3. Results
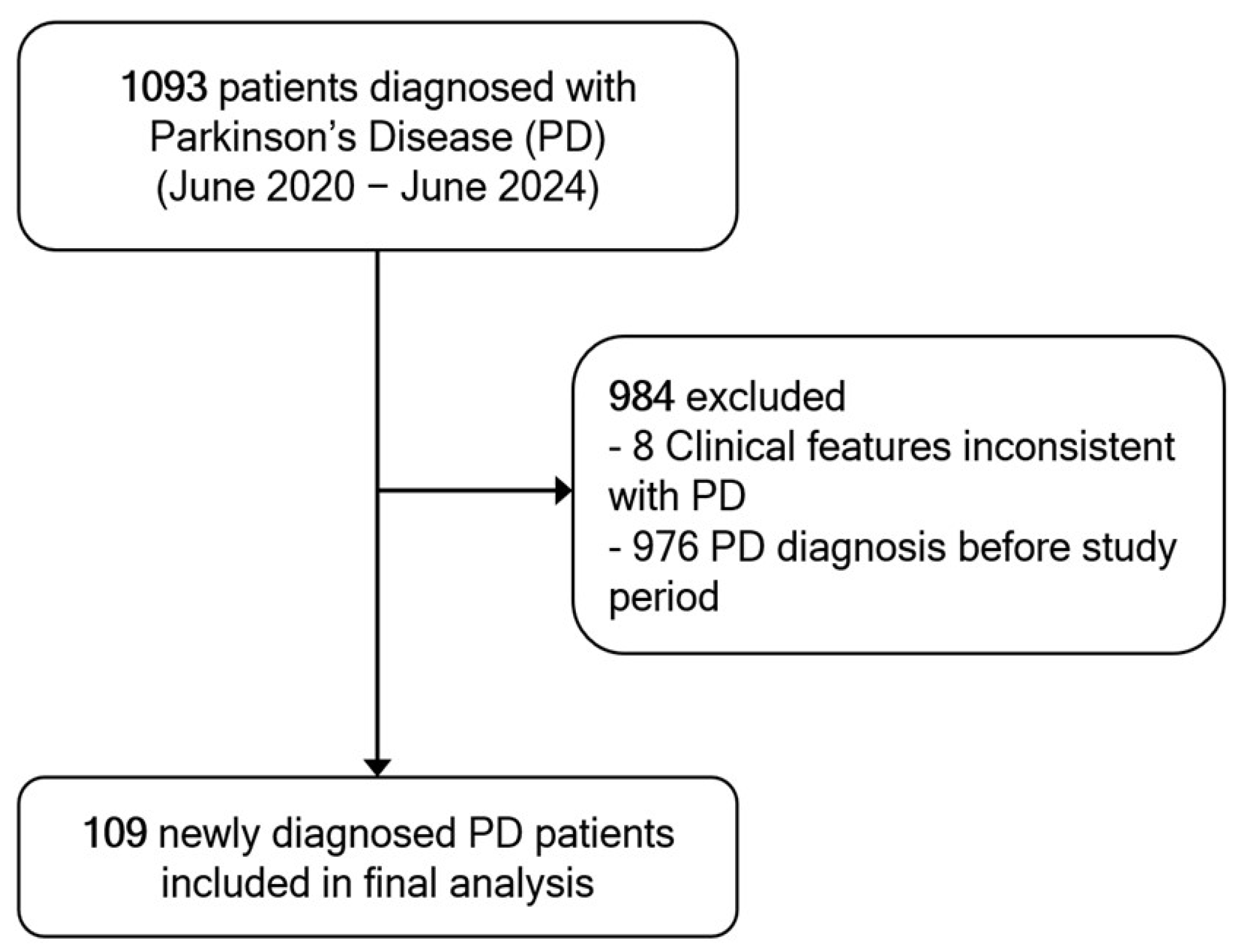
A total of 1093 patients were diagnosed with PD during the study period. Of these, 976 were excluded due to a prior PD diagnosis before the study period, and 8 patients were excluded because their clinical features were inconsistent with a probable PD diagnosis upon review. Consequently, 109 newly diagnosed patients met the inclusion criteria and were included in the final analysis (
Figure 1).
