Short-Term In Vitro Culture of Human Ovarian Tissue: A Comparative Study of Serum Supplementation for Primordial Follicle Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient History
2.2. Ovarian Tissue Collection, Freezing, and Thawing
2.3. Media Preparation
2.4. Ovarian Tissue Culture
2.5. Histological Examination
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| OCFs | Ovarian Cortical Fragments |
| HSA | Human Serum Albumin |
| HS | Human Serum |
| SSS | Serum Substitute Supplement |
| PMFs | Primordial Follicles |
| POI | Primary Ovarian Insufficiency |
| OTC-T | Cryopreservation And Transplantation |
| BCM | Basic Culture Medium |
| GCs | Granulosa Cells |
| PFs | Primary Follicles |
| GFs | Growing Follicles |
| GLMM | Generalized Linear Mixed Model |
| IVF | In Vitro Fertilization |
References
- Data Visualization Tools for Exploring the Global Cancer Burden in 2022. Available online: https://gco.iarc.fr/today/en (accessed on 5 September 2025).
- Lee, S.; Ozkavukcu, S.; Ku, S.-Y. Current and Future Perspectives for Improving Ovarian Tissue Cryopreservation and Transplantation Outcomes for Cancer Patients. Reprod. Sci. 2021, 28, 1746–1758. [Google Scholar] [CrossRef]
- Markham, M.J.; Wachter, K.; Agarwal, N.; Bertagnolli, M.M.; Chang, S.M.; Dale, W.; Diefenbach, C.S.M.; Rodriguez-Galindo, C.; George, D.J.; Gilligan, T.D.; et al. Clinical Cancer Advances 2020: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. JCO 2020, 38, 1081. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Oktay, K. Options on Fertility Preservation in Female Cancer Patients. Cancer Treat. Rev. 2012, 38, 354–361. [Google Scholar] [CrossRef]
- Jeong, K.; Aslan, E.; Ozkaya, E.; Sonmezer, M.; Oktay, K. Ovarian Cryopreservation. Minerva Med. 2012, 103, 37–46. [Google Scholar]
- Dolmans, M.-M.; Hossay, C.; Nguyen, T.Y.T.; Poirot, C. Fertility Preservation: How to Preserve Ovarian Function in Children, Adolescents and Adults. J. Clin. Med. 2021, 10, 5247. [Google Scholar] [CrossRef] [PubMed]
