; Prather, A.A.; Lin, J.; Oxendine, J.D.; Guintivano, J.; Xia, K.; Rubinow, D.R.; Wolkowitz, O.; Epel, E.S.; Zannas, A.S. HPA axis regulation and epigenetic programming of immune-related genes in chronically stressed and non-stressed mid-life women. Brain Behav. Immun. 2020, 92, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.; Diorio, J.; Liu, D.; Meaney, M.J. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999, 286, 1155–1158. [Google Scholar] [CrossRef]
- Gold, P.W.; Chrousos, G.P. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol. Psychiatry 2002, 7, 254–275. [Google Scholar] [CrossRef]
- Johannsen, A.; Rydmark, I.; Söder, B.; Asberg, M. Gingival inflammation, increased periodontal pocket depth and elevated interleukin-6 in gingival crevicular fluid of depressed women on long-term sick leave. J. Periodontal. Res. 2007, 42, 546–552. [Google Scholar] [CrossRef]
- Warren, K.R.; Postolache, T.T.; Groer, M.E.; Pinjari, O.; Kelly, D.L.; Reynolds, M.A. Role of chronic stress and depression in periodontal diseases. Periodontology 2000 2014, 64, 127–138. [Google Scholar] [CrossRef]
- Sundararajan, S.; Muthukumar, S.; Rao, S.R. Relationship between depression and chronic periodontitis. J. Indian. Soc. Periodontol. 2015, 19, 294–296. [Google Scholar]
- Tanveer, S.; Afaq, A.; Alqutub, M.N.; Aldahiyan, N.; AlMubarak, A.M.; Shaikh, A.C.; Naseem, M.; Vohra, F.; Abduljabbar, T. Association of self-perceived psychological stress with the periodontal health of socially deprived women in shelter homes. Int. J. Environ. Res. Public Health 2021, 18, 5160. [Google Scholar] [CrossRef]
- Aldosari, M.; Helmi, M.; Kennedy, E.N.; Badamia, R.; Odani, S.; Agaku, I.; Vardavas, C. Depression, periodontitis, caries and missing teeth in the USA.; NHANES 2009–2014. Fam. Med. Commun. Health 2020, 8, e000583. [Google Scholar] [CrossRef] [PubMed]
- Decker, A.; Askar, H.; Tattan, M.; Taichman, R.; Wang, H.L. The assessment of stress, depression, and inflammation as a collective risk factor for periodontal diseases: A systematic review. Clin. Oral Investig. 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Postolache, T.T.; García-Bueno, B.; Leza, J.C.; Figuero, E.; Lowry, C.A.; Malan-Müller, S. The Role of the Oral Microbiota Related to Periodontal Diseases in Anxiety, Mood and Trauma- and Stress-Related Disorders. Front. Psychiatry 2022, 12, 814177. [Google Scholar] [CrossRef]
- Neupane, S.P.; Virtej, A.; Myhren, L.E.; Bull, V.H. Biomarkers common for inflammatory periodontal disease and depression: A systematic review. Brain Behav. Immun. Health 2022, 21, 100450. [Google Scholar] [CrossRef]
- Nagai, Y.; Kisaka, Y.; Nomura, K.; Nishitani, N.; Andoh, C.; Koda, M.; Kawai, H.; Seiriki, K.; Nagayasu, K.; Kasai, A.; et al. Dorsal raphe serotonergic neurons preferentially reactivate dorsal dentate gyrus cell ensembles associated with positive experience. Cell Rep. 2023, 42, 112149. [Google Scholar] [CrossRef]
- Hugoson, A.; Ljungquist, B.; Breivik, T. The relationship of some negative events and psychological factors to periodontal disease in an adult Swedish population 50 to 80 years of age. J. Clin. Periodontol. 2002, 29, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Baganz, N.L.; Blakely, R.D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 2013, 4, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in the activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Szałach, Ł.P.; Lisowska, K.A.; Cubała, W.J. The influence of antidepressants on the immune system. Arch. Immunol. Ther. Exp. 2019, 67, 143–151. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Role of 5-HT7 receptors in the immune system in health and disease. Mol. Med. 2019, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Martino, M.; Rocchi, G.; Escelsior, A.; Fornaro, M. Immunomodulation mechanism of antidepressants: Interactions between serotonin/norepinephrine balance and Th1/Th2 balance. Curr. Neuropharmacol. 2012, 10, 97–123. [Google Scholar] [CrossRef]
- Stasi, C.; Sadalla, S.; Milani, S. The relationship between the serotonin metabolism, gut-microbiota and the gut-brain axis. Curr. Drug Metab. 2019, 20, 646–655. [Google Scholar] [CrossRef]
- Baldwin, D.; Rudge, S. The role of serotonin in depression and anxiety. Int. Clin. Psychopharmacol. 1995, 9 (Suppl. S4), 41–45. [Google Scholar] [CrossRef]
- Branco-de-Almeida, L.S.; Franco, G.C.; Castro, M.L.; dos Santos, J.G.; Anbinder, A.L.; Cortelli, S.C.; Kajiya, M.; Kawai, T.; Rosalen, P.L. Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. J. Periodontol. 2012, 83, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Zhang, L.; Kirkwood, C.L.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Inhibition of acid sphingomyelinase by imipramine abolishes the synergy between metabolic syndrome and periodontitis on alveolar bone loss. J. Periodontal. Res. 2022, 57, 173–185. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.; Zhao, H.; Zhang, F.; Wang, J.; Liu, Y.; Lin, J.; Huang, Y.; Pan, W.; Qi, J.; et al. Midbrain FA initiates neuroinflammation and depression onset in both acute and chronic LPS-induced depressive model mice. Brain Behav. Immun. 2024, 117, 356–375. [Google Scholar] [CrossRef]
- Feldman, S.; Weidenfeld, J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res. Bull. 1998, 45, 389–393. [Google Scholar] [CrossRef]
- Nakajima, K.; Hamada, N.; Takahashi, Y.; Sasaguri, K.; Tsukinoki, K.; Umemoto, T.; Sato, S. Restraint stress enhances alveolar bone loss in an experimental rat model. J. Periodontal. Res. 2006, 41, 527–534. [Google Scholar] [CrossRef]
- Siebler, P.H.; Heinze, J.D.; Kienzle, D.M.; Hale, M.W.; Lukkes, J.L.; Donner, N.C.; Kopelman, J.M.; Rodriguez, O.A.; Lowry, C.A. Acute administration of the nonpathogenic, saprophytic bacterium, Mycobacterium vaccae, induces activation of serotonergic neurons in the dorsal raphe nucleus and antidepressant-like behavior in association with mild hypothermia. Cell Mol. Neurobiol. 2018, 38, 289–304. [Google Scholar] [CrossRef]
- Kayama, T.; Ikegaya, Y.; Sasaki, T. Phasic firing of dopaminergic neurons in the ventral tegmental area triggers peripheral immune responses. Sci. Rep. 2022, 12, 1447. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaanan, T.L.; Azulay-Debby, H.; Dubovik, T.; Starosvetsky, E.; Korin, B.; Schiller, M.; Green, N.L.; Admon, Y.; Hakim, F.; Shen-Orr, S.S.; et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 2016, 22, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaanan, T.L.; Schiller, M.; Azulay-Debby, H.; Korin, B.; Boshnak, N.; Koren, T.; Krot, M.; Shakya, J.; Rahat, M.A.; Hakim, F.; et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 2018, 9, 2723. [Google Scholar] [CrossRef]