The cohort exhibited a slight male predominance (59.63%). The mean age at motor symptom onset was 69.13 ± 9.29 years. Most patients (62.38%) were covered by the Civil Servant Medical Benefit Scheme (CSMBS), followed by self-pay/private insurance (16.51%) and the Universal Coverage Scheme (UCS) (15.59%). A family history of PD was documented in 46.79% of cases; 11.76% confirmed as positive. Approximately 75.23% of patients resided within 80 km of the hospital. Regarding initial healthcare access, 62.39% of patients were first assessed by non-neurologists in outpatient clinics, whereas 37.61% were directly evaluated by neurologists (
Table 1).
Movement disorder specialists confirmed the diagnosis in 55.05% of cases, followed by general neurologists (39.45%), internal medicine physicians (4.59%), and general practitioners (0.92%). Because the time intervals exhibited high variability and a skewed distribution, the median was chosen as the most appropriate measure of central tendency. The median OTV duration was 360 days (IQR 150–720), while the median VTD duration was 10 days (IQR 1–30). The median VTD and OTD durations were significantly shorter in patients who were initially assessed by neurologists, whereas the median OTV duration did not differ significantly between groups (
Table 2).
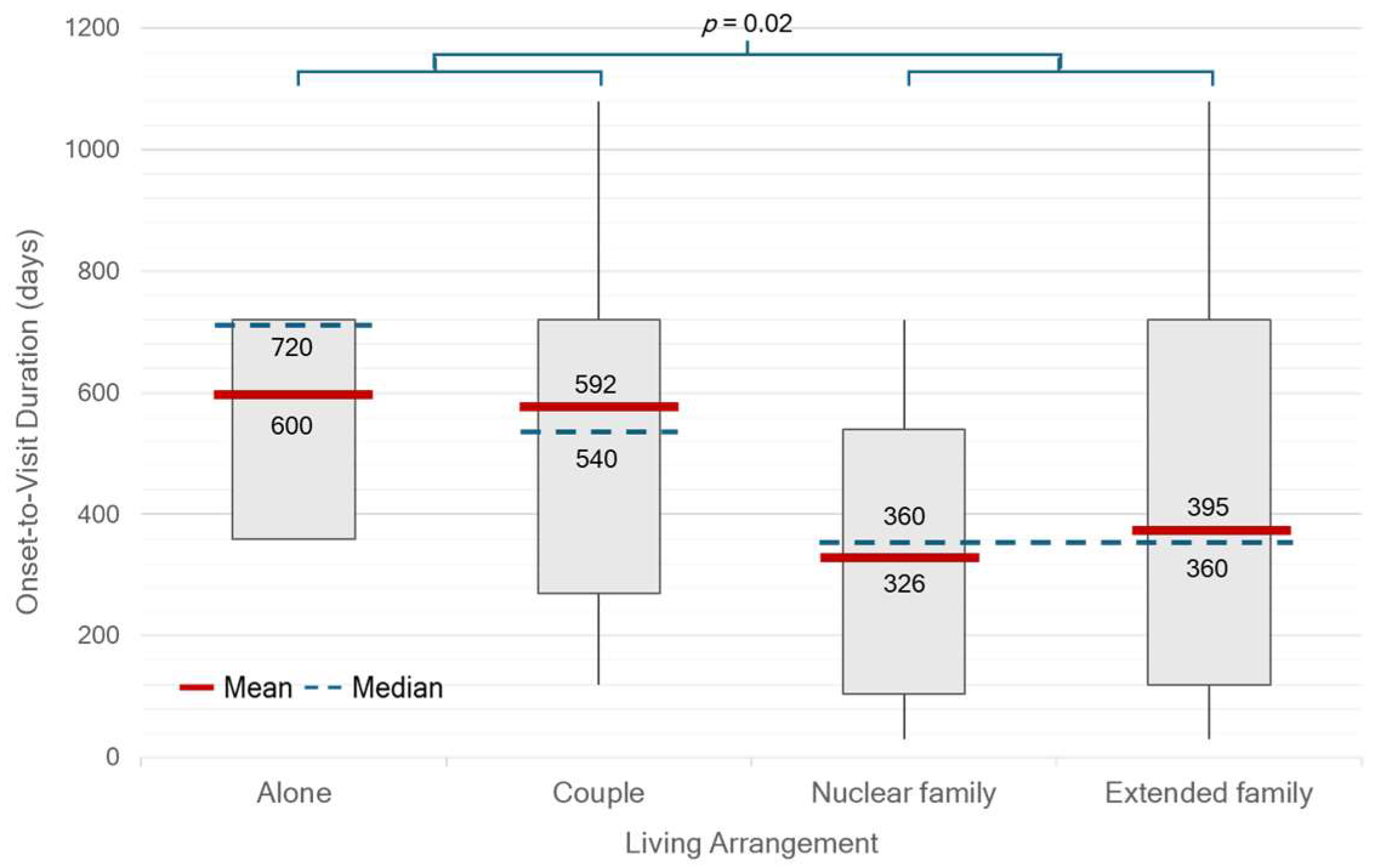
There were significant differences in median OTV duration between single- or couple-households and family households (
p = 0.02) (
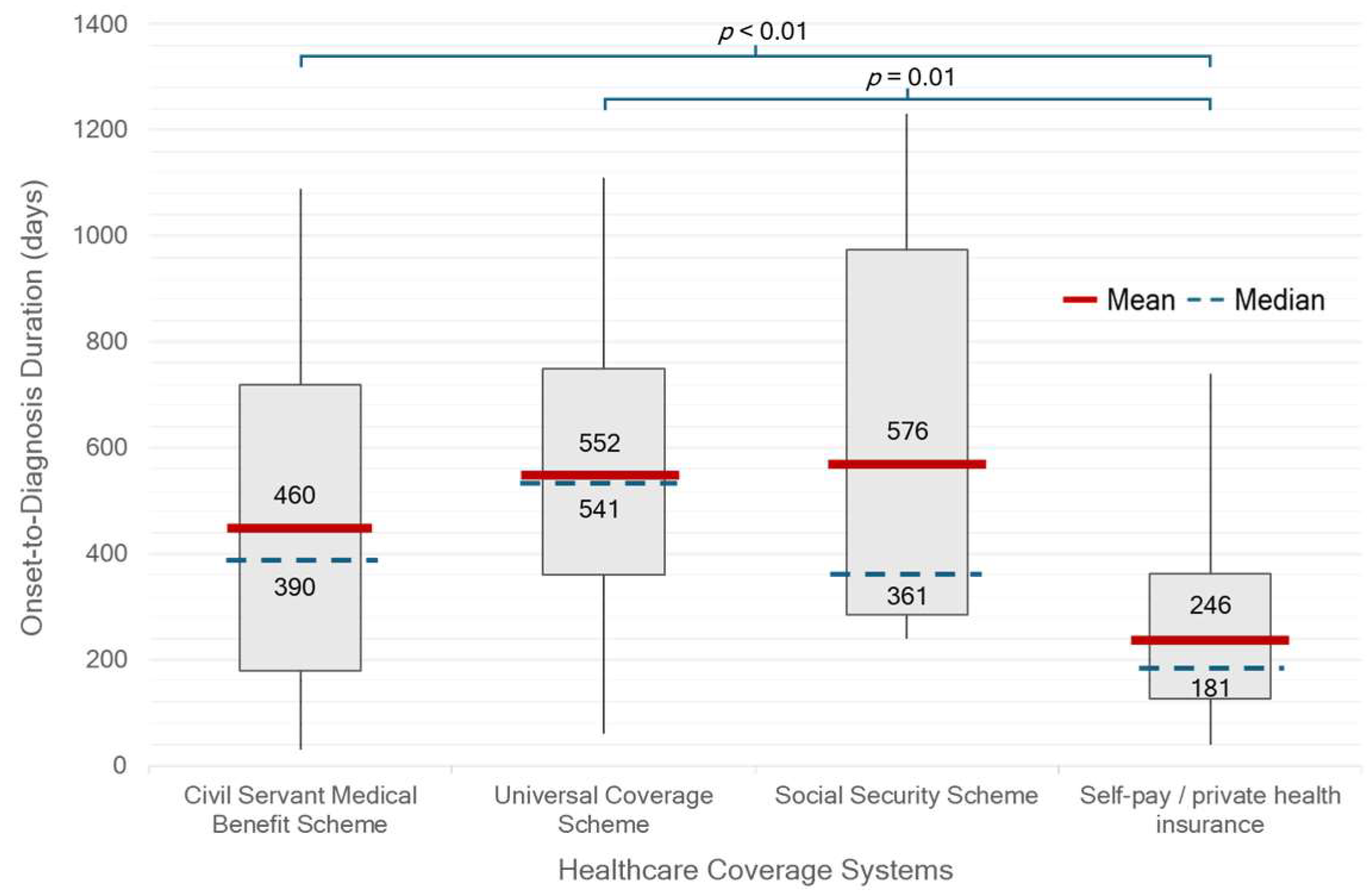
Figure 2). Patients who were self-paying or covered by private insurance had a significantly shorter median OTD duration of 181 days (IQR 127–362) compared with those under other healthcare schemes (390 days, IQR 241–721;
p < 0.01). In contrast, patients receiving care under the UCS had the longest median duration of 541 days (IQR 361–750) (
Figure 3). There was no statistically significant difference in median OTD duration between early-onset Parkinson’s disease (EOPD, <50 years), regular-onset PD (50–79 years), and very-late-onset PD (≥80 years). However, diagnostic delays appeared longer in patients with very-late-onset PD (
Figure 4). No significant differences in OTV duration, VTD duration, age at onset, or HY stage were observed between male and female patients.
