Breast Cancer Treatments: Drugs Targeting the PI3K/AKT/mTOR Pathway, TNBC Therapy and Future Directions: A Review
Abstract
1. Introduction
2. Selected Therapeutic Approaches in Breast Cancer
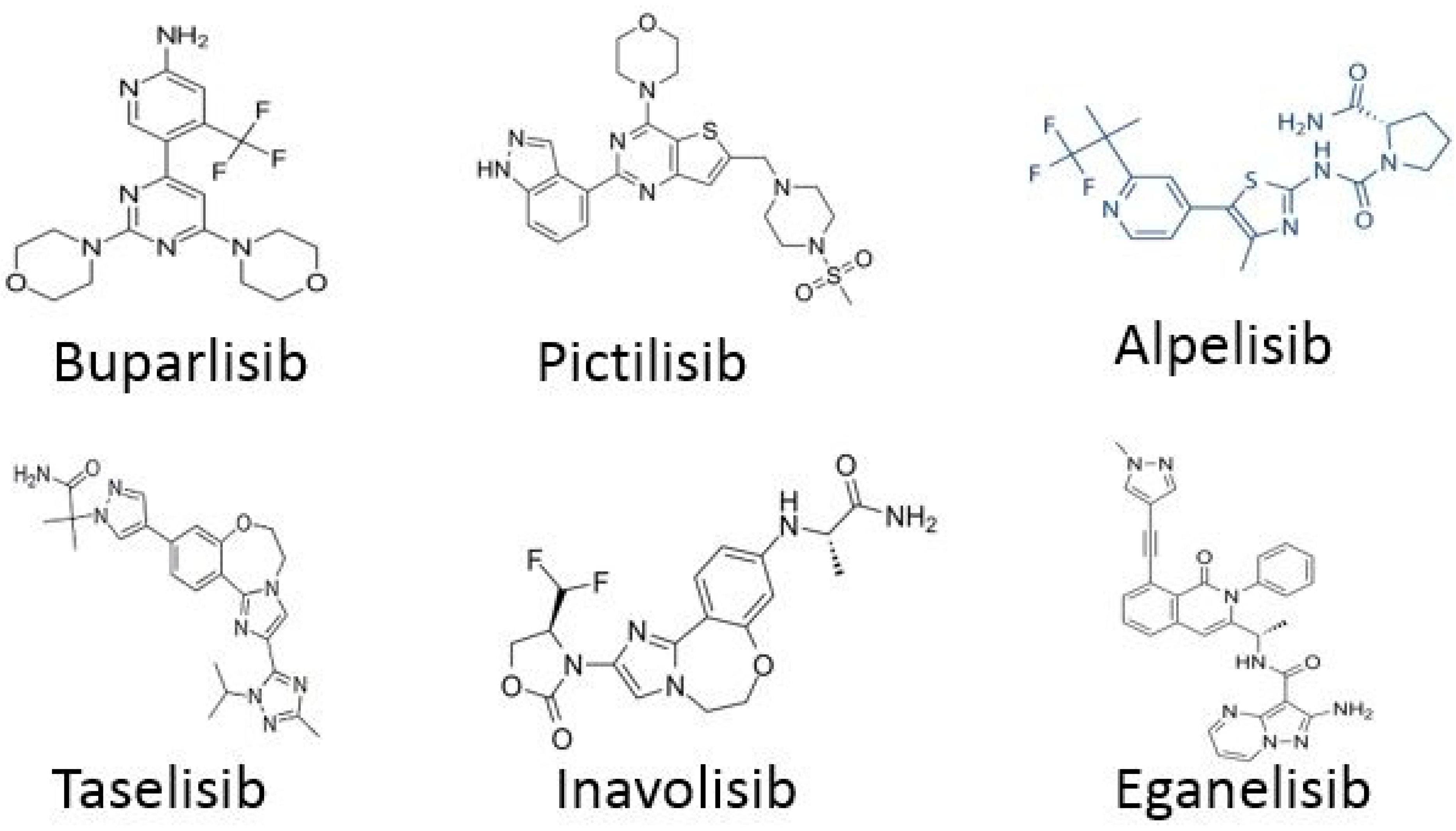
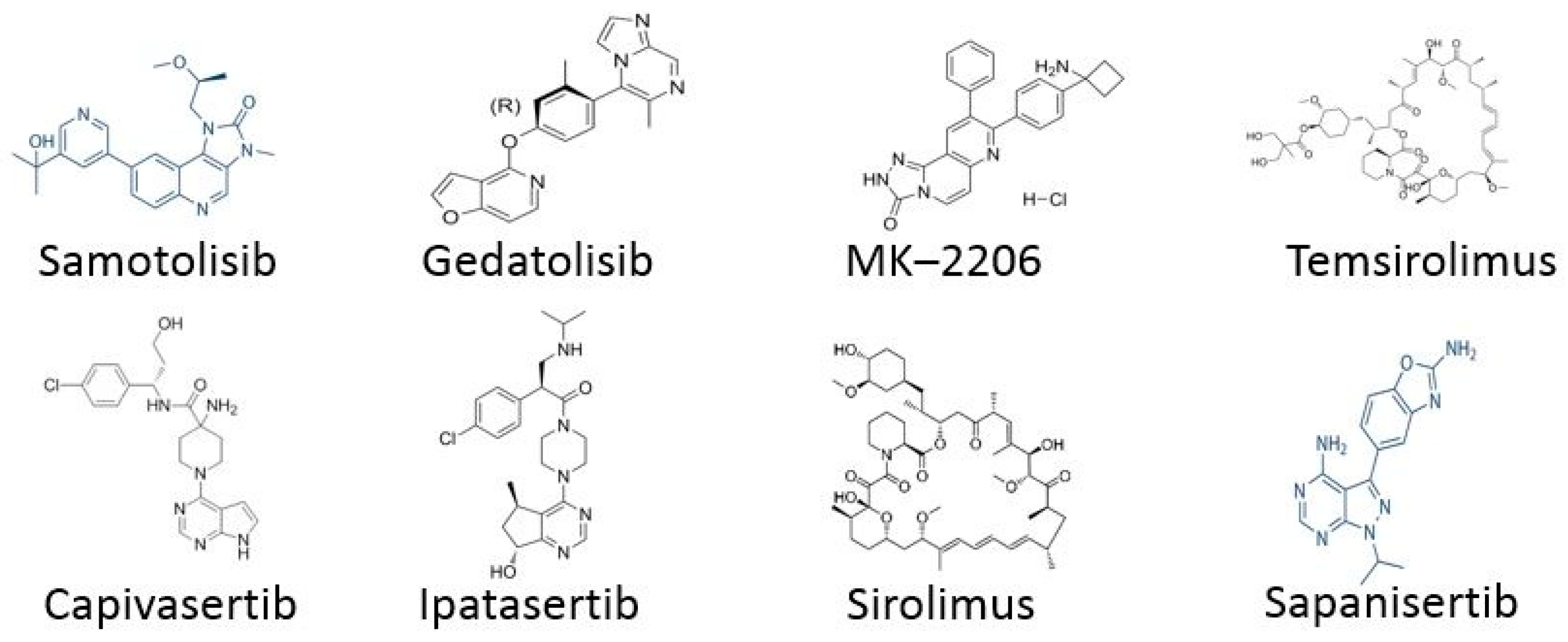
2.1. Targeting PI3K/AKT/mTOR Pathway
TNBC Drugs
- For TNBC, the following drugs are used: Samotolisib and Ipatasertib.