- Kentner, A.C.; Takeuchi, A.; James, J.S.; Miki, T.; Seino, S.; Hayley, S.; Bielajew, C. The effects of rewarding ventral tegmental area stimulation and environmental enrichment on lipopolysaccharide-induced sickness behavior and cytokine expression in female rats. Brain Res. 2008, 1217, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Ronström, J.W.; Williams, S.B.; Payne, A.; Obray, D.J.; Hafen, C.; Burris, M.; Scott Weber, K.; Steffensen, S.C.; Yorgason, J.T. Interleukin-10 enhances the activity of ventral tegmental area dopamine neurons, resulting in increased dopamine release. Brain Behav. Immun. 2023, 113, 145–155. [Google Scholar] [CrossRef]
- Cerniauskas, I.; Winterer, J.; de Jong, J.W.; Lukacsovich, D.; Yang, H.; Khan, F.; Peck, J.R.; Obayashi, S.K.; Lilascharoen, V.; Lim, B.K.; et al. Chronic Stress Induces Activity, Synaptic, and Transcriptional Remodeling of the Lateral Habenula Associated with Deficits in Motivated Behaviors. Neuron 2019, 104, 899–915.e8. [Google Scholar] [CrossRef] [PubMed]
- Gogolla, N. The insular cortex. Curr. Biol. 2017, 27, R580–R586. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; Labrenz, F.; Kotulla, S.; Brotte, L.; Rödder, P.; Tebbe, B.; Theysohn, N.; Engler, H.; Elsenbruch, S. Amplified gut feelings under inflammation and depressed mood: A randomized fMRI trial on interoceptive pain in healthy volunteers. Brain Behav. Immun. 2023, 112, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef]
- Lekander, M.; Karshikoff, B.; Johansson, E.; Soop, A.; Fransson, P.; Lundström, J.N.; Andreasson, A.; Ingvar, M.; Petrovic, P.; Axelsson, J.; et al. Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain Behav. Immun. 2016, 56, 34–44. [Google Scholar] [CrossRef]
- Diaz-Castro, B.; Bernstein, A.M.; Coppola, G.; Sofroniew, M.V.; Khakh, B.S. Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep. 2021, 36, 109508. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Wada, Y.; Matsui, S.; Ohtsuki, G. Microglia-triggered hyperexcitability plasticity of pyramidal neurons in the rat medial prefrontal cortex. Curr. Res. Neurobiol. 2022, 3, 100028. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, X.X.; Jin, S.Y.; He, X.Y.; Li, X.W.; Yang, J.M.; Gao, T.M.; Chen, Y.H. Dopamine D2 receptors in pyramidal neurons in the medial prefrontal cortex regulate social behavior. Pharmacol. Res. 2024, 199, 107042. [Google Scholar] [CrossRef]
- McEwen, B.S.; Karatsoreos, I.N. Sleep Deprivation and Circadian Disruption: Stress, Allostasis, and Allostatic Load. Sleep Med. Clin. 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Iwasaki, M.; Usui, M.; Ariyoshi, W.; Nakashima, K.; Nagai-Yoshioka, Y.; Inoue, M.; Kobayashi, K.; Nishihara, T. Sleep duration and severe periodontitis in middle-aged Japanese workers. J. Clin. Periodontol. 2022, 49, 59–66. [Google Scholar] [CrossRef]
- Han, D.H.; Kim, M.S.; Kim, S.; Yoo, J.W.; Shen, J.J. Sleep time and duration are associated with periodontitis in a representative sample of Koreans. J. Periodontol. 2022, 93, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, X.; Duan, Z.; Wu, Y.; Shu, J.; Wu, P.; Zhao, Y.; Wang, X.; Wang, Y. Circadian rhythm disruption exacerbates the progression of macrophage dysfunction and alveolar bone loss in periodontitis. Int. Immunopharmacol. 2023, 116, 109796. [Google Scholar] [CrossRef]
- Nakada, T.; Kato, T.; Numabe, Y. Effects of fatigue from sleep deprivation on experimental periodontitis in rats. J. Periodontal. Res. 2015, 50, 131–137. [Google Scholar] [CrossRef]
