Beyond PSA: The Future of Prostate Cancer Diagnosis Using Artificial Intelligence, Novel Biomarkers, and Advanced Imagery
Abstract
1. Introduction
2. Limitations of PSA Screening for PCa Diagnosis
3. AI and Machine Learning in Prostate Cancer Diagnosis
3.1. AI in Imaging
3.2. AI in Pathology
3.3. AI in Risk Prediction Models
4. Integrating AI, Biomarkers, and Imaging for a Unified Diagnostic Model
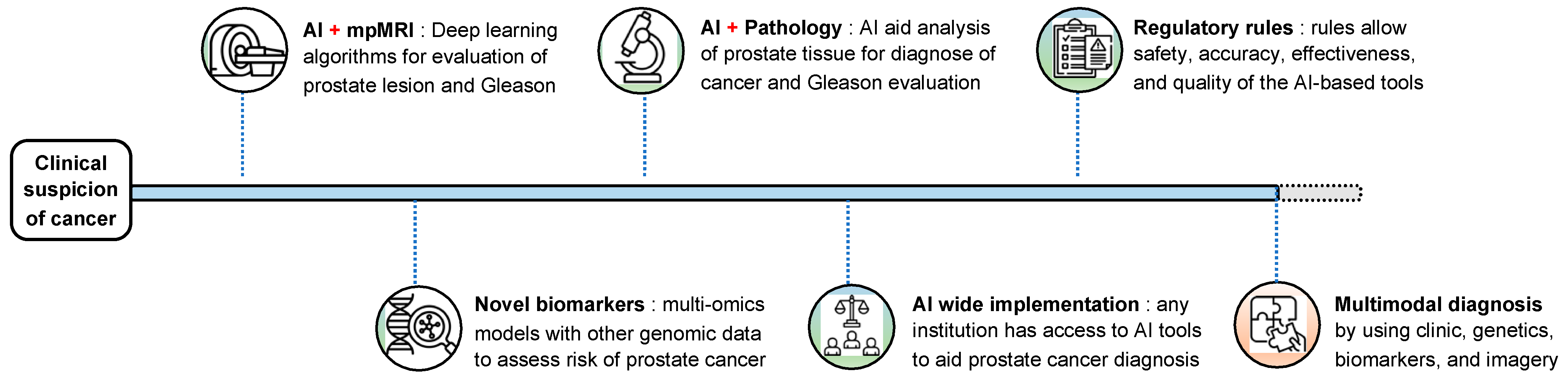
5. Future Perspectives and Clinical Implementation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.C.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Purification of a human prostate specific antigen. Investig. Urol. 1979, 17, 159–163. [Google Scholar] [CrossRef]
- Catalona, W.J. History of the discovery and clinical translation of prostate-specific antigen. Asian J. Urol. 2014, 1, 12–14. [Google Scholar] [CrossRef]
- Quantitation of Prostate-Specific Antigen in Serum by a Sensitive Enzyme Immunoassay1|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/40/12/4658/484620/Quantitation-of-Prostate-specific-Antigen-in-Serum (accessed on 27 March 2025).