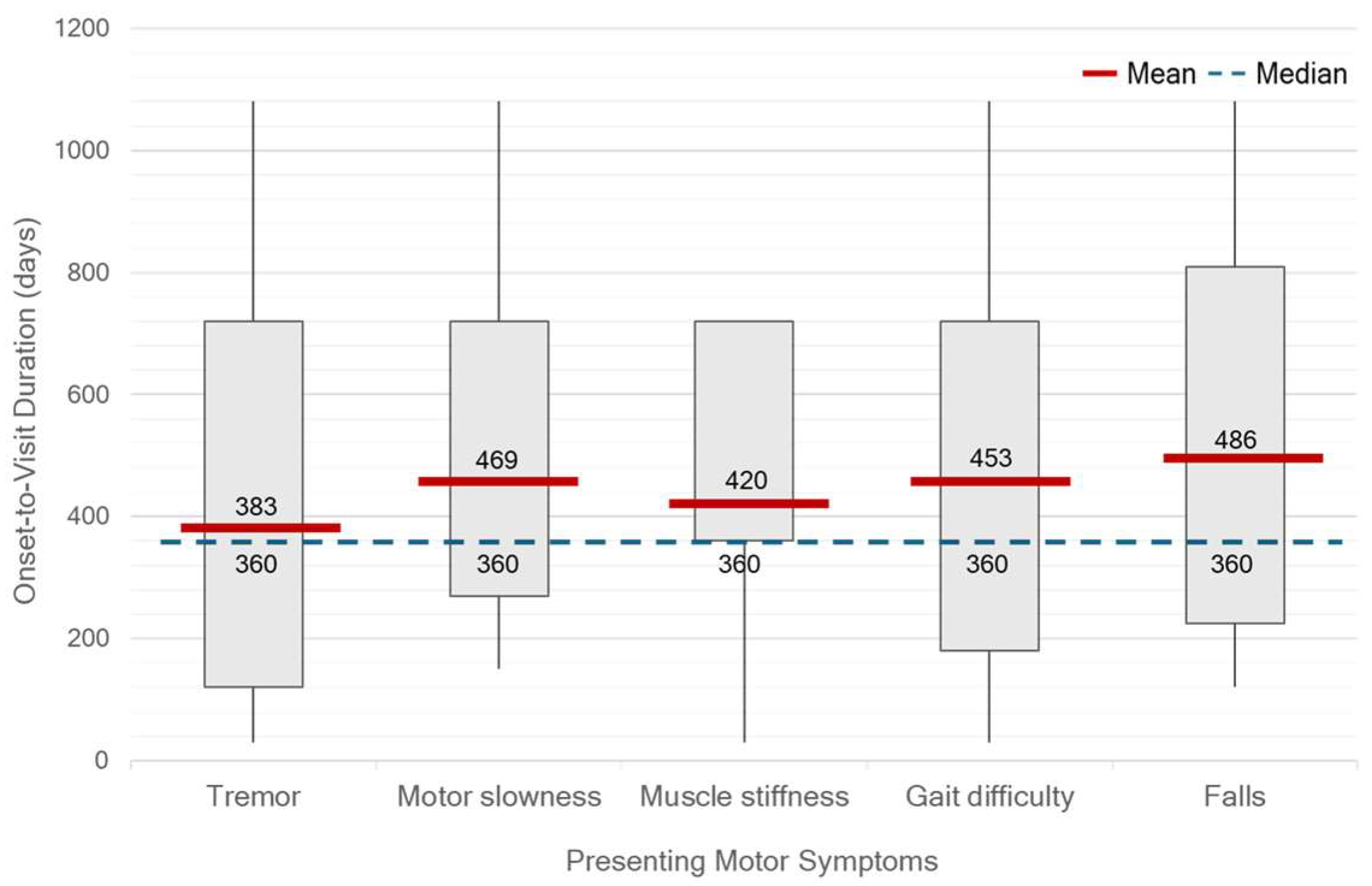
At the time of diagnosis, the median HY stage was 2 (range, 1–4). Only 32.10% of patients were classified as HY stage 1, while 9.17% were diagnosed at advanced stages (HY stage 3–4). Unilateral motor symptom predominance was observed in 93.57% of patients, most commonly on the right side (52.29%). Tremor was the most frequent initial symptom, reported in 75.22% of patients, followed by gait difficulty (38.45%), slowness of movement (37.61%), muscle stiffness (16.51%), and falls (9.17%). No significant differences in OTV duration were observed across different initial motor symptoms. However, patients presenting with tremor tended to seek clinical evaluation earlier than others. (
Figure 5.) On neurological examination, bradykinesia was identified in 85.32% of patients, resting tremor in 80.73%, rigidity in 73.39%, and postural instability in 22.02%. A significant discrepancy was found between self-reported slowness of movement and bradykinesia identified during clinical examination (
p < 0.01).