- Bostan, S.A.; Yemenoglu, H.; Kose, O.; Akyildiz, K.; Mercantepe, T.; Saral, S.; Tumkaya, L.; Yilmaz, A. Preventive effects of melatonin on periodontal tissue destruction due to psychological stress in rats with experimentally induced periodontitis. J. Periodontal. Res. 2024, 59, 500–511. [Google Scholar] [CrossRef]
- Short, M.A.; Louca, M. Sleep deprivation leads to mood deficits in healthy adolescents. Sleep Med. 2015, 16, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.A.; Huecker, M.R. Sleep Deprivation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cao, D.; Zhao, Y.; Wang, Y.; Wei, D.; Yan, M.; Su, S.; Pan, H.; Wang, Q. Effects of sleep deprivation on anxiety-depressive-like behavior and neuroinflammation. Brain Res. 2024, 1836, 148916. [Google Scholar] [CrossRef]
- Palmer, C.A.; Bower, J.L.; Cho, K.W.; Clementi, M.A.; Lau, S.; Oosterhoff, B.; Alfano, C.A. Sleep loss and emotion: A systematic review and meta-analysis of over 50 years of experimental research. Psychol. Bull. 2024, 150, 440–463. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wei, R.M.; Feng, Y.Z.; Zhang, K.X.; Ge, Y.J.; Kong, X.Y.; Li, X.Y.; Chen, G.H. Sleep deprivation aggravates lipopolysaccharide-induced anxiety, depression and cognitive impairment: The role of pro-inflammatory cytokines and synaptic plasticity-associated proteins. J. Neuroimmunol. 2024, 386, 578252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Sun, J.; Fang, D.; Wang, Z.; Liu, Y. Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. Int. J. Mol. Sci. 2023, 24, 9603. [Google Scholar] [CrossRef]
- Guo, X.; Keenan, B.T.; Sarantopoulou, D.; Lim, D.C.; Lian, J.; Grant, G.R.; Pack, A.I. Age attenuates the transcriptional changes that occur with sleep in the medial prefrontal cortex. Aging Cell 2019, 18, e13021. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, X.; Li, Y.; Xi, K.; Han, Y.; Mao, H.; Ren, K.; Wang, W.; Wu, Z. TNF signaling pathway-mediated microglial activation in the PFC underlies acute paradoxical sleep deprivation-induced anxiety-like behaviors in mice. Brain Behav. Immun. 2022, 100, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Peyton, L.; Oliveros, A.; Tufvesson-Alm, M.; Schwieler, L.; Starski, P.; Engberg, G.; Erhardt, S.; Choi, D.S. Lipopolysaccharide Increases Cortical Kynurenic Acid and Deficits in Reference Memory in Mice. Int. J. Tryptophan Res. 2019, 12, 1178646919891169. [Google Scholar] [CrossRef]
- Horning, G.M.; Cohen, M.E. Necrotizing ulcerative gingivitis, periodontitis, and stomatitis: Clinical staging and predisposing factors. J. Periodontol. 1995, 66, 990–998. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Necrotizing Periodontal Diseases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- de Carvalho, M.; Swash, M. Upper and lower motor neuron neurophysiology and motor control. Handb. Clin. Neurol. 2023, 195, 17–29. [Google Scholar] [PubMed]
- Terpou, B.A.; Densmore, M.; Thome, J.; Frewen, P.; McKinnon, M.C.; Lanius, R.A. The Innate Alarm System and Subliminal Threat Presentation in Posttraumatic Stress Disorder: Neuroimaging of the Midbrain and Cerebellum. Chronic Stress 2019, 3, 2470547018821496. [Google Scholar] [CrossRef]
- Schutter, D.J. The cerebello-hypothalamic-pituitary-adrenal axis dysregulation hypothesis in depressive disorder. Med. Hypotheses 2012, 79, 779–783. [Google Scholar] [CrossRef]
- Rizzi, A.; Saccia, M.; Benagiano, V. Is the Cerebellum Involved in the Nervous Control of the Immune System Function? Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 546–557. [Google Scholar] [CrossRef]