- Gutman, A.B.; Gutman, E.B. An “ACID” Phosphatase Occurring in the Serum of Patients With Metastasizing Carcinoma of the Prostate Gland. J. Clin. Investig. 1938, 17, 473–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N. Engl. J. Med. 1991, 324, 1156–1161, Erratum in N. Engl. J. Med. 1991, 325, 1324. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; Dekernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of Digital Rectal Examination and Serum Prostate Specific Antigen in the Early Detection of Prostate Cancer: Results of a Multicenter Clinical Trial of 6630 Men. J. Urol. 1994, 151, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, R.; Tsodikov, A.; Mariotto, A.; Szabo, A.; Falcon, S.; Wegelin, J.; Ditommaso, D.; Karnofski, K.; Gulati, R.; Penson, D.F.; et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2007, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Merriel, S.W.D.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.M.; Spencer, A.; Hamilton, W. Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients. BMC Med. 2022, 20, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornford, P.; Bergh, R.C.v.D.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2024, 86, 148. [Google Scholar] [CrossRef]
- Bizzarri, F.P.; Campetella, M.; Ragonese, M.; Scarciglia, E.; Russo, P.; Marino, F.; Filomena, G.B.; Gavi, F.; Rossi, F.; D’Amico, L.; et al. The role of alternative medicine and complimentary therapies in urologic disease: New horizons. Urol. J. 2024, 91, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, K3519. [Google Scholar] [CrossRef]
- Brawer, M.K.; Chetner, M.P.; Beatie, J.; Buchner, D.M.; Vessella, R.L.; Lange, P.H. Screening for prostatic carcinoma with prostate specific antigen. J. Urol. 1992, 147 Pt 2, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Ankerst, D.P.; Chi, C.; Lucia, M.S.; Goodman, P.J.; Crowley, J.J.; Parnes, H.L.; Coltman, C.A. Operating Characteristics of Prostate-Specific Antigen in Men With an Initial PSA Level of 3.0 ng/mL or Lower. JAMA 2005, 294, 66–70. [Google Scholar] [CrossRef]
- Loeb, S.; Carter, H.B.; Berndt, S.I.; Ricker, W.; Schaeffer, E.M. Complications Following Prostate Biopsy: Data from SEER-Medicare. J. Urol. 2011, 186, 1830. [Google Scholar] [CrossRef]
- Rodríguez, L.V.; Terris, M.K. Risks and complications of transrectal ultrasound guided prostate needle biopsy: A prospective study and review of the literature. J Urol. 1998, 160 Pt 1, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Wolters, T.; van der Kwast, T.H.; Vissers, C.J.; Bangma, C.H.; Roobol, M.M.; Schröder, F.H.; van Leenders, G.J.L.H. False-negative prostate needle biopsies: Frequency, histopathologic features, and follow-up. Am. J. Surg. Pathol. 2010, 34, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, M.; Ahmed, H.; Nam, R.; Schaeffer, E.; Schiavina, R.; Taneja, S.; Weidner, W.; Loeb, S. Complications After Systematic, Random, and Image-guided Prostate Biopsy. Eur. Urol. 2017, 71, 353–365. [Google Scholar] [CrossRef]
- Warner, J.; Whitmore, W.F. Expectant management of clinically localized prostatic cancer. J. Urol. 1994, 152, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Zhang, L.; Lam, A.; Nam, R.; Mamedov, A.; Loblaw, A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J. Clin. Oncol. 2010, 28, 126–131. [Google Scholar] [CrossRef]
- Heijnsdijk, E.A.; Wever, E.M.; Auvinen, A.; Hugosson, J.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Villers, A.; Páez, A.; Moss, S.M.; et al. Quality-of-life effects of prostate-specific antigen screening. N. Engl. J. Med. 2012, 367, 595–605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, D.P.; King, M.T.; Egger, S.; Berry, M.P.; Stricker, P.D.; Cozzi, P.; Ward, J.; O’Connell, D.L.; Armstrong, B.K. Quality of life three years after diagnosis of localised prostate cancer: Population based cohort study. BMJ 2009, 339, 195. [Google Scholar] [CrossRef]