- The ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva De Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE Guideline: Female Fertility Preservation†. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoshioka, N.; Takae, S.; Sugishita, Y.; Tamura, M.; Hashimoto, S.; Morimoto, Y.; Kawamura, K. Successful Fertility Preservation Following Ovarian Tissue Vitrification in Patients with Primary Ovarian Insufficiency. Hum. Reprod. 2015, 30, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.J.; DeRosa, M.; Goldsmith, S.; Fan, Y.; Castleman, L.; Melnick, J. Cryopreservation and Transplantation of Ovarian Tissue: Results from One Center in the USA. J. Assist. Reprod. Genet. 2018, 35, 2205–2213. [Google Scholar] [CrossRef]
- Sänger, N.; John, J.; Einenkel, R.; Schallmoser, A. First Report on Successful Delivery after Retransplantation of Vitrified, Rapid Warmed Ovarian Tissue in Europe. Reprod. Biomed. Online 2024, 49, 103940. [Google Scholar] [CrossRef]
- Keros, V.; Milenkovic, M.; Hultenby, K. The Art of Cryopreservation-Live Birth after Transplantation of Vitrified Ovarian Tissue. Cryobiology 2024, 117, 105085. [Google Scholar] [CrossRef]
- Fabbri, R.; Pasquinelli, G.; Bracone, G.; Orrico, C.; Di Tommaso, B.; Venturoli, S. Cryopreservation of Human Ovarian Tissue. Cell Tissue Bank 2006, 7, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of Cryopreserved Ovarian Tissue in a Series of 285 Women: A Review of Five Leading European Centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Wakimoto, Y.; Colmorn, L.B.; Dueholm, M.; Pors, S.E.; Macklon, K.T.; Mamsen, L.S.; Nikiforov, D.; Cadenas, J.; Greve, V.H.; et al. Use of Cryopreserved Ovarian Tissue in the Danish Fertility Preservation Cohort. Fertil. Steril. 2021, 116, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Lotz, L.; Bender-Liebenthron, J.; Dittrich, R.; Häberle, L.; Beckmann, M.W.; Germeyer, A.; Korell, M.; Sänger, N.; Kruessel, J.S.; Von Wolff, M.; et al. Determinants of Transplantation Success with Cryopreserved Ovarian Tissue: Data from 196 Women of the FertiPROTEKT Network. Hum. Reprod. 2022, 37, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Feinmesser, M.; Yaniv, I.; Fisch, B.; Cohen, I.J.; Ben-Haroush, A.; Meirow, D.; Felz, C.; Avigad, S. Occasional Involvement of the Ovary in Ewing Sarcoma. Hum. Reprod. 2010, 25, 1708–1712. [Google Scholar] [CrossRef]