- Aslankoc, R.; Savran, M.; Ozmen, O.; Asci, S. Hippocampus and cerebellum damage in sepsis induced by lipopolysaccharide in aged rats-Pregabalin can prevent damage. Biomed. Pharmacother. 2018, 108, 1384–1392. [Google Scholar] [CrossRef]
- Johnson, J. Effect of emotions on learning, memory, and disorders associated with the changes in expression levels: A narrative review. Brain Circ. 2024, 10, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Sanderson, D.J. Malaise in the water maze: Untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav. Immun. 2008, 22, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, I.R.; Cloutier, C.J.; Tyson, C.D.; Matic, V.M.; Kavaliers, M.; Ossenkopp, K.P. Infection, learning, and memory: Focus on immune activation and aversive conditioning. Neurosci. Biobehav. Rev. 2022, 142, 104898. [Google Scholar] [CrossRef]
- Koren, T.; Rolls, A. Immunoception: Defining brain-regulated immunity. Neuron 2022, 110, 3425–3428. [Google Scholar] [CrossRef]
- Fields, R.D.; Stevens-Graham, B. New insights into neuron-glia communication. Science 2002, 298, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef]
- Miyao, M.; Hirotsu, A.; Tatsumi, K.; Tanaka, T. Prior exposure to stress exacerbates neuroinflammation and causes long-term behavior changes in sepsis. Heliyon 2023, 9, e16904. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef]
- Sulhan, S.; Lyon, K.A.; Shapiro, L.A.; Huang, J.H. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: Pathophysiology and potential therapeutic targets. J. Neurosci. Res. 2020, 98, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.Y.; Hong, D.Y.; Lee, E.C.; Park, S.W.; Lee, Y.K.; Oh, J.S. Pharmacological Treatment for Neuroinflammation in Stress-Related Disorder. Biomedicines 2022, 10, 2518. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Bari, B.A.; Chokshi, V.; Schmidt, K. Locus coeruleus-norepinephrine: Basic functions and insights into Parkinson’s disease. Neural. Regen. Res. 2020, 15, 1006–1013. [Google Scholar] [CrossRef]
- Wang, Q.; Oyarzabal, E.A.; Song, S.; Wilson, B.; Santos, J.H.; Hong, J.S. Locus coeruleus neurons are most sensitive to chronic neuroinflammation-induced neurodegeneration. Brain Behav. Immun. 2020, 87, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Wu, Y.; Zhou, Y.; Ju, L.; Liu, Y.; Ju, R.; Duan, D.; Xu, Q. Lesion of the locus coeruleus aggravates dopaminergic neuron degeneration by modulating microglial function in mouse models of Parkinson’s disease. Brain Res. 2015, 1625, 255–274. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Mittli, D.; Tukacs, V.; Ravasz, L.; Csősz, É.; Kozma, T.; Kardos, J.; Juhász, G.; Kékesi, K.A. LPS-induced acute neuroinflammation, involving interleukin-1 beta signalling, causes proteomic, cellular, and network-level changes in the prefrontal cortex of mice. Brain Behav. Immun.-Health 2023, 28, 100594. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Zhang, X.; Chen, H.; Dong, J.; Lu, W.; Song, Z.; Zhou, W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflamm. 2018, 15, 37. [Google Scholar] [CrossRef]