- Madalinska, J.B.; Essink-Bot, M.L.; De Koning, H.J.; Kirkels, W.J.; Van der Maas, P.J.; Schröder, F.H. Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J. Clin. Oncol. 2001, 19, 1619–1628. [Google Scholar] [CrossRef]
- Mols, F.; Korfage, I.J.; Vingerhoets, A.J.; Kil, P.J.; Coebergh, J.W.W.; Essink-Bot, M.-L.; van de Poll-Franse, L.V. Bowel, urinary, and sexual problems among long-term prostate cancer survivors: A population-based study. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 30–38. [Google Scholar] [CrossRef]
- Mazzone, E.; Stabile, A.; Pellegrino, F.; Basile, G.; Cignoli, D.; Cirulli, G.O.; Sorce, G.; Barletta, F.; Scuderi, S.; Bravi, C.A.; et al. Positive Predictive Value of Prostate Imaging Reporting and Data System Version 2 for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2021, 4, 697–713. [Google Scholar] [CrossRef]
- Feng, X.; Chen, X.; Peng, P.; Zhou, H.; Hong, Y.; Zhu, C.; Lu, L.; Xie, S.; Zhang, S.; Long, L. Values of multiparametric and biparametric MRI in diagnosing clinically significant prostate cancer: A multivariate analysis. BMC Urol. 2024, 24, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Bergh, R.C.V.D.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- van Schie, M.A.; Dinh, C.V.; van Houdt, P.J.; Pos, F.J.; Heijmink, S.W.; Kerkmeijer, L.G.; Kotte, A.N.; Oyen, R.; Haustermans, K.; van der Heide, U.A. Contouring of prostate tumors on multiparametric MRI: Evaluation of clinical delineations in a multicenter radiotherapy trial. Radiother. Oncol. 2018, 128, 321–326. [Google Scholar] [CrossRef]
- Garvey, B.; Türkbey, B.; Truong, H.; Bernardo, M.; Periaswamy, S.; Choyke, P.L. Clinical value of prostate segmentation and volume determination on MRI in benign prostatic hyperplasia. Diagn. Interv. Radiol. 2014, 20, 229. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Toth, R.; van de Ven, W.; Hoeks, C.; Kerkstra, S.; van Ginneken, B.; Vincent, G.; Guillard, G.; Birbeck, N.; Zhang, J.; et al. Evaluation of prostate segmentation algorithms for MRI: The PROMISE12 challenge. Med. Image Anal. 2014, 18, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Rundo, L.; Militello, C.; Russo, G.; Garufi, A.; Vitabile, S.; Gilardi, M.C.; Mauri, G. Automated Prostate Gland Segmentation Based on an Unsupervised Fuzzy C-Means Clustering Technique Using Multispectral T1w and T2w MR Imaging. Information 2017, 8, 49. [Google Scholar] [CrossRef]
- Arif, M.; Schoots, I.G.; Tovar, J.C.; Bangma, C.H.; Krestin, G.P.; Roobol, M.J.; Niessen, W.; Veenland, J.F. Clinically significant prostate cancer detection and segmentation in low-risk patients using a convolutional neural network on multi-parametric MRI. Eur. Radiol. 2020, 30, 6582–6592. [Google Scholar] [CrossRef]
- Pellicer-Valero, O.J.; Jiménez, J.L.M.; Gonzalez-Perez, V.; Ramón-Borja, J.L.C.; García, I.M.; Benito, M.B.; Gómez, P.P.; Rubio-Briones, J.; Rupérez, M.J.; Martín-Guerrero, J.D. Deep learning for fully automatic detection, segmentation, and Gleason grade estimation of prostate cancer in multiparametric magnetic resonance images. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Algohary, A.; Shiradkar, R.; Pahwa, S.; Purysko, A.; Verma, S.; Moses, D.; Shnier, R.; Haynes, A.-M.; Delprado, W.; Thompson, J.; et al. Combination of Peri-Tumoral and Intra-Tumoral Radiomic Features on Bi-Parametric MRI Accurately Stratifies Prostate Cancer Risk: A Multi-Site Study. Cancers 2020, 12, 2200. [Google Scholar] [CrossRef]