Among non-motor symptoms, constipation was the most prevalent (32.11%), followed by rapid eye movement sleep behavior disorder (RBD) (23.85%), anxiety (12.84%), insomnia (12.84%), and depression (4.58%). Regarding imaging for diagnosis, approximately one-third of patients (30.28%) did not undergo any neuroimaging studies. Among those who did, brain magnetic resonance imaging (MRI) was performed more frequently (47.71%) than computed tomography (CT) (22.02%). Levodopa was the most initiated treatment following diagnosis, prescribed in 88.07% of patients, followed by dopamine agonists in 11.01%, and no treatment (0.92%).
In 55.96% of patients, initial Parkinsonian features were not recognized as such. The most common alternative diagnoses included tremor disorders (21.10%), orthopedic conditions (17.76%), and ischemic stroke (6.42%) (
Table 3). The rate of initially unrecognized parkinsonism was significantly higher among patients first evaluated by non-neurologists compared to neurologists (81.97% vs. 18.03%,
p < 0.01). Furthermore, patients who initially presented with muscle stiffness (77.78% vs. 51.65%,
p = 0.04) and gait difficulty (69.05% vs. 47.76%,
p = 0.03) were more likely to be misdiagnosed.
Correlation coefficients analysis revealed a moderate negative correlation between the OTV duration and years of education (r = −0.37, p < 0.01). Weak positive correlations were observed between OTV duration and isolated living arrangement (r = 0.29, p = 0.02) and bilateral motor symptom involvement (r = 0.26, p = 0.05), HY stage at diagnosis (r = 0.19, p = 0.04), and the presence of slowness of movement (r = 0.20, p = 0.04). For the VTD duration, a moderate positive correlation was found with initial assessment by non-neurologists (r = 0.38, p < 0.01), and a mild positive correlation was noted for the presence of rigidity symptoms (r = 0.22, p = 0.02).
4. Discussion
The results of this study indicate that the longest delay in the diagnostic process for PD occurs between the onset of motor symptoms and the patient’s decision to seek medical attention. On average, patients waited approximately 12 months before their first medical consultation, underscoring delays in early symptom recognition and potential barriers to accessing healthcare services in Thailand. Compared to earlier Western studies conducted over a decade ago, which reported median durations of 4 months in New York City and 11 months in Cambridge [
5,
6], the delay observed in our contemporary Thai cohort is notably longer, despite overall improvements in global health literacy and healthcare infrastructure.
In contrast, the median duration from the first medical visit to diagnosis in our cohort was relatively short. The overall duration was 10 days, and among patients who were initially assessed by non-neurologists, the median duration was 30 days. These intervals are comparable to Western cohorts [
5,
6]. This efficiency reflects the availability of neurologists at a tertiary hospital but may not be generalizable to settings with limited specialist access.
We found no gender-related differences in time to PD diagnosis, in contrast to previous studies that report delayed diagnosis among women [
6]. This finding may reflect comparable healthcare-seeking behaviors and more equitable access to medical services between sexes in Thailand. The predominance of elderly male patients in our cohort aligns with the typical demographic profile of PD reported in both Thai and global populations [
5,
6,
7,
8].