- Yamawaki, Y.; So, H.; Oue, K.; Asano, S.; Furusho, H.; Miyauchi, M.; Tanimoto, K.; Kanematsu, T. Imipramine prevents Porphyromonas gingivalis lipopolysaccharide-induced microglial neurotoxicity. Biochem. Biophys. Res. Commun. 2022, 634, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Kang, X.N.; Cao, Y.; Zheng, D.-X.; Lu, Y.-M.; Pang, C.-F.; Wang, Z.; Cheng, B.; Peng, Y. Porphyromonas gingivalis induces depression via downregulating p75NTR-mediated BDNF maturation in astrocytes. Brain Behav. Immun. 2019, 81, 523–534. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF; Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Sohroforouzani, A.M.; Shakerian, S.; Ghanbarzadeh, M.; Alaei, H. Effect of forced treadmill exercise on stimulation of BDNF expression, depression symptoms, tactile memory and working memory in LPS-treated rats. Behav. Brain Res. 2022, 418, 113645. [Google Scholar] [CrossRef]
- Song, T.; Song, X.; Zhu, C.; Patrick, R.; Skurla, M.; Santangelo, I.; Green, M.; Harper, D.; Ren, B.; Forester, B.P.; et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 2021, 72, 101503. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Jackson, T.C.; Kotermanski, S.E.; Kochanek, P.M.; Jackson, E.K. Oxidative stress induces release of 2′-AMP from microglia. Brain Res. 2019, 1706, 101–109. [Google Scholar] [CrossRef]
- Verma, A.; Azhar, G.; Zhang, X.; Patyal, P.; Kc, G.; Sharma, S.; Che, Y.; Wei, J.Y. P. gingivalis-LPS Induces Mitochondrial Dysfunction Mediated by Neuroinflammation through Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 950. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug. Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Jin, H.J.; Xue, Y.; Chen, G.; Wu, Z.Y. Effect of coenzyme Q10 on the expression of tumor necrosis factor-α and interleukin-10 in gingival tissue of experimental periodontitis in rats. Zhonghua Kou Qiang Yi Xue Za Zhi 2013, 48, 660–663. [Google Scholar]
- Merle, C.L.; Lenzen, C.; Schmalz, G.; Ziebolz, D. Systematic Review on Protocols of Coenzyme Q10 Supplementation in Non-Surgical Periodontitis Therapy. Nutrients 2023, 15, 1585. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, H.; Mat, A.F.; Ozhan, Y.; Aydin, A. The Interrelation between Oxidative Stress, Depression and Inflammation through the Kynurenine Pathway. Curr. Top. Med. Chem. 2023, 23, 415–425. [Google Scholar] [CrossRef]
- Cunha, F.A.; Cota, L.O.M.; Cortelli, S.C.; Miranda, T.B.; Neves, F.S.; Cortelli, J.R.; Costa, F.O. Periodontal condition and levels of bacteria associated with periodontitis in individuals with bipolar affective disorders: A case-control study. J. Periodontal. Res. 2019, 54, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Hammen, C. Risk factors for depression: An autobiographical review. Annu. Rev. Clin. Psychol. 2018, 14, 1–28. [Google Scholar] [CrossRef]
- Reyes-Martínez, S.; Segura-Real, L.; Gómez-García, A.P.; Tesoro-Cruz, E.; Constantino-Jonapa, L.A.; Amedei, A.; Aguirre-García, M.M. Neuroinflammation, Microbiota-Gut-Brain Axis, and Depression: The Vicious Circle. J. Integr. Neurosci. 2023, 22, 65. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Oršolić, N.; Karlović, D.; Peitl, V. Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 6888. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; de Jonge, P.; Beekman, A.T.; Penninx, B.W. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 2013, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W.; Chrousos, G.P. Melancholic and atypical subtypes of depression represent distinct pathophysiological entities: CRH.; neural circuits, and the diathesis for anxiety and depression. Mol. Psychiatry 2013, 18, 632–634. [Google Scholar] [CrossRef]