2.2. Anti-HER2 Therapy
2.3. Immunotherapy
2.4. TNBC Cancer Therapy
2.5. Limitations and Challenges
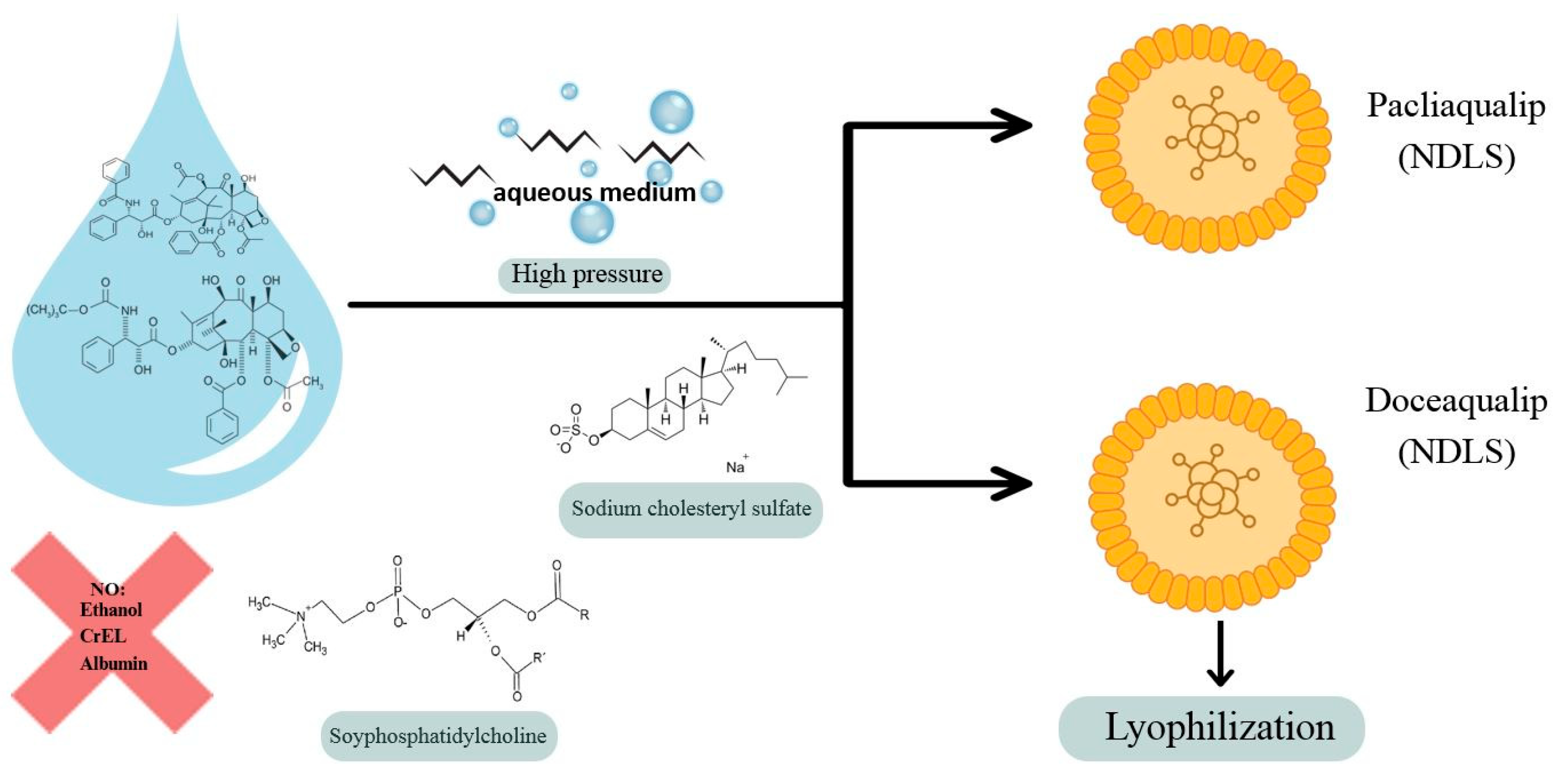
3. Future Research Directions in Breast Cancer Treatment—Nanomedicine
Nanoaqualip Technology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nwosu, N. Cancer: A Disease of Modern Times? Cureus 2024, 16, e74666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients. 2024, 16, 800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotsifaki, A.; Maroulaki, S.; Armakolas, A. Exploring the Immunological Profile in Breast Cancer: Recent Advances in Diagnosis and Prognosis through Circulating Tumor Cells. Int. J. Mol. Sci. 2024, 25, 4832. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer 2023, 15, 721–730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Rossi, L.; Mazzara, C.; Pagani, O. Diagnosis and Treatment of Breast Cancer in Young Women. Curr. Treat. Options Oncol. 2019, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Habibi, S.; Bahramian, S.; Zare Jalise, S.; Mehri, S.; Ababzadeh, S.; Kavianpour, M. Novel strategies in breast cancer management: From treatment to long-term remission. Crit. Rev. Oncol. Hematol. 2025, 211, 104715. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, Q.; Khan, M.U.; Fatima, T.; Khalid, S.; Malik, Z.I. Recent Insights Into Breast Cancer: Molecular Pathways, Epigenetic Regulation, and Emerging Targeted Therapies. Breast Cancer 2025, 19, 1–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.; Graff, S.L.; Wang, Y. New Emerging Therapies Targeting PI3K/AKT/mTOR/PTEN Pathway in Hormonal Receptor-Positive and HER2-Negative Breast Cancer-Current State and Molecular Pathology Perspective. Cancers 2024, 17, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stanciu, I.M.; Parosanu, A.I.; Orlov-Slavu, C.; Iaciu, I.C.; Popa, A.M.; Olaru, C.M.; Pirlog, C.F.; Vrabie, R.C.; Nitipir, C. Mechanisms of Resistance to CDK4/6 Inhibitors and Predictive Biomarkers of Response in HR+/HER2-Metastatic Breast Cancer-A Review of the Literature. Diagnostics 2023, 13, 987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Rampioni Vinciguerra, G.L.; Sonego, M.; Segatto, I.; Dall’Acqua, A.; Vecchione, A.; Baldassarre, G.; Belletti, B. CDK4/6 Inhibitors in Combination Therapies: Better in Company Than Alone: A Mini Review. Front. Oncol. 2022, 12, 891580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piezzo, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Gioia, G.D.; Lauro, V.D.; Fusco, G.; Martinelli, C.; Nuzzo, F.; Pensabene, M.; et al. Targeting Cell Cycle in Breast Cancer: CDK4/6 Inhibitors. Int. J. Mol. Sci. 2020, 21, 6479. [Google Scholar] [CrossRef]
- Choudhary, N.; Bawari, S.; Burcher, J.T.; Sinha, D.; Tewari, D.; Bishayee, A. Targeting Cell Signaling Pathways in Lung Cancer by Bioactive Phytocompounds. Cancers 2023, 15, 3980. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Molecular targets for the treatment of pancreatic cancer: Clinical and experimental studies. World J. Gastroenterol. 2016, 22, 776–789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, W.; Wang, H.; Li, C. Signal pathways of melanoma and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01610284 (accessed on 20 May 2025).