- Rosendahl, M.; Andersen, M.T.; Ralfkiær, E.; Kjeldsen, L.; Andersen, M.K.; Andersen, C.Y. Evidence of Residual Disease in Cryopreserved Ovarian Cortex from Female Patients with Leukemia. Fertil. Steril. 2010, 94, 2186–2190. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Marinescu, C.; Saussoy, P.; Van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of Cryopreserved Ovarian Tissue from Patients with Acute Lymphoblastic Leukemia Is Potentially Unsafe. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Ovarian Tissue Freezing: Current Status. Curr. Opin. Obstet. Gynecol. 2015, 27, 222–230. [Google Scholar] [CrossRef]
- Subiran Adrados, C.; Cadenas, J.; Zheng, M.; Lund, S.; Larsen, E.C.; Tanvig, M.H.; Greve, V.H.; Blanche, P.; Andersen, C.Y.; Kristensen, S.G. Human Platelet Lysate Improves the Growth and Survival of Cultured Human Pre-Antral Follicles. Reprod. Biomed. Online 2023, 47, 103256. [Google Scholar] [CrossRef]
- Schallmoser, A.; Emrich, N.; Einenkel, R.; Sänger, N. Explorative 3-D Culture of Early Secondary Follicles in a Time Lapse System for up to 36 Days Gives Valuable, but Limited Insights in Follicular Development. Placenta 2025, 164, 50–63. [Google Scholar] [CrossRef]
- De Vos, M.; Grynberg, M.; Ho, T.M.; Yuan, Y.; Albertini, D.F.; Gilchrist, R.B. Perspectives on the Development and Future of Oocyte IVM in Clinical Practice. J. Assist. Reprod. Genet. 2021, 38, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Segers, I.; Mateizel, I.; Wouters, K.; Van Moer, E.; Anckaert, E.; De Munck, N.; De Vos, M. Ovarian Tissue Oocyte-In Vitro Maturation for Fertility Preservation. J. Vis. Exp. 2024, 207, 65255. [Google Scholar] [CrossRef]
- Salama, M.; Anazodo, A.; Woodruff, T.K. Preserving Fertility in Female Patients with Hematological Malignancies: A Multidisciplinary Oncofertility Approach. Ann. Oncol. 2019, 30, 1760–1775. [Google Scholar] [CrossRef]
- Hovatta, O.; Silye, R.; Abir, R.; Krausz, T.; Winston, R.M. Extracellular Matrix Improves Survival of Both Stored and Fresh Human Primordial and Primary Ovarian Follicles in Long-Term Culture. Hum. Reprod. 1997, 12, 1032–1036. [Google Scholar] [CrossRef]