- Dunjic-Kostic, B.; Ivkovic, M.; Radonjic, N.V.; Petronijevic, N.D.; Pantovic, M.; Damjanovic, A.; Poznanovic, S.T.; Jovanovic, A.; Nikolic, T.; Jasovic-Gasic, M. Melancholic and atypical major depression–connection between cytokines, psychopathology and treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 1–6. [Google Scholar] [CrossRef]
- Genco, R.J.; Ho, A.W.; Grossi, S.G.; Dunford, R.G.; Tedesco, L.A. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. J. Periodontol. 1999, 70, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, G.; Janda, M.; Wieselmann-Penkner, K.; Jakse, N.; Polansky, R.; Pertl, C. Coping with stress: Its influence on periodontal disease. J. Periodontol. 2002, 73, 1343–1351. [Google Scholar] [CrossRef]
- Islam, M.M.; Ekuni, D.; Yoneda, T.; Yokoi, A.; Morita, M. Influence of occupational stress and coping style on periodontitis among Japanese workers: A cross-sectional study. Int. J. Environ. Res. Public Health 2019, 16, 3540. [Google Scholar] [CrossRef] [PubMed]
- Aragão, W.A.B.; Souza-Monteiro, D.; Frazão, D.R.; Né, Y.G.D.S.; Ferreira, R.D.O.; Rivera, L.F.S.; Saito, M.T.; Rösing, C.K.; Fagundes, N.C.F.; Maia, L.C.; et al. Is there any association between chronic periodontitis and anxiety in adults? A systematic review. Front. Psychiatry 2021, 12, 710606. [Google Scholar] [CrossRef]
- Breivik, T.; Thrane, P.S.; Murison, R.; Gjermo, P. Emotional stress effects on immunity, gingivitis and periodontitis. Eur. J. Oral Sci. 1996, 104 Pt 1, 327–334. [Google Scholar] [CrossRef]
- Breivik, T.; Thrane, P.S. Chapter 63: Psychoneuroimmune interactions in periodontal disease. In Psychoneuroimmunology, 3rd ed.; Ader, R., Felten, D.L., Cohen, N., Eds.; Academic Press: New York, NY, USA, 2000; pp. 627–644. [Google Scholar]
- Folkman, S.; Moskowitz, J.T. Coping: Pitfalls and promise. Annu. Rev. Psychol. 2004, 55, 745–774. [Google Scholar] [CrossRef]
- Braun-Lewensohn, O.; Mayer, C.H. Salutogenesis and Coping: Ways to Overcome Stress and Conflict. Int. J. Environ. Res. Public Health 2020, 17, 6667. [Google Scholar] [CrossRef]
- Algorani, E.B.; Gupta, V. Coping Mechanisms. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wood, S.K.; Bhatnagar, S. Resilience to the effects of social stress: Evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol. Stress 2015, 1, 164–173. [Google Scholar] [CrossRef]
- de Boer, S.F.; Buwalda, B.; Koolhaas, J.M. Untangling the neurobiology of coping styles in rodents: Towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci. Biobehav. Rev. 2017, 74 Pt B, 401–422. [Google Scholar] [CrossRef]
- Deo, V.; Bhongade, M.L. Pathogenesis of periodontitis: Role of cytokines in host response. Dent. Today 2010, 29, 60–62. [Google Scholar] [PubMed]
- Chapple, I.L.; Van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Buschdorf, J.P.; Meaney, M.J. Epigenetics/programming in the HPA axis. Compr. Physiol. 2015, 6, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Finken, M.J.J.; van der Voorn, B.; Hollanders, J.J.; Ruys, C.A.; de Waard, M.; van Goudoever, J.B.; Rotteveel, J. Programming of the hypothalamus-pituitary-adrenal axis by very preterm birth. Ann. Nutr. Metab. 2017, 70, 170–174. [Google Scholar] [CrossRef]
- Suchecki, D. Maternal regulation of the infant’s hypothalamic-pituitary-adrenal axis stress response: Seymour ‘Gig’ Levine’s legacy to neuroendocrinology. J. Neuroendocrinol. 2018, 30, e12610. [Google Scholar] [CrossRef] [PubMed]
- van Seventer, J.M.; Hochberg, N.S. Principles of Infectious Diseases: Transmission, Diagnosis, Prevention, and Control. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 22–39. [Google Scholar] [CrossRef]