Beyond sex-related patterns, social context played a critical role in influencing diagnostic timing. Patients with higher educational attainment and those living in extended family settings, common in Thai and other Asian societies, were more likely to seek medical attention earlier, likely because of greater disease awareness and encouragement from family members. In addition, cultural factors also contribute to delays. In Thai society, patients often tolerate mild or non-disabling symptoms and avoid seeking medical care until symptoms become more severe or disruptive. This reluctance may be reinforced by fear of diagnosis, concerns about treatment costs, or the perceived inconvenience of hospital visits. Such health-seeking behaviors likely prolong the OTV duration and delay timely recognition of PD.
Healthcare entitlement also emerged as a key determinant of diagnostic delay. Patients with private insurance experienced a significantly shorter median duration from symptom onset to diagnosis compared to those enrolled in other benefit schemes, particularly the UCS. Although UCS is Thailand’s national insurance program intended to ensure universal access regardless of socioeconomic status, its multi-tiered referral system can create delays before patients reach specialist care. In contrast, patients with financial flexibility or access to streamlined schemes such as the CSMBS can more readily consult neurologists or tertiary care centers, leading to earlier diagnosis. Indeed, CSMBS was the most common healthcare coverage among patients in this cohort, indicating that many received full reimbursement for medical expenses. This facet likely contributed to the relatively shorter diagnostic timelines observed in our study population, compared to what might be expected in the broader Thai population.
Educational level and household income also appear to contribute meaningfully to diagnostic disparities. Lower education levels among members of older generations may partly explain reduced symptom recognition, a notion consistent with the observed correlation between education and diagnostic delay. The household income data, while incomplete, revealed that over half of the patients had a monthly income below 50,000 Thai Baht. These lower-income patients are more likely to be covered under UCS, further compounding diagnostic delays due to structural barriers.
Taken together, these findings illustrate how disparities in education, income, and healthcare coverage intersect to shape the diagnostic journeys of PD patients. Addressing these systemic inequities through public health education, enhanced primary care screening, and more efficient referral mechanisms, particularly within the UCS, will be essential in promoting earlier diagnosis and ensuring more equitable brain health outcomes.
A family history of PD was documented in less than half of the patients, a finding that may be attributable to incomplete medical records or insufficient emphasis on family history during clinical assessment. Nevertheless, the proportion of patients with a confirmed positive family history was consistent with previous reports [
5,
7]. Although the majority of PD cases are considered sporadic, they are likely influenced by complex interactions between genetic predisposition and environmental factors [
9]. Documenting family history may provide valuable insights into hereditary risk and facilitate the identification of genetic variants and inheritance patterns in future research.
This study found substantial variation in the distance between patients’ residences and the hospital, ranging from being within the same province to being in other provinces and even in different regions. These differences may reflect disparities in healthcare coverage. Patients receiving care under the UCS were generally referred from nearby provincial hospitals, whereas those covered by the CSMBS or who self-paid were more likely to choose their healthcare providers based on the hospital’s reputation and the availability of specialists. These findings suggest that healthcare entitlement plays an important role in determining both referral patterns and access to expert care.
Interestingly, Parkinsonian features were initially unrecognized or misdiagnosed for more than half of the patients with PD, most commonly by non-neurologists during their first medical assessment. Patients who presented with unilateral muscle stiffness and gait difficulty were frequently misdiagnosed as having suffered a stroke or being afflicted with other orthopedic conditions. Initial misdiagnosis not only delays appropriate treatment but may also result in unnecessary medication use, excessive diagnostic investigations, and unwarranted surgical interventions. These findings underscore the urgent need to improve awareness and diagnostic proficiency related to PD among a broader range of healthcare providers. Movement disorder specialists and neurologists played a pivotal role in establishing an accurate diagnosis of PD. Therefore, expanding access to neurologists and movement disorder specialists, along with implementing efficient consultation and referral systems, is crucial for ensuring early and accurate diagnosis of PD.
In terms of motor symptoms, a tremor was the most common presenting feature in this study, a result consistent with previous findings [
5,
10]. It often served as the main symptom prompting patients to seek medical attention and guiding physicians toward a diagnosis of PD. However, it is important to recognize that tremors are not required for diagnosis, as approximately 15 to 20 percent of patients with PD do not exhibit tremors [
10]. Additionally, patients with EOPD associated with gene mutations, such as PRKN (Parkin) mutations, may exhibit slower disease progression, dystonic features involving the lower limbs, and often an absence of tremors. These atypical clinical presentations may contribute to diagnostic delays [
11,
12].