- Bjarkadottir, B.D.; Walker, C.A.; Fatum, M.; Lane, S.; Williams, S.A. Analysing Culture Methods of Frozen Human Ovarian Tissue to Improve Follicle Survival. Reprod. Fertil. 2021, 2, 59–68. [Google Scholar] [CrossRef]
- Scott, J.E.; Carlsson, I.B.; Bavister, B.D.; Hovatta, O. Human Ovarian Tissue Cultures: Extracellular Matrix Composition, Coating Density and Tissue Dimensions. Reprod. Biomed. Online 2004, 9, 287–293. [Google Scholar] [CrossRef]
- Anderson, R.A.; McLaughlin, M.; Wallace, W.H.B.; Albertini, D.F.; Telfer, E.E. The Immature Human Ovary Shows Loss of Abnormal Follicles and Increasing Follicle Developmental Competence through Childhood and Adolescence. Hum. Reprod. 2014, 29, 97–106. [Google Scholar] [CrossRef]
- McLaughlin, M.; Kinnell, H.L.; Anderson, R.A.; Telfer, E.E. Inhibition of Phosphatase and Tensin Homologue (PTEN) in Human Ovary in Vitro Results in Increased Activation of Primordial Follicles but Compromises Development of Growing Follicles. Mol. Hum. Reprod. 2014, 20, 736–744. [Google Scholar] [CrossRef]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II Oocytes from Human Unilaminar Follicles Grown in a Multi-Step Culture System. MHR Basic Sci. Reprod. Med. 2018, 24, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hreinsson, J.G.; Scott, J.E.; Rasmussen, C.; Swahn, M.L.; Hsueh, A.J.W.; Hovatta, O. Growth Differentiation Factor-9 Promotes the Growth, Development, and Survival of Human Ovarian Follicles in Organ Culture. J. Clin. Endocrinol. Metab. 2002, 87, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.L.T. Density and Distribution of Primordial Follicles in Single Pieces of Cortex from 21 Patients and in Individual Pieces of Cortex from Three Entire Human Ovaries. Hum. Reprod. 2003, 18, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.; Liu, J.; Morgan, S.; Matthews, R.; Nevin, L.; Anderson, R.A.; Spears, N. Single and Combined Effects of Cisplatin and Doxorubicin on the Human and Mouse Ovary in Vitro. Reproduction 2020, 159, 193–204. [Google Scholar] [CrossRef]