- Campone, M.; Im, S.A.; Iwata, H.; Clemons, M.; Ito, Y.; Awada, A.; Chia, S.; Jagiełło-Gruszfeld, A.; Pistilli, B.; Tseng, L.M.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur. J. Cancer 2018, 103, 147–154. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01633060 (accessed on 20 May 2025).
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lonning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.A.; Kalev, D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar]
- Krop, I.E.; Mayer, I.A.; Ganju, V.; Dickler, M.; Johnston, S.; Morales, S.; Yardley, D.A.; Melichar, B.; Forero-Torres, A.; Lee, S.C.; et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 811–821. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01437566 (accessed on 20 May 2025).
- Schmid, P.; Pinder, S.E.; Wheatley, D.; Macaskill, J.; Zammit, C.; Hu, J.; Price, R.; Bundred, N.; Hadad, S.; Shia, A.; et al. Phase II Randomized Preoperative Window-of-Opportunity Study of the PI3K Inhibitor Pictilisib Plus Anastrozole Compared with Anastrozole Alone in Patients with Estrogen Receptor-Positive Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1987–1994. [Google Scholar] [CrossRef]
- Schöffski, P.; Cresta, S.; Mayer, I.A.; Wildiers, H.; Damian, S.; Gendreau, S.; Rooney, I.; Morrissey, K.M.; Spoerke, J.M.; Ng, V.W.; et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res. 2018, 20, 109. [Google Scholar]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01740336 (accessed on 20 May 2025).
- Vuylsteke, P.; Huizing, M.; Petrakova, K.; Roylance, R.; Laing, R.; Chan, S.; Abell, F.; Gendreau, S.; Rooney, I.; Apt, D.; et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann. Oncol. 2016, 27, 2059–2066. [Google Scholar] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. SOLAR-1 Study Group. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [PubMed]
- Copur, M.S. Alpelisib to treat breast cancer. Drugs Today (Barc.) 2020, 56, 357–363. [Google Scholar]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03056755 (accessed on 20 May 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01923168 (accessed on 20 May 2025).
- Mayer, I.A.; Prat, A.; Egle, D.; Blau, S.; Fidalgo, J.A.P.; Gnant, M.; Fasching, P.A.; Colleoni, M.; Wolff, A.C.; Winer, E.P.; et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2975–2987. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02340221 (accessed on 20 May 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04191499 (accessed on 20 May 2025).
- Arabshomali, A.; Goswami, S.; Masurkar, P.P. Inavolisib for HR-Positive, HER2-Negative Advanced Breast Cancer: Clinical Trials and Patient Access Implication. Am. J. Clin. Oncol. 2025, 48, 496–500. [Google Scholar]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03961698 (accessed on 20 May 2025).