Notably, a clear discrepancy was observed between self-reported slowness of movement and bradykinesia identified during clinical examination, highlighting the under-recognition of subtle or mild bradykinetic symptoms by patients. In addition, patients with very-late-onset PD appeared to be diagnosed later than those in other age groups. This finding may reflect a tendency to attribute symptoms to the normal aging process rather than a pathological condition. Taken together, these findings emphasize the need to improve both public and clinical awareness of non-tremor motor manifestations of PD. Additionally, approximately half of the patients were diagnosed at a moderate stage, as indicated by HY stage 2, suggesting that the symptoms prompting medical consultation often emerge at a later stage of the disease, typically when bilateral motor involvement has already developed. Therefore, greater emphasis should be placed on the recognition of early-stage symptoms, including those in the prodromal phase.
In terms of non-motor symptoms (NMSs), the prevalence observed in this study was lower than that previously reported [
6], suggesting potential under-recognition or under-documentation of NMSs by physicians. A comprehensive evaluation of NMSs should be an integral part of routine clinical PD assessment. Continuing medical education on the broad spectrum of PD manifestations is essential to improve diagnostic accuracy and optimize patient care.
Regarding the use of neuroimaging in diagnosis, approximately 30% of patients did not undergo any brain imaging studies. This finding is consistent with clinical practice when the presentation is typical and fulfills established diagnostic criteria. In such cases, neuroimaging may be deemed unnecessary, particularly in settings where resources are limited.
In the context of diagnostic disparities, biomarkers offer a promising avenue for improving early recognition of PD [
13]. While traditional imaging biomarkers such as dopamine transporter single-photon emission computed tomography (DAT-SPECT) remain costly and limited in availability, digital biomarkers present a more scalable and accessible alternative in middle-income countries like Thailand. With high smartphone penetration in Thailand [
14], nationwide digital health initiatives, such as a “Check PD” campaign, could empower individuals aged 40 and older to self-screen using a smartphone-based application [
15]. These platforms, which assess tremor, dexterity, gait, and voice, may help identify subtle abnormalities long before patients seek medical care, reducing reliance on physician expertise and mitigating disparities in under-resourced areas.
Awareness of prodromal symptoms, including constipation and REM sleep behavior disorder, also remains limited. Education and training for both the public and primary care physicians could improve timely recognition and referral. Locally developed tools, such as the non-motor symptoms questionnaire (NMSQ) application [
16], further demonstrate the potential of digital health solutions to increase awareness and patient engagement. While not diagnostic, these approaches can act as diagnostic equalizers, expanding access to early detection strategies and advancing brain health equity.
In this cohort, levodopa was the most frequently initiated treatment, prescribed for almost 90% of patients following diagnosis. This high rate reflects current clinical practices and recommendations, particularly in resource-limited settings where levodopa remains the most effective and accessible therapy for the motor symptoms of PD. Its early and widespread use in this population also suggests that most patients presented with symptoms that were advanced enough to warrant pharmacologic intervention, further underscoring the need for earlier detection and timely referral in order to minimize functional impairment at treatment initiation.
Despite the apparent familiarity of diagnostic delay issues in PD, the context-specific challenges faced by patients in LMICs like Thailand remain underexplored. Our study highlights how health entitlement schemes, sociocultural dynamics, and systemic referral structures uniquely influence the diagnostic journey in this population. By providing empirical data from a Southeast Asian setting, our study emphasizes the need for localized evidence in order to guide equitable healthcare policies. Addressing diagnostic delays represents a “known but neglected” issue in brain health equity. Reducing delays through public education, primary care training, and streamlined referral systems is essential to ensure that no patient is left behind.
Several limitations should be acknowledged when interpreting the findings of this study. First, the retrospective design may have introduced missing-data and documentation bias, although the use of standardized diagnostic criteria and structured data abstraction enhanced consistency and reliability. Second, as a single-tertiary-care-center study, its generalizability may be limited. However, the hospital serves a wide catchment area with both urban and rural populations, so the findings likely reflect real-world diagnostic challenges in similar settings. Finally, although the sample size was modest, it was drawn from a clearly defined cohort of newly diagnosed PD patients, allowing for robust analysis of diagnostic intervals, symptomatology, and system-level factors.