- Fabbri, R.; Vicenti, R.; Macciocca, M.; Pasquinelli, G.; Lima, M.; Parazza, I.; Magnani, V.; Venturoli, S. Cryopreservation of Ovarian Tissue in Pediatric Patients. Obstet. Gynecol. Int. 2012, 2012, 910698. [Google Scholar] [CrossRef]
- Fabbri, R.; Vicenti, R.; Macciocca, M.; Martino, N.A.; Dell’Aquila, M.E.; Pasquinelli, G.; Morselli-Labate, A.M.; Seracchioli, R.; Paradisi, R. Morphological, Ultrastructural and Functional Imaging of Frozen/Thawed and Vitrified/Warmed Human Ovarian Tissue Retrieved from Oncological Patients. Hum. Reprod. 2016, 31, 1838–1849. [Google Scholar] [CrossRef]
- Fabbri, R.; Macciocca, M.; Vicenti, R.; Caprara, G.; Piccinni, M.; Paradisi, R.; Terzano, P.; Papi, A.; Seracchioli, R. Epigallocatechin-3-Gallate Inhibits Doxorubicin-Induced Inflammation on Human Ovarian Tissue. Biosci. Rep. 2019, 39, BSR20181424. [Google Scholar] [CrossRef]
- Martino, N.A.; Vicenti, R.; Macciocca, M.; Seracchioli, R.; Marzano, G.; Mastrorocco, A.; Lacalandra, G.M.; Tomaiuolo, M.; Marchesani, G.; Chiaravalle, E.A.; et al. Effects of Low-Dose X-Ray Medical Diagnostics on Female Gonads: Insights from Large Animal Oocytes and Human Ovaries as Complementary Models. PLoS ONE 2021, 16, e0253536. [Google Scholar] [CrossRef] [PubMed]
- Boone, W.R.; Keck, B.B.; Catlin, J.C.; Casey, K.J.; Boone, E.T.; Dye, P.S.; Schuett, R.J.; Tsubota, T.; Bahr, J.C. Evidence That Bears Are Induced Ovulators. Theriogenology 2004, 61, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Van Langendonckt, A.; Demylle, D.; Wyns, C.; Nisolle, M.; Donnez, J. Comparison of G1.2/G2.2 and Sydney IVF Cleavage/Blastocyst Sequential Media for the Culture of Human Embryos: A Prospective, Randomized, Comparative Study. Fertil. Steril. 2001, 76, 1023–1031. [Google Scholar] [CrossRef]
- Meintjes, M.; Chantilis, S.J.; Ward, D.C.; Douglas, J.D.; Rodriguez, A.J.; Guerami, A.R.; Bookout, D.M.; Barnett, B.D.; Madden, J.D. A Randomized Controlled Study of Human Serum Albumin and Serum Substitute Supplement as Protein Supplements for IVF Culture and the Effect on Live Birth Rates. Hum. Reprod. 2008, 24, 782–789. [Google Scholar] [CrossRef]