- Zhuang, H.; Chatterjee, A.; Fan, X.; Qi, S.; Qian, W.; He, D. A radiomics based method for prediction of prostate cancer Gleason score using enlarged region of interest. BMC Med. Imaging 2023, 23, 205. [Google Scholar] [CrossRef]
- Gutsche, R.; Gülmüs, G.; Mottaghy, F.M.; Gärtner, F.; Essler, M.; von Mallek, D.; Ahmadzadehfar, H.; Lohmann, P.; Heinzel, A. Multicentric 68Ga-PSMA PET radiomics for treatment response assessment of 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. Front. Nucl. Med. 2023, 3, 1234853. [Google Scholar] [CrossRef] [PubMed]
- Telecan, T.; Chiorean, A.; Sipos-Lascu, R.; Caraiani, C.; Boca, B.; Hendea, R.M.; Buliga, T.; Andras, I.; Crisan, N.; Lupsor-Platon, M. ISUP Grade Prediction of Prostate Nodules on T2WI Acquisitions Using Clinical Features, Textural Parameters and Machine Learning-Based Algorithms. Cancers 2025, 17, 2035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayerl, N.; Adams, L.C.; Cavallaro, A.; Bäuerle, T.; Schlicht, M.; Wullich, B.; Hartmann, A.; Uder, M.; Ellmann, S. Assessment of a fully-automated diagnostic AI software in prostate MRI: Clinical evaluation and histopathological correlation. Eur. J. Radiol. 2024, 181, 111790. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; van Ginneken, B.; Bjartell, A.; Bonekamp, D.; Villeirs, G.; Salomon, G.; Giannarini, G.; Kalpathy-Cramer, J.; Barentsz, J.; Rusu, M.; et al. Artificial intelligence and radiologists in prostate cancer detection on MRI (PI-CAI): An international, paired, non-inferiority, confirmatory study. Lancet Oncol. 2024, 25, 879–887. [Google Scholar] [CrossRef]
- Müller, D.; Meyer, P.; Rentschler, L.; Manz, R.; Bäcker, J.; Cramer, S.; Wengenmayr, C.; Märkl, B.; Huss, R.; Soto-Rey, I.; et al. DeepGleason: A System for Automated Gleason Grading of Prostate Cancer using Deep Neural Networks. arXiv 2024, arXiv:2403.16678. Available online: https://arxiv.org/abs/2403.16678 (accessed on 1 June 2025). [CrossRef]
- Morozov, A.; Taratkin, M.; Bazarkin, A.; Rivas, J.G.; Puliatti, S.; Checcucci, E.; Belenchon, I.R.; Kowalewski, K.-F.; Shpikina, A.; Singla, N.; et al. A systematic review and meta-analysis of artificial intelligence diagnostic accuracy in prostate cancer histology identification and grading. Prostate Cancer Prostatic Dis. 2023, 26, 681–692. [Google Scholar] [CrossRef]
- Steiner, D.F.; Nagpal, K.; Sayres, R.; Foote, D.J.; Wedin, B.D.; Pearce, A.; Cai, C.J.; Winter, S.R.; Symonds, M.; Yatziv, L.; et al. Evaluation of the Use of Combined Artificial Intelligence and Pathologist Assessment to Review and Grade Prostate Biopsies. JAMA Netw. Open 2020, 3, e2023267. [Google Scholar] [CrossRef]
- Spratt, D.E.; Sun, Y.; Van der Wal, D.; Huang, S.-C.; Mohamad, O.; Armstrong, A.J.; Tward, J.D.; Nguyen, P.; Chen, E.; DeVries, S.; et al. An AI-derived digital pathology-based biomarker to predict the benefit of androgen deprivation therapy in localized prostate cancer with validation in NRG/RTOG 9408. J. Clin. Oncol. 2022, 40 (Suppl. S6), 223. [Google Scholar] [CrossRef]
- Wessels, F.; Kuntz, S.; Krieghoff-Henning, E.; Schmitt, M.; Braun, V.; Worst, T.S.; Neuberger, M.; Steeg, M.; Gaiser, T.; Fröhling, S.; et al. Artificial intelligence to predict oncological outcome directly from hematoxylin and eosin-stained slides in urology. Minerva Urol. Nephrol. 2022, 74, 538–550. [Google Scholar] [CrossRef]