- Hatem, S.; Hargis, J.; Elias, A.; Lee, A.; Swart, R.; Dahkil, S.; Drakaki, A.; Phan, V.; Kass, F.; Cobleigh, M.; et al. Updated efficacy, safety and translational data from MARIO-3, a phase II open-label study evaluating a novel triplet combination of eganelisib (IPI-549), atezolizumab (atezo), and nab-paclitaxel (nab-pac) as first-line (1L) therapy for locally advanced or metastatic triple-negative breast cancer (TNBC). Cancer Res. 2022, 82, 5-16-02. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/show/NCT02684032 (accessed on 20 May 2025).
- Layman, R.M.; Han, H.S.; Rugo, H.S.; Stringer-Reasor, E.M.; Specht, J.M.; Dees, E.C.; Kabos, P.; Suzuki, S.; Mutka, S.C.; Sullivan, B.F.; et al. Gedatolisib in combination with palbociclib and endocrine therapy in women with hormone receptor-positive, HER2-negative advanced breast cancer: Results from the dose expansion groups of an open-label, phase 1b study. Lancet Oncol. 2024, 25, 474–487. [Google Scholar]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05501886 (accessed on 20 May 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06757634 (accessed on 20 May 2025).
- Chien, A.J.; Tripathy, D.; Albain, K.S.; Symmans, W.F.; Rugo, H.S.; Melisko, M.E.; Wallace, A.M.; Schwab, R.; Helsten, T.; Forero-Torres, A.; et al. I-SPY 2 Consortium. MK-2206 and Standard Neoadjuvant Chemotherapy Improves Response in Patients with Human Epidermal Growth Factor Receptor 2-Positive and/or Hormone Receptor-Negative Breast Cancers in the I-SPY 2 Trial. J. Clin. Oncol. 2020, 38, 1059–1069. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/study/NCT04305496 (accessed on 20 May 2025).
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. CAPItello-291 Study Group. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, B.; Sun, X.; Rong, G.; Wang, W.; Li, H.; Guan, X.; Li, L.; Zhai, J.; Li, C.; et al. Safety and efficacy of sirolimus combined with endocrine therapy in patients with advanced hormone receptor-positive breast cancer and the exploration of biomarkers. Breast 2020, 52, 17–22. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06957379 (accessed on 20 May 2025).
- Wolff, A.C.; Lazar, A.A.; Bondarenko, I.; Garin, A.M.; Brincat, S.; Chow, L.; Sun, Y.; Neskovic-Konstantinovic, Z.; Guimaraes, R.C.; Fumoleau, P.; et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2013, 31, 195–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Sáenz, J.Á.; Martínez-Jáñez, N.; Cubedo, R.; Jerez, Y.; Lahuerta, A.; González-Santiago, S.; Ferrer, N.; Ramos, M.; Alonso-Romero, J.L.; Antón, A.; et al. Sapanisertib plus Fulvestrant in Postmenopausal Women with Estrogen Receptor-Positive/HER2-Negative Advanced Breast Cancer after Progression on Aromatase Inhibitor. Clin. Cancer Res. 2022, 28, 1107–1116. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02756364 (accessed on 20 May 2025).
- Schmid, P.; Zaiss, M.; Harper-Wynne, C.; Ferreira, M.; Dubey, S.; Chan, S.; Makris, A.; Nemsadze, G.; Brunt, A.M.; Kuemmel, S.; et al. Fulvestrant Plus Vistusertib vs Fulvestrant Plus Everolimus vs Fulvestrant Alone for Women with Hormone Receptor-Positive Metastatic Breast Cancer: The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1556–1564. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02216786 (accessed on 20 May 2025).
- Hong, D.S.; Moore, K.N.; Bendell, J.C.; Karp, D.D.; Wang, J.S.; Ulahannan, S.V.; Jones, S.; Wu, W.; Donoho, G.P.; Ding, Y.; et al. Preclinical evaluation and phase ib study of prexasertib, a chk1 inhibitor, and samotolisib (LY3023414), a dual PI3K/mTOR inhibitor. Clin. Cancer Res. 2021, 27, 1864–1874. [Google Scholar] [CrossRef]
- Kim, S.B.; Dent, R.; Im, S.A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02162719 (accessed on 20 May 2025).
- Dent, R.A.; Kim, S.B.; Oliveira, M.; Barrios, C.; O’Shaughnessy, J.; Isakoff, S.J.; Saji, S.; Freitas-Junior, R.; Philco, M.; Bondarenko, I.; et al. Ipatasertib plus Paclitaxel for Patients with PIK3CA/AKT1/PTEN-Altered Locally Advanced Unresectable or Metastatic Triple-Negative Breast Cancer in the IPATunity130 Phase III Trial. Clin Cancer Res. 2024, 30, 4329–4338. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03337724 (accessed on 20 May 2025).