- Fabbri, R.; Pasquinelli, G.; Keane, D.; Magnani, V.; Paradisi, R.; Venturoli, S. Optimization of Protocols for Human Ovarian Tissue Cryopreservation with Sucrose, 1,2-Propanediol and Human Serum. Reprod. Biomed. Online 2010, 21, 819–828. [Google Scholar] [CrossRef]
- Gougeon, A. Regulation of Ovarian Follicular Development in Primates: Facts and Hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef]
- Cheng, H.; Wei, F.; Del Valle, J.S.; Stolk, T.H.R.; Huirne, J.A.; Asseler, J.D.; Pilgram, G.S.K.; Van Der Westerlaken, L.A.J.; Van Mello, N.M.; Chuva De Sousa Lopes, S.M. In Vitro Growth of Secondary Follicles from Cryopreserved-Thawed Ovarian Cortex. Hum. Reprod. 2024, 39, 2743–2753. [Google Scholar] [CrossRef]
- Xu, F.; Lawson, M.S.; Bean, Y.; Ting, A.Y.; Pejovic, T.; De Geest, K.; Moffitt, M.; Mitalipov, S.M.; Xu, J. Matrix-Free 3D Culture Supports Human Follicular Development from the Unilaminar to the Antral Stage in Vitro Yielding Morphologically Normal Metaphase II Oocytes. Hum. Reprod. 2021, 36, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Hovatta, O.; Margara, R.; Trew, G.; Winston, R.M.; Franks, S.; Hardy, K. Effects of Follicle-Stimulating Hormone and Serum Substitution on the in-Vitro Growth of Human Ovarian Follicles. Hum. Reprod. 1999, 14, 1555–1562. [Google Scholar] [CrossRef]
- Nonowaki, S.; Takahashi, K.; Horiuchi, T. Culture Media Affect Follicle Survival and Oocyte Maturation in Preantral Mouse Follicle Cultures. J. Mamm. Ova Res. 2010, 27, 35–41. [Google Scholar] [CrossRef]
- Simon, L.E.; Kumar, T.R.; Duncan, F.E. In Vitro Ovarian Follicle Growth: A Comprehensive Analysis of Key Protocol Variables†. Biol. Reprod. 2020, 103, 455–470. [Google Scholar] [CrossRef]
- Moshrefi, M.; Aflatoonian, A.; Ghasemi-Esmailabad, S.; Karimi-Zarchi, M.; Sadeghian-Nodoushan, F.; Shahmohamadi, S.; Nikukar, H. How to Support the In Vitro Culture of Human Ovarian Cells and Follicles? A Preliminary Step for Assembling an Artificial Ovary. 2021. Available online: https://www.researchsquare.com/article/rs-415982/v1 (accessed on 1 August 2025).