- Huang, W.; Randhawa, R.; Jain, P.; Hubbard, S.; Eickhoff, J.; Kummar, S.; Wilding, G.; Basu, H.; Roy, R. A Novel Artificial Intelligence-Powered Method for Prediction of Early Recurrence of Prostate Cancer After Prostatectomy and Cancer Drivers. JCO Clin. Cancer Inform. 2022, 6, e2100131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marletta, S.; Eccher, A.; Martelli, F.M.; Santonicco, N.; Girolami, I.; Scarpa, A.; Pagni, F.; L’Imperio, V.; Pantanowitz, L.; Gobbo, S.; et al. Artificial intelligence-based algorithms for the diagnosis of prostate cancer: A systematic review. Am. J. Clin. Pathol. 2024, 161, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Hachem, S.; Yehya, A.; El Masri, J.; Mavingire, N.; Johnson, J.R.; Dwead, A.M.; Kattour, N.; Bouchi, Y.; Kobeissy, F.; Rais-Bahrami, S.; et al. Contemporary Update on Clinical and Experimental Prostate Cancer Biomarkers: A Multi-Omics-Focused Approach to Detection and Risk Stratification. Biology 2024, 13, 762. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, D.; Goddard, A.D.; Natraj, N.; Cherbavaz, D.B.; Clark-Langone, K.M.; Snable, J.; Watson, D.; Falzarano, S.M.; Magi-Galluzzi, C.; A Klein, E.; et al. Analytical validation of the Oncotype DX prostate cancer assay—A clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genom. 2013, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Esengur, O.T.; Yilmaz, E.C.; Ozyoruk, K.B.; Chen, A.; Lay, N.S.; Gelikman, D.G.; Merino, M.J.; Gurram, S.; Wood, B.J.; Choyke, P.L.; et al. Multimodal approach to optimize biopsy decision-making for PI-RADS 3 lesions on multiparametric MRI. Clin Imaging 2025, 117, 110363. [Google Scholar] [CrossRef]
- Fountzilas, E.; Pearce, T.; Baysal, M.A.; Chakraborty, A.; Tsimberidou, A.M. Convergence of evolving artificial intelligence and machine learning techniques in precision oncology. Npj Digit. Med. 2025, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Menengola, E.; Gabardo, E.; González Sanmiguel, N.N. The proposal of the european regulation on artificial intelligence. Sequência 2023, 43, e91435. [Google Scholar] [CrossRef]
- Far, B.F. Artificial intelligence ethics in precision oncology: Balancing advancements in technology with patient privacy and autonomy. Explor. Target. Antitumor Ther. 2023, 4, 685. [Google Scholar] [CrossRef]
- Waldman, C.E.; Hermel, M.; A Hermel, J.; Allinson, F.; Pintea, M.N.; Bransky, N.; Udoh, E.; Nicholson, L.; Robinson, A.; Gonzalez, J.; et al. Artificial Intelligence in Healthcare: A Primer for Medical Education in Radiomics. Pers. Med. 2022, 19, 445–456. [Google Scholar] [CrossRef] [PubMed]
| Study | Objectives | Main Findings |
|---|---|---|
| Arif, M. et al. (2020) [32] | csPCa detection and segmentation in 292 low-risk patients by a convolutional neural network model on prostate MRI | The model achieved a high sensitivity (82–92%) with a variable specificity (43–76%) based on lesion volume (AUC of 0.65–0.89) |
| Pellicer-Valero, O et al. (2022) [33] | Testing a fully automated DL model for csPCa detection, lesion segmentation, and Gleason grade evaluation on MRI | High sensitivity and specificity for prostate lesion segmentation (100% and 79%) and for PCa detection (100% and 80%) on MRI |
| Algohary, A. et al. (2020) [35] | Evaluation of combining peri-tumoral and intra-tumoral radiomics of prostate MRI images for risk stratification | Radiomics using the peri-tumoral and intra-tumoral features had accuracy of 53% (vs. 48% PI-RADS) for PCa risk stratification |
| Zhuang, H. et al. (2023) [36] | Evaluation of radiomics features for Gleason estimation using enlarged ROIs in 26 biopsy-proven PCa patients | Radiomics achieved 73.96% accuracy (Gleason ≥ 3+4 vs. 3+3) vs. 83.72% (Gleason 3+4 vs. ≥4+3) for radiologist-drawn ROIs |