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer. 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Owonikoko, T.K.; Khuri, F.R. Targeting the PI3K/AKT/mTOR pathway: Biomarkers of success and tribulation. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, e395–e401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keating, G.M. Pertuzumab: In the first-line treatment of HER2-positive metastatic breast cancer. Drugs 2012, 72, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Maadi, H.; Soheilifar, M.H.; Choi, W.S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ovcinnikovs, V.; Dijkman, K.; Zom, G.G.; Beurskens, F.J.; Trouw, L.A. Enhancing complement activation by therapeutic anti-tumor antibodies: Mechanisms, strategies, and engineering approaches. Semin. Immunol. 2025, 77, 101922. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.C.; Crosby, E.J.; Trotter, T.N.; Wei, J.; Wang, T.; Yang, X.; Summers, A.N.; Lei, G.; Rabiola, C.A.; Chodosh, L.A.; et al. Trastuzumab/pertuzumab combination therapy stimulates antitumor responses through complement-dependent cytotoxicity and phagocytosis. JCI Insight 2022, 7, e155636. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. APHINITY Steering Committee and Investigators. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131, Erratum in N. Engl. J. Med. 2017, 377, 702; https://doi.org/10.1056/NEJMx170011. Erratum in N. Engl. J. Med. 2018, 379, 1585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasso, J.M.; Tenchov, R.; Bird, R.; Iyer, K.A.; Ralhan, K.; Rodriguez, Y.; Zhou, Q.A. The Evolving Landscape of Antibody-Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjug Chem. 2023, 34, 1951–2000. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biswas, G.; Subudhi, G.C.; Biswas, S.; Das, B. Long-Term Success with a TDM-1 Biosimilar in Recurrent HER2-Positive Breast Cancer with Multisite Metastases: A Comprehensive Case Study. Cureus 2024, 16, e67047. [Google Scholar] [CrossRef]
- von Arx, C.; De Placido, P.; Caltavituro, A.; Di Rienzo, R.; Buonaiuto, R.; De Laurentiis, M.; Arpino, G.; Puglisi, F.; Giuliano, M.; Del Mastro, L. The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer. Cancer Treat Rev. 2023, 113, 102500. [Google Scholar]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Fabi, A.; De Laurentiis, M.; Caruso, M.; Valle, E.; Moscetti, L.; Santini, D.; Cannita, K.; Carbognin, L.; Ciccarese, M.; Rossello, R.; et al. Efficacy and safety of T-DM1 in the ‘common-practice’ of HER2+ advanced breast cancer setting: A multicenter study. Oncotarget 2017, 8, 64481–64489. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, L.; Li, J.; Xu, Q.; Gao, Z.; Yang, M.; Wu, X.; Li, X. HER2/PI3K/AKT pathway in HER2-positive breast cancer: A review. Medicine 2024, 103, e38508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xuhong, J.C.; Qi, X.W.; Zhang, Y.; Jiang, J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am. J. Cancer Res. 2019, 9, 2103–2119. [Google Scholar] [PubMed] [PubMed Central]
- Li, J.; Wang, H.; Li, J.; Bao, J.; Wu, C. Discovery of a Potential HER2 Inhibitor from Natural Products for the Treatment of HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1055. [Google Scholar] [CrossRef]
- Yang, X.; Wu, D.; Yuan, S. Tyrosine Kinase Inhibitors in the Combination Therapy of HER2 Positive Breast Cancer. Technol. Cancer Res. Treat. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Yang, T.; Kang, L.; Li, D.; Song, Y. Immunotherapy for HER-2 positive breast cancer. Front. Oncol. 2023, 13, 1097983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abaza, A.; Sid Idris, F.; Anis Shaikh, H.; Vahora, I.; Moparthi, K.P.; Al Rushaidi, M.T.; Muddam, M.R.; Obajeun, O.A.; Jaramillo, A.P.; Khan, S. Programmed Cell Death Protein 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) Immunotherapy: A Promising Breakthrough in Cancer Therapeutics. Cureus 2023, 15, e44582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jie, H.; Ma, W.; Huang, C. Diagnosis, Prognosis, and Treatment of Triple-Negative Breast Cancer: A Review. Breast Cancer 2025, 17, 265–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, A.; Barge, A.; Parris, C.N. Combination strategies with PARP inhibitors in BRCA-mutated triple-negative breast cancer: Overcoming resistance mechanisms. Oncogene 2025, 44, 193–207, Erratum in Oncogene 2025, 44, 1063. https://doi.org/10.1038/s41388-025-03364-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, J.; Tang, B.; Shen, P.; Zeng, H.; Wei, Q. Empowering PARP inhibition through rational combination: Mechanisms of PARP inhibitors and combinations with a focus on the treatment of metastatic castration-resistant prostate cancer. Crit. Rev. Oncol. Hematol. 2025, 210, 104698. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Saini, K.S.; Vidal, L.; Peg, V.; Slebe, F.; Loibl, S.; Curigliano, G.; Schmid, P.; Cortes, J. Window of opportunity trials with immune checkpoint inhibitors in triple-negative breast cancer. ESMO Open 2024, 9, 103713. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Wang, Y.; Minden, A. Current Molecular Combination Therapies Used for the Treatment of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 11046. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asleh, K.; Riaz, N.; Nielsen, T.O. Heterogeneity of triple negative breast cancer: Current advances in subtyping and treatment implications. J. Exp. Clin. Cancer Res. 2022, 41, 265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: A patient-level meta-analysis of 100,000 women from 86 randomised trials. Lancet 2023, 401, 1277–1292. [Google Scholar] [CrossRef]
- Yu, J.; Mu, Q.; Fung, M.; Xu, X.; Zhu, L.; Ho, R.J.Y. Challenges and opportunities in metastatic breast cancer treatments: Nano-drug combinations delivered preferentially to metastatic cells may enhance therapeutic response. Pharmacol. Ther. 2022, 236, 108108. [Google Scholar] [CrossRef]
- Turner, N.C.; Im, S.A.; Saura, C.; Juric, D.; Loibl, S.; Kalinsky, K.; Schmid, P.; Loi, S.; Sunpaweravong, P.; Musolino, A.; et al. Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer. N. Engl. J. Med. 2024, 391, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Royce, M.; Shah, M.; Zhang, L.; Cheng, J.; Bonner, M.K.; Pegues, M.; Miller, C.P.; Leu, L.; Price, L.S.L.; Qiu, J.; et al. FDA Approval Summary: Datopotamab deruxtecan-dlnk for treatment of patients with unresectable or metastatic, HR-positive, HER2-negative breast cancer. Clin Cancer Res. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tokunaga, E.; Iwata, H.; Itoh, M.; Taira, T.; Toyama, T.; Mizuno, T.; Osaki, A.; Yanagita, Y.; Nakamura, S.; Nakamura, R.; et al. Capivasertib and fulvestrant for patients with HR-positive/HER2-negative advanced breast cancer: Analysis of the subgroup of patients from Japan in the phase 3 CAPItello-291 trial. Breast Cancer 2025, 32, 132–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Liu, Q. Narrative review on the role of immunotherapy in early triple negative breast cancer: Unveiling opportunities and overcoming challenges. Transl. Breast Cancer Res. 2023, 4, 16. [Google Scholar] [CrossRef]
- Medina, A.; Carballo, J.; González-Marcano, E.; Blanca, I.; Convit, A.F. Breast cancer immunotherapy: Realities and advances. Cancer Innov. 2024, 3, e140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oncology. In Clinical Practice. Available online: https://journals.viamedica.pl/oncology_in_clinical_practice/article/view/106843/83567 (accessed on 2 October 2025).