- Telfer, E.E.; Andersen, C.Y. In Vitro Growth and Maturation of Primordial Follicles and Immature Oocytes. Fertil. Steril. 2021, 115, 1116–1125. [Google Scholar] [CrossRef]
- Weathersbee, P.S.; Pool, T.B.; Ord, T. Synthetic Serum Substitute (SSS): A Globulin-Enriched Protein Supplement for Human Embryo Culture. J. Assist. Reprod. Genet. 1995, 12, 354–360. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Lu, X.; Wang, X.; Xi, H.; Zhu, C.; Zhang, F.; Lv, J.; Ge, H. The Effect of Protein Supplement Concentration in Embryo Transfer Medium on Clinical Outcome of IVF/ICSI Cycles: A Prospective, Randomized Clinical Trial. Reprod. Biomed. Online 2016, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Carrell, D.; Cobo, A.; Meseguer, M.; Rubio, C.; Smith, G.D. Optimizing the Culture Environment and Embryo Manipulation to Help Maintain Embryo Developmental Potential. Fertil. Steril. 2016, 105, 571–587. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Tzeng, S.-L.; Chen, W.-J.; Huang, C.-C.; Chen, H.-H.; Lee, T.-H.; Lee, M.-S. Effects of Synthetic Serum Supplementation in Sperm Preparation Media on Sperm Capacitation and Function Test Results. Oxidative Med. Cell. Longev. 2016, 2016, 1027158. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.; Lichtblau, I.; Elder, K. New Insights into Human Pre-Implantation Metabolism in Vivo and in Vitro. J. Assist. Reprod. Genet. 2013, 30, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative Stress-Induced Apoptosis in Granulosa Cells Involves JNK, P53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef] [PubMed]
- Eijkenboom, L.; Saedt, E.; Zietse, C.; Braat, D.; Beerendonk, C.; Peek, R. Strategies to Safely Use Cryopreserved Ovarian Tissue to Restore Fertility after Cancer: A Systematic Review. Reprod. Biomed. Online 2022, 45, 763–778. [Google Scholar] [CrossRef]
- Grosbois, J.; Bailie, E.C.; Kelsey, T.W.; Anderson, R.A.; Telfer, E.E. Spatio-Temporal Remodelling of the Composition and Architecture of the Human Ovarian Cortical Extracellular Matrix during in Vitro Culture. Hum. Reprod. 2023, 38, 444–458. [Google Scholar] [CrossRef]
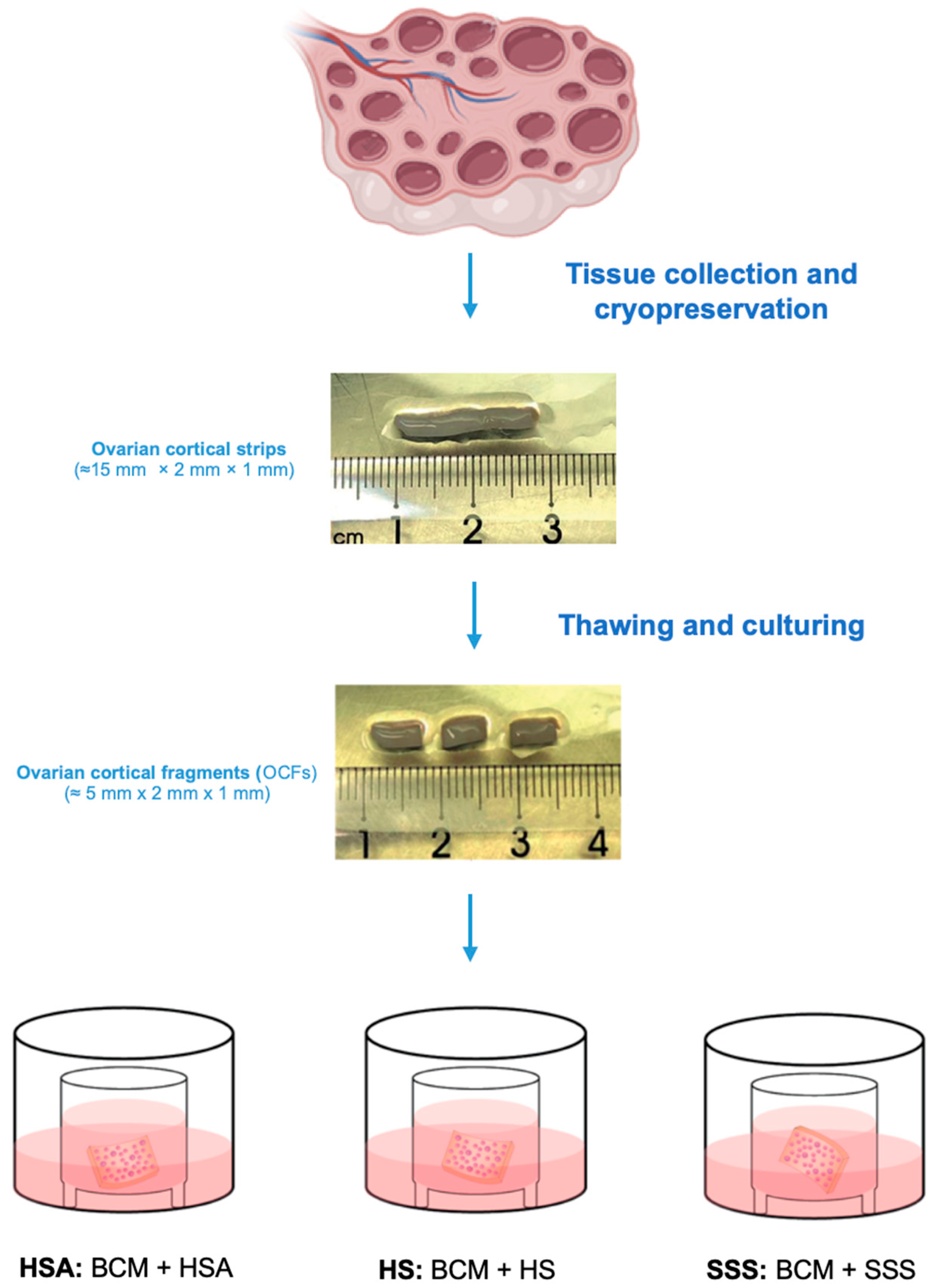
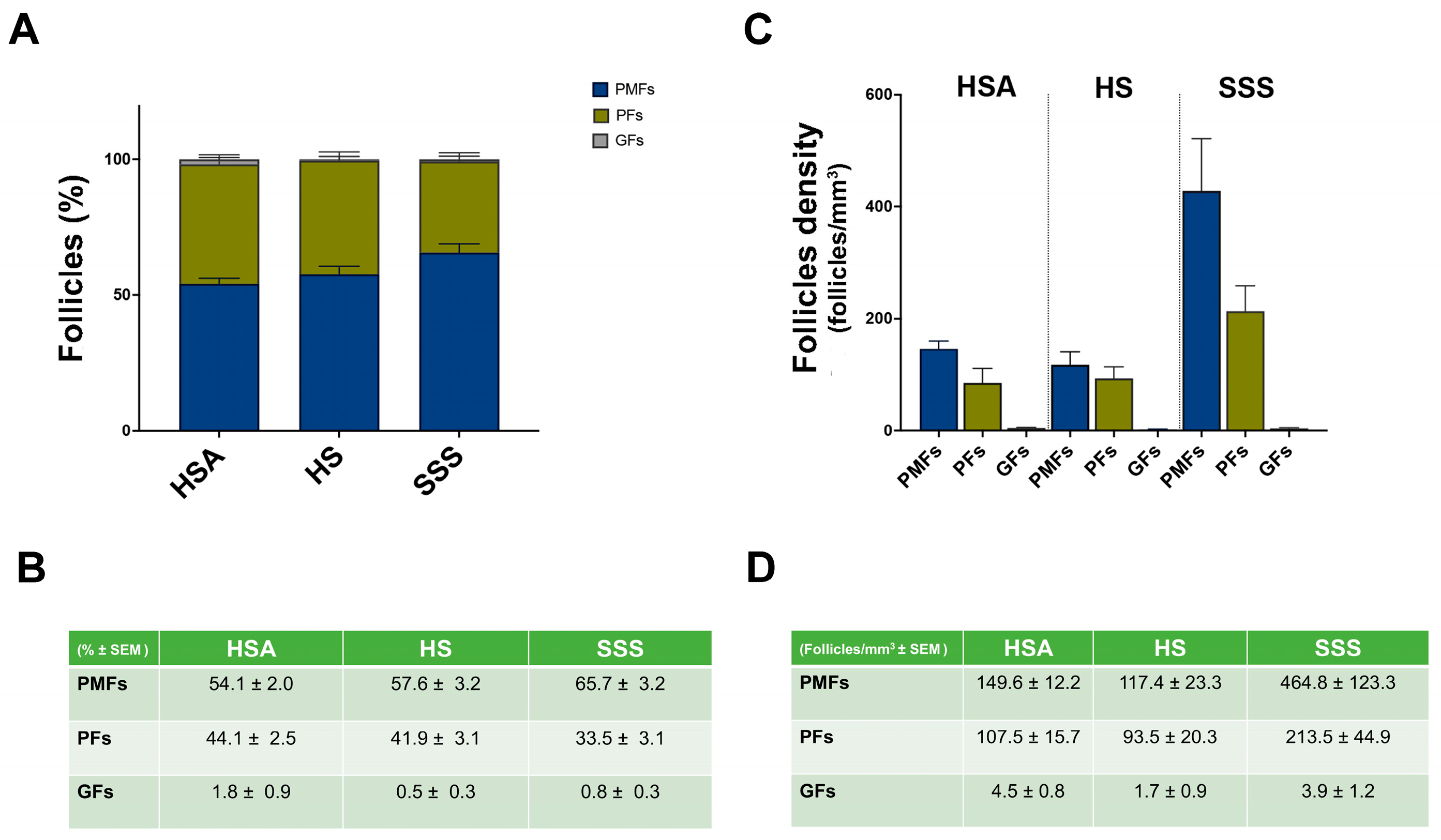