| Bayerl, N. et al. (2024) [39] | Assessment of a fully automated diagnostic AI software for prostate MRI evaluation and pathological correlation | AI software showed 100% sensitivity for PI-RADS ≥ 2 lesions and 85.5% sensitivity with 63.2% specificity for PI-RADS ≥ 4 lesions |
| Saha, A. et al. (2024) [40] | International large study with 10,207 studies over 10 years comparing AI and radiologists for csPCa detection on MRI | In 400 cases, AI outperformed 62 radiologists for PCa detection |
| Study | Objectives | Results |
|---|---|---|
| Steiner, D. F. et al. (2020) [43] | Evaluation of merging AI and pathologist evaluation to review and grade prostate biopsy cores in 240 patients | AI reviews showed a 5.6% improvement in agreement with pathologist (69.7% to 75.3%; p < 0.001). AI reviews also improved tumoral detection, time needed for review, self-confidence, and pathologists’ inter-agreement |
| Spratt, D. E. et al. (2022) [44] | Evaluation of an AI-powered digital pathology-based biomarker to predict ADT results in localized PCa with validation in NRG/RTOG 9408 studies with 1719 patients | AI-derived ADT biomarker evaluation showed benefit in the ADT group (HR 0.62, p = 0.006). In the biomarker-positive subgroup (39%), the ADT improved outcomes (HR 0.33, p < 0.001). Median follow-up of 17.4 years |
| Morozov, A. et al. (2023) [42] | Meta-analysis of AI diagnostic accuracy in diagnosing PCa and evaluating Gleason grades based on 24 studies with 8000 biopsies and 458 prostatectomy specimens | AI had high sensitivity (87–100%) and high specificity (68–99%) for PCa diagnosis. Meta-analysis pooled sensitivity of 0.96 and specificity of 0.95 |
| Müller, D. et al. (2024) [41] | Evaluating a neural network AI system (DeepGleason©) trained on 34,264 tiles from 369 slides for automated Gleason grading of PCa on pathologic prostate samples | High accuracy (97.4%) with outperformance of conventional methods to separate benign from malignant lesions (sensitivity 94%, specificity 98%) and Gleason 3 from Gleason 4–5 lesions (sensitivity 91%, specificity 75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Barajraji, M.; Coscarella, M.; Svistakov, I.; Flôres Soares da Silva, H.; Déniz, P.M.; Marugan, M.J.; González-Santander, C.; Fernández Montarroso, L.; Galante, I.; Rivas, J.G.; et al. Beyond PSA: The Future of Prostate Cancer Diagnosis Using Artificial Intelligence, Novel Biomarkers, and Advanced Imagery. Life 2025, 15, 1508. https://doi.org/10.3390/life15101508
Al Barajraji M, Coscarella M, Svistakov I, Flôres Soares da Silva H, Déniz PM, Marugan MJ, González-Santander C, Fernández Montarroso L, Galante I, Rivas JG, et al. Beyond PSA: The Future of Prostate Cancer Diagnosis Using Artificial Intelligence, Novel Biomarkers, and Advanced Imagery. Life. 2025; 15(10):1508. https://doi.org/10.3390/life15101508
Chicago/Turabian StyleAl Barajraji, Moncef, Mathieu Coscarella, Ilyas Svistakov, Helena Flôres Soares da Silva, Paula Mata Déniz, María Jesús Marugan, Claudia González-Santander, Lorena Fernández Montarroso, Isabel Galante, Juan Gómez Rivas, and et al. 2025. "Beyond PSA: The Future of Prostate Cancer Diagnosis Using Artificial Intelligence, Novel Biomarkers, and Advanced Imagery" Life 15, no. 10: 1508. https://doi.org/10.3390/life15101508
APA StyleAl Barajraji, M., Coscarella, M., Svistakov, I., Flôres Soares da Silva, H., Déniz, P. M., Marugan, M. J., González-Santander, C., Fernández Montarroso, L., Galante, I., Rivas, J. G., & Moreno Sierra, J. (2025). Beyond PSA: The Future of Prostate Cancer Diagnosis Using Artificial Intelligence, Novel Biomarkers, and Advanced Imagery. Life, 15(10), 1508. https://doi.org/10.3390/life15101508