- Storme, G.A. Breast Cancer: Impact of New Treatments? Cancers 2023, 15, 2205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin Nanoformulations for Colorectal Cancer: A Review. Front. Pharmacol. 2019, 10, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdullah, K.M.; Sharma, G.; Singh, A.P.; Siddiqui, J.A. Nanomedicine in Cancer Therapeutics: Current Perspectives from Bench to Bedside. Mol. Cancer. 2025, 24, 169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahimkhoei, V.; Alzaidy, A.H.; Abed, M.J.; Rashki, S.; Salavati-Niasari, M. Advances in inorganic nanoparticles-based drug delivery in targeted breast cancer theranostics. Adv. Colloid. Interface Sci. 2024, 329, 103204. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, W.; Huang, P.; Zeng, X.; Mei, L. Smart Materials for Drug Delivery and Cancer Therapy. VIEW 2021, 2, 20200042. [Google Scholar] [CrossRef]
- Petrovic, S.; Bita, B.; Barbinta-Patrascu, M.E. Nanoformulations in Pharmaceutical and Biomedical Applications: Green Perspectives. Int. J. Mol. Sci. 2024, 25, 5842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Fan, S.; Wang, W.; Che, W.; Xu, Y.; Jin, C.; Dong, L.; Xia, Q. Nanomedicines Targeting Metabolic Pathways in the Tumor Microenvironment: Future Perspectives and the Role of AI. Metabolites 2025, 15, 201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Eerden, R.A.G.; Mathijssen, R.H.J.; Koolen, S.L.W. Recent Clinical Developments of Nanomediated Drug Delivery Systems of Taxanes for the Treatment of Cancer. Int. J. Nanomed. 2020, 15, 8151–8166. [Google Scholar] [CrossRef]
- Gao, G.; Guo, Q.; Zhi, J. Nanodiamond-Based Theranostic Platform for Drug Delivery and Bioimaging. Small 2019, 15, e1902238. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Wang, H.; Zhang, M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin. Drug Deliv. 2017, 14, 123–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Rahmani Moghadam, E.; Raei, M.; Kalantari, M.; Tavakol, S.; Mohammadinejad, R.; Najafi, M.; et al. Progress in Natural Compounds/siRNA Co-delivery Employing Nanovehicles for Cancer Therapy. ACS Comb. Sci. 2020, 22, 669–700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sousa, C.; Videira, M. Dual Approaches in Oncology: The Promise of siRNA and Chemotherapy Combinations in Cancer Therapies. Onco 2025, 5, 2. [Google Scholar] [CrossRef]
- Ali Zaidi, S.S.; Fatima, F.; Ali Zaidi, S.A.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: Challenges and strategies. J. Nanobiotechnology 2023, 21, 381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.; Rehman, U.; Parveen, N.; Kumar, S.; Baboota, S.; Ali, J. siRNA therapeutics: Insights, challenges, remedies and future prospects. Expert Opin. Drug Deliv. 2023, 20, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Ngamcherdtrakul, W.; Castro, D.J.; Gu, S.; Morry, J.; Reda, M.; Gray, J.W.; Yantasee, W. Current development of targeted oligonucleotide-based cancer therapies: Perspective on HER2-positive breast cancer treatment. Cancer Treat. Rev. 2016, 45, 19–29. [Google Scholar] [CrossRef]
- Jones, S.K.; Merkel, O.M. Tackling breast cancer chemoresistance with nano-formulated siRNA. Gene Ther. 2016, 23, 821–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ngamcherdtrakul, W.; Yantasee, W. siRNA therapeutics for breast cancer: Recent efforts in targeting metastasis, drug resistance, and immune evasion. Transl. Res. J. Lab. Clin. Med. 2019, 214, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Badiginchala, R.; Dattatreya, P.S.; Suresh, A.V.S.; Nirni, S.S.; Andra, V.V.; Bunger, D.; Chaturvedi, A. Efficacy and Safety of Nanosomal Docetaxel Lipid Suspension (NDLS) versus Conventional Docetaxel as Neoadjuvant and Adjuvant Therapy for Primary Operable Breast Cancer. Onco Targets Ther. 2023, 16, 215–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoogevest, P.; Tiemessen, H.; Metselaar, J.M.; Drescher, S.; Fahr, A. The Use of Phospholipids to Make Pharmaceutical form Line Extensions. Eur. J. Lipid Sci. Technol. 2021, 123, 2000297. [Google Scholar] [CrossRef]
- Satheesh, C.T.; Taran, R.; Singh, J.K.; Shrivastav, S.P.; Vithalani, N.K.; Mukherjee, K.K.; Nagarkar, R.V.; Maksud, T.; Mehta, A.O.; Srinivasan, K.; et al. Treatment with nanosomal paclitaxel lipid suspension versus conventional paclitaxel in metastatic breast cancer patients—A multicenter, randomized, comparative, phase II/III clinical study. Ther. Adv. Med. Oncol. 2024, 16, 17588359241236442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subramanian, S.; Majumdar, S.K.D.; Biswas, G.; Joshi, N.; Bunger, D.; Khan, M.A.; Ahmad, I. Efficacy and safety of nanosomal docetaxel lipid suspension based chemotherapy in gastric and gastroesophageal junction adenocarcinoma. Mol. Clin. Oncol. 2020, 13, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiley. Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002%2Fmog2.67 (accessed on 2 October 2025).