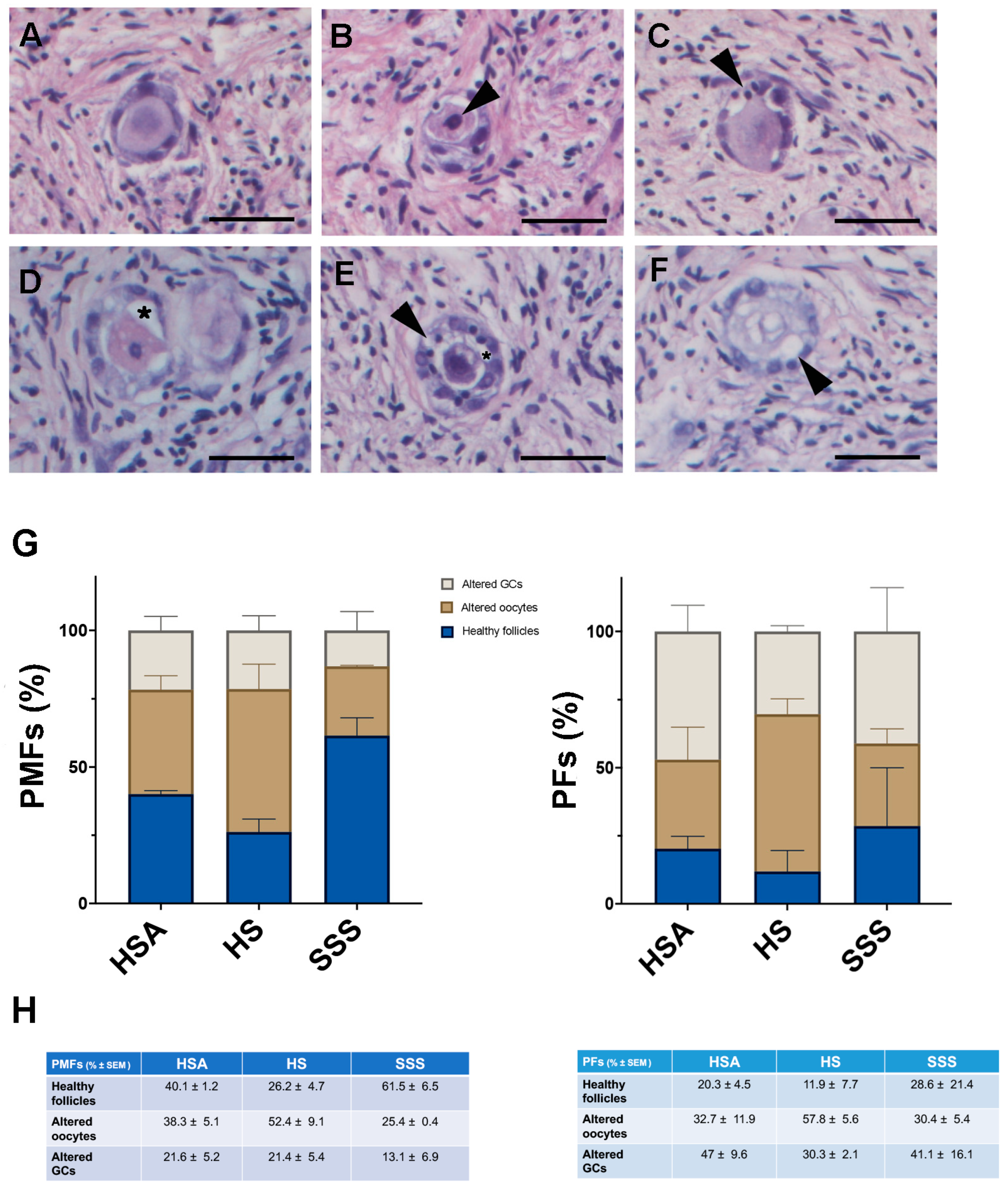
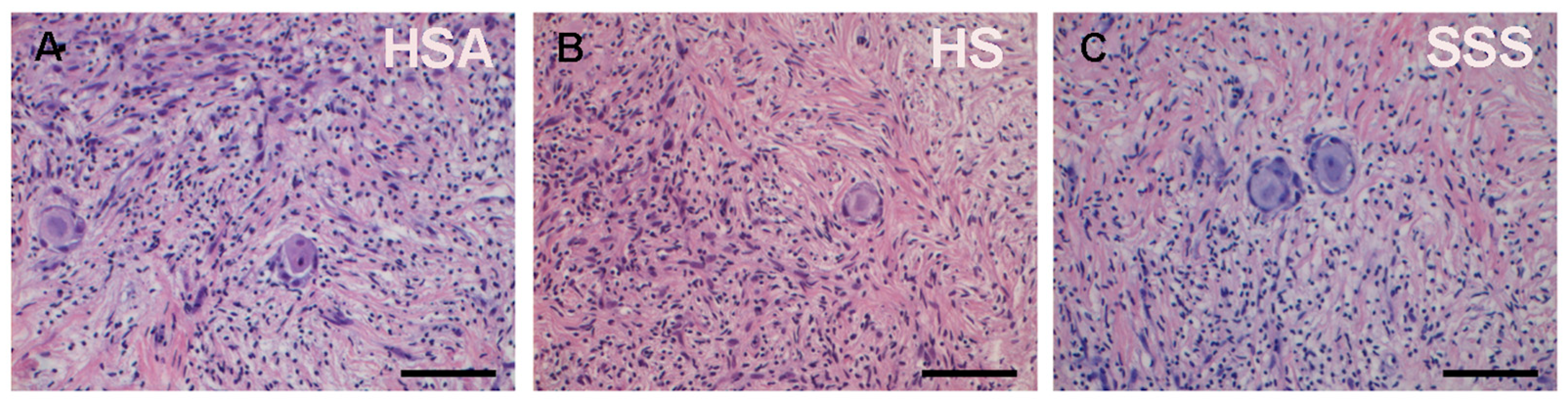
| Subject | Age (Years) | Pathology | Total Follicles Analyzed | Total PMFs Analyzed | Total PFs Analyzed | Total GFs Analyzed |
|---|---|---|---|---|---|---|
| 1 | 14 | Hemophagocytic syndrome | 8244 | 4230 | 3888 | 126 |
| 2 | 15 | Medulloblastoma | 33,067 | 22,991 | 9943 | 133 |
| 3 | 25 | Ewing’s sarcoma | 24,675 | 16,997 | 7583 | 95 |
| Predictors | Coefficient | Std. Error | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|
| Treatment HSA | 0.413 | 0.204 | 0.042 | 0.014 | 0.812 |
| Treatment SSS | 0.883 | 0.201 | <0.001 | 0.490 | 1.276 |
| Patient age | 0.044 | 0.016 | 0.005 | 0.013 | 0.075 |
| Intercept (cons) | −0.088 | 0.357 | 0.804 | −0.788 | 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcozzi, S.; Vicenti, R.; La Sala, G.; Lamsira, H.K.; Scarica, C.; Bertani, N.; De Felici, M.; Fabbri, R.; Klinger, F.G. Short-Term In Vitro Culture of Human Ovarian Tissue: A Comparative Study of Serum Supplementation for Primordial Follicle Survival. Life 2025, 15, 1509. https://doi.org/10.3390/life15101509
Marcozzi S, Vicenti R, La Sala G, Lamsira HK, Scarica C, Bertani N, De Felici M, Fabbri R, Klinger FG. Short-Term In Vitro Culture of Human Ovarian Tissue: A Comparative Study of Serum Supplementation for Primordial Follicle Survival. Life. 2025; 15(10):1509. https://doi.org/10.3390/life15101509
Chicago/Turabian StyleMarcozzi, Serena, Rossella Vicenti, Gina La Sala, Harpreet Kaur Lamsira, Catello Scarica, Nicole Bertani, Massimo De Felici, Raffaella Fabbri, and Francesca Gioia Klinger. 2025. "Short-Term In Vitro Culture of Human Ovarian Tissue: A Comparative Study of Serum Supplementation for Primordial Follicle Survival" Life 15, no. 10: 1509. https://doi.org/10.3390/life15101509
APA StyleMarcozzi, S., Vicenti, R., La Sala, G., Lamsira, H. K., Scarica, C., Bertani, N., De Felici, M., Fabbri, R., & Klinger, F. G. (2025). Short-Term In Vitro Culture of Human Ovarian Tissue: A Comparative Study of Serum Supplementation for Primordial Follicle Survival. Life, 15(10), 1509. https://doi.org/10.3390/life15101509