- Shaler, C.R.; Parco, A.A.; Elhenawy, W.; Dourka, J.; Jury, J.; Verdu, E.F.; Coombes, B.K. Psychological stress impairs IL22-driven protective gut mucosal immunity against colonising pathobionts. Nat. Commun. 2021, 12, 6664. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.L.; Barnich, N.; Bringer, M.A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Dufty, J.; Gkranias, N.; Petrie, A.; McCormick, R.; Elmer, T.; Donos, N. Prevalence and treatment of necrotising ulcerative gingivitis (NUG) in the British Armed Forces: A case-control study. Clin. Oral Investig. 2017, 21, 1935–1944. [Google Scholar] [CrossRef]
- Kanwar, B.; Favre, D.; McCune, J.M. Th17 and regulatory T cells: Implications for AIDS pathogenesis. Curr. Opin. HIV AIDS 2010, 5, 151–157. [Google Scholar] [CrossRef]
- Planas, D.; Routy, J.P.; Ancuta, P. New Th17-specific therapeutic strategies for HIV remission. Curr. Opin. HIV AIDS 2019, 14, 85–92. [Google Scholar] [CrossRef]
- Godbout, J.P.; Glaser, R. Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. J. Neuroimmune Pharmacol. 2006, 1, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Zhang, X.; Zhao, L.; Zhao, X.; Li, Z.; Song, T.; Huang, C. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem. Biophys. Res. Commun. 2013, 439, 471–476. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Kubberød, J.O.; Torgersen, G.R.; Gjermo, P.; Baelum, V.; Preus, H.R. Five-year radiological findings from a randomized controlled trial of four periodontitis treatment strategies. Eur. J. Oral Sci. 2023, 131, e12949. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558 Pt 1, 263–275. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Mistry, R.; Kounatidis, I.; Ligoxygakis, P. Interaction between familial transmission and a constitutively active immune system gut microbiota in Drosophila melanogaster. Genetics 2017, 206, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Dianan, T.G.; Cryan, J.F. Microbes, immunity, and behavior: Psychoneuroimmunology meets the microbiome. Neuropsychopharmacology 2017, 42, 178–192. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Baptista, A.P.; Tamoutounour, S.; Zhuang, L.; Bouladoux, N.; Martins, A.J.; Huang, Y.; Gerner, M.Y.; Belkaid, Y.; Germain, R.N. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 2018, 554, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breivik, T.J.; Gjermo, P.; Opstad, P.K.; Murison, R.; von Hörsten, S.; Fristad, I. Brain Structures, Circuits, and Networks Involved in Immune Regulation, Periodontal Health, and Disease. Life 2025, 15, 1572. https://doi.org/10.3390/life15101572
Breivik TJ, Gjermo P, Opstad PK, Murison R, von Hörsten S, Fristad I. Brain Structures, Circuits, and Networks Involved in Immune Regulation, Periodontal Health, and Disease. Life. 2025; 15(10):1572. https://doi.org/10.3390/life15101572
Chicago/Turabian StyleBreivik, Torbjørn Jarle, Per Gjermo, Per Kristian Opstad, Robert Murison, Stephan von Hörsten, and Inge Fristad. 2025. "Brain Structures, Circuits, and Networks Involved in Immune Regulation, Periodontal Health, and Disease" Life 15, no. 10: 1572. https://doi.org/10.3390/life15101572
APA StyleBreivik, T. J., Gjermo, P., Opstad, P. K., Murison, R., von Hörsten, S., & Fristad, I. (2025). Brain Structures, Circuits, and Networks Involved in Immune Regulation, Periodontal Health, and Disease. Life, 15(10), 1572. https://doi.org/10.3390/life15101572