- Đorđević, S.; Gonzalez, M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, H.J.; De Geest, B.G. Nanomedicine and cancer immunotherapy. Acta Pharmacol. Sin. 2020, 41, 879–880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.; Kumar, P.; Reda, M.; Wallstrum, A.G.; Crumrine, N.A.; Ngamcherdtrakul, W.; Yantasee, W. Nanotechnology Applications in Breast Cancer Immunotherapy. Small. 2024, 20, e2308639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fallatah, M.M.; Alradwan, I.; Alfayez, N.; Aodah, A.H.; Alkhrayef, M.; Majrashi, M.; Jamous, Y.F. Nanoparticles for Cancer Immunotherapy: Innovations and Challenges. Pharmaceuticals 2025, 18, 1086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Countries | Study Type | Phase | Official Title | Detailed Description | Interventions | Years and Status |
|---|---|---|---|---|---|---|
| Beijing, China | Interventional | Not Applicable | Safety Assessment of Concurrent Radiotherapy and Novel Systemic Therapy for Breast Cancer | The aim of the study is to evaluate the safety of combination therapy in patients with lymphatic drainage within the chest wall/breast requiring capecitabine, a CDK 4/6 inhibitor, HER2-targeted therapy, or immunotherapy. | Radiation HER2 inhibitors, CDK4/6 inhibitors, PARP inhibitors, ICIs | 2024–2027 |
| Jacksonville, FL, USA | Observational | Not Applicable | Observational Basket Trial to Collect Tissue to Train and Validate a Live Tumor Diagnostic Platform | This study is being done to collect tissue samples to test how accurately a tumor response platform, Elephas, can predict clinical response across multiple types of immunotherapies, chemoimmunotherapy and tumor types. | Patients will receive standard treatment with checkpoint inhibitors and undergo standard tumor assessment during screening and follow-up. | 2024–2027 |
| This study has locations in Greece | Observational | Not applicable | Mechanisms of Response and Resistance to Innovative Treatments in Patients With Locally Advanced or Metastatic Breast Cancer | The study aims to utilize modern methods such as DNA sequencing, analysis of circulating genetic material, digital imaging, and radiological analysis, as well as data from patient medical records. Based on this, a machine learning algorithm will be developed that will predict whether a patient will respond well to treatment or develop resistance, depending on the genetics and molecular profile of the tumor. | Patients receiving treatment with an Antibody–Drug conjugate (e.g., Trastuzumab–Deruxtecan, Sacituzumab–Govitecan) Patients receiving treatment with an immune checkpoint inhibitor (ICI) (e.g., pembrolizumab) Patients receiving treatment with a PARP inhibitor (e.g., Olaparib) | 2024–2029 |
| Drugs | Structure | Characteristics | Indications |
|---|---|---|---|
| Capivasertib | 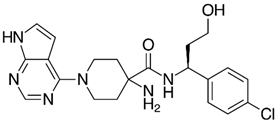 | Capivasertib is a kinase inhibitor drug that works by blocking specific proteins that play a key role in the development and survival of cancer cells. This substance selectively inhibits the activity of the AKT protein (also known as protein kinase), a key component of the PI3K/AKT/mTOR signaling pathway. Overactivity of this pathway in cancer promotes uncontrolled cell growth and survival. Inhibition of AKT by capivasertib aims to limit or stop cancer cell proliferation. | Approved for the treatment of hormone receptor-positive, HER2-negative breast cancer with an abnormal PIK3CA gene |
| Datopotamab deruxtecan-dlnk | 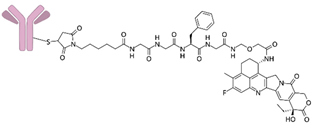 | This drug binds to Trop-2 and internalizes the receptor, enabling transport of the active drug into the cell. The release of exatecan inhibits cell replication, leading to apoptosis. The process of exatecan penetration into neighboring cells triggers a series of processes that ultimately result in cell death. | Approved for the treatment of: hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer. It is also being studied for the treatment of other types of cancer. |
| Inavolisib | 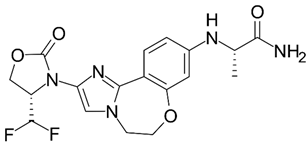 | Inavolisib is a potent and selective inhibitor of the PI3K-alpha enzyme (phosphoinositide 3-kinase alpha). It is designed to inhibit the PI3K pathway through HER2-dependent degradation. It aims to inhibit tumor growth in patients whose cancers are caused by PI3K mutations. | Approved for the treatment of hormone receptor-positive, HER2-negative breast cancer with an abnormal PIK3CA gene. It is also being studied for the treatment of other types of cancer |
| Kisqali Femara Co-Pack (Ribociclib Succinate and Letrozole) | 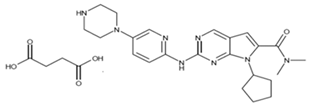  | It inhibits the growth of cancer cells, which are ultimately destroyed. | Approved for the treatment of hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer. Used for stage II or III breast cancer. |
| Countries | Study Type | Phase | Official Title | Detailed Description | Interventions | Years and Status |
|---|---|---|---|---|---|---|
| Shanghai, China | Interventional | 2 | A Phase II Study to Explore the Safety, Tolerability, and Preliminary Antitumor Activity of Sitravatinib Plus Tislelizumab or Combination With Nab-paclitaxel in Patients With Locally Recurrent or Metastatic Triple Negative Breast Cancer (TNBC) | The aim of the study is to evaluate the efficacy of sitravatinib in combination with tislelizumab and nab-paclitaxel in patients with previously untreated metastatic breast cancer (TNBC) or with recurrence/metastasis after surgery. | Drug: Sitravatinib Drug: Tislelizumab Drug: Nab-paclitaxel | 2021–2024 |
| Gothenburg, Sweden | Interventional | 2 | Sentinel Lymph Node Localisation With an Ultra-low Dose of Superparamagnetic Iron Oxide Nanoparticles in Patients With Breast Cancer | The main objective is to demonstrate that the use of superparamagnetic iron oxide nanoparticles (SPIO) as a tracer is equally effective in detecting sentinel lymph nodes (SLNs) in breast cancer patients. | Drug: Superparamagnetic Iron Oxide Device: Technetium99 | 2023–2027 |
| This study has locations in USA, Hong Kong, and Sweden | Interventional | 3 | Sentinel Lymph Node Biopsy in Ductal Cancer in Situ or Unclear Lesions of the Breast and How to Not do it. An Open-label, Phase 3, Randomised Controlled Trial. (SentiNot 2.0). | The aim of this study is to investigate the use of superparamagnetic iron oxide nanoparticles as a marker for delayed sentinel lymph node dissection in patients for whom initial axillary surgery is considered oncologically unnecessary and should be avoided. This includes patients with a preoperative diagnosis of ductal carcinoma in situ. | Diagnostic Test: Delayed SLND Diagnostic Test: Late SLND | 2020–2027 |
| Houston, TX, USA | Interventional | 2 | A Phase-2, Two-Cohort Trial of Neoadjuvant Nab-Paclitaxel and Alpelisib in Anthracycline Refractory Triple Negative Breast Cancer With PIK3CA or PTEN Alterations | The aim of the study is to evaluate the efficacy of nab-paclitaxel and alpelisib in the treatment of patients with triple-negative breast cancer with mutations in the PIK3CA or PTEN gene that does not respond to anthracycline chemotherapy (anthracycline-resistant). | Drug: Alpelisib Drug: Nab-paclitaxel | 2020–2025 |
| Gothenburg, Sweden | Interventional | 1, 2 | Sentinel Node Localization and Staging with Low Dose Superparamagnetic Iron Oxide-enhanced Magnetic Resonance Imaging and Magnetic Probe in Patients with Breast Cancer | The aim of this study was to determine whether detection of sentinel node status using ultralow dose superparamagnetic iron oxide nanoparticles is feasible. | Drug: Superparamagnetic Iron Oxide | 2021–2024 |
| This study has 30 locations in USA | Interventional | 2 | Randomized Phase 2 Clinical Trial of Nab-Paclitaxel + Durvalumab (MEDI4736) + Tremelimumab + Neoantigen Vaccine Vs. Nab-Paclitaxel + Durvalumab (MEDI4736) + Tremelimumab in Patients With Metastatic Triple Negative Breast Cancer | The Phase II study is examining the efficacy of the drugs nab-paclitaxel, durvalumab, and tremelimumab, in combination with or without a personalized synthetic long-peptide vaccine (neoantigen vaccine), in the treatment of patients with metastatic triple-negative breast cancer. | Procedure: Biopsy Procedure Procedure: Biospecimen Collection Drug: Carboplatin Procedure: Computed Tomography Biological: Durvalumab Drug: Gemcitabine Hydrochloride Procedure: Magnetic Resonance Imaging Drug: Nab-paclitaxel Biological: Personalized Synthetic Long Peptide Vaccine Drug: Poly ICLC Biological: Sacituzumab Govitecan Biological: Tremelimumab | 2021–2025 |
| This study has 1008 locations in USA and Puerto Rico | Interventional | 2 | (CompassHER2-pCR): Preoperative THP and Postoperative HP in Patients Who Achieve a Pathologic Complete Response | The aim of the study is to evaluate the efficacy of paclitaxel, trastuzumab, and pertuzumab in eliminating further chemotherapy after surgery in patients with HER2-positive stage II-IIIa breast cancer who, after preoperative chemotherapy and HER2-targeted therapy, had no cancer detected at the time of surgery (neither in the breast nor in the axillary lymph nodes). | Drug: Docetaxel Procedure: Lumpectomy Procedure: Mastectomy Drug: Nab-paclitaxel Drug: Paclitaxel Biological: Pertuzumab Radiation: Radiation Therapy Biological: Trastuzumab Biological: Trastuzumab Emtansine | 2020–2038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dynarowicz, K.; Bartusik-Aebisher, D.; Koszarska, K.; Kotlińska, A.; Aebisher, D. Breast Cancer Treatments: Drugs Targeting the PI3K/AKT/mTOR Pathway, TNBC Therapy and Future Directions: A Review. Life 2025, 15, 1583. https://doi.org/10.3390/life15101583
Dynarowicz K, Bartusik-Aebisher D, Koszarska K, Kotlińska A, Aebisher D. Breast Cancer Treatments: Drugs Targeting the PI3K/AKT/mTOR Pathway, TNBC Therapy and Future Directions: A Review. Life. 2025; 15(10):1583. https://doi.org/10.3390/life15101583
Chicago/Turabian StyleDynarowicz, Klaudia, Dorota Bartusik-Aebisher, Katarzyna Koszarska, Aleksandra Kotlińska, and David Aebisher. 2025. "Breast Cancer Treatments: Drugs Targeting the PI3K/AKT/mTOR Pathway, TNBC Therapy and Future Directions: A Review" Life 15, no. 10: 1583. https://doi.org/10.3390/life15101583
APA StyleDynarowicz, K., Bartusik-Aebisher, D., Koszarska, K., Kotlińska, A., & Aebisher, D. (2025). Breast Cancer Treatments: Drugs Targeting the PI3K/AKT/mTOR Pathway, TNBC Therapy and Future Directions: A Review. Life, 15(10), 1583. https://doi.org/10.3390/life15101583









