Non-Inferiority Study of Two Capsaicin Formulations for Painful Diabetic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Patient Involvement
2.4. Eligibility Criteria
2.5. Study Procedures
2.6. Sample Size Calculations
2.7. Randomization
2.8. Statistical Analysis
2.9. Ethical Procedures
3. Results
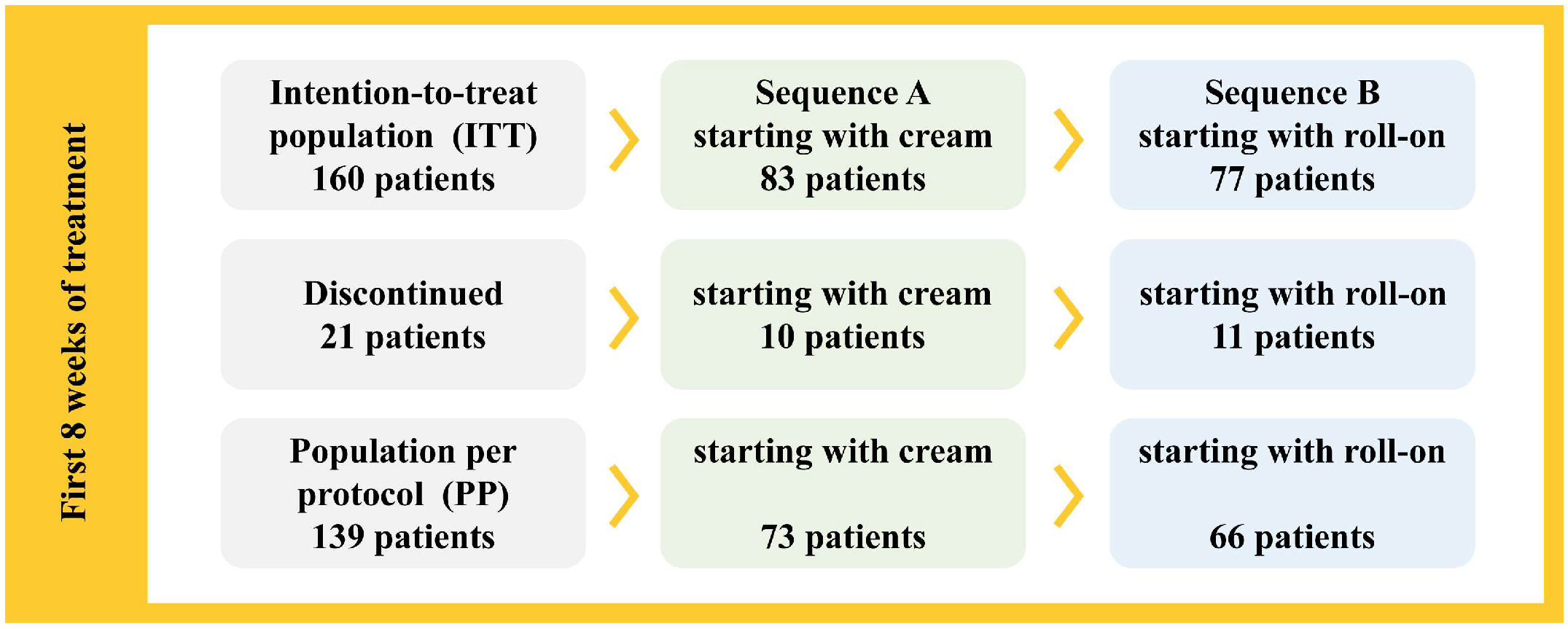
3.1. Population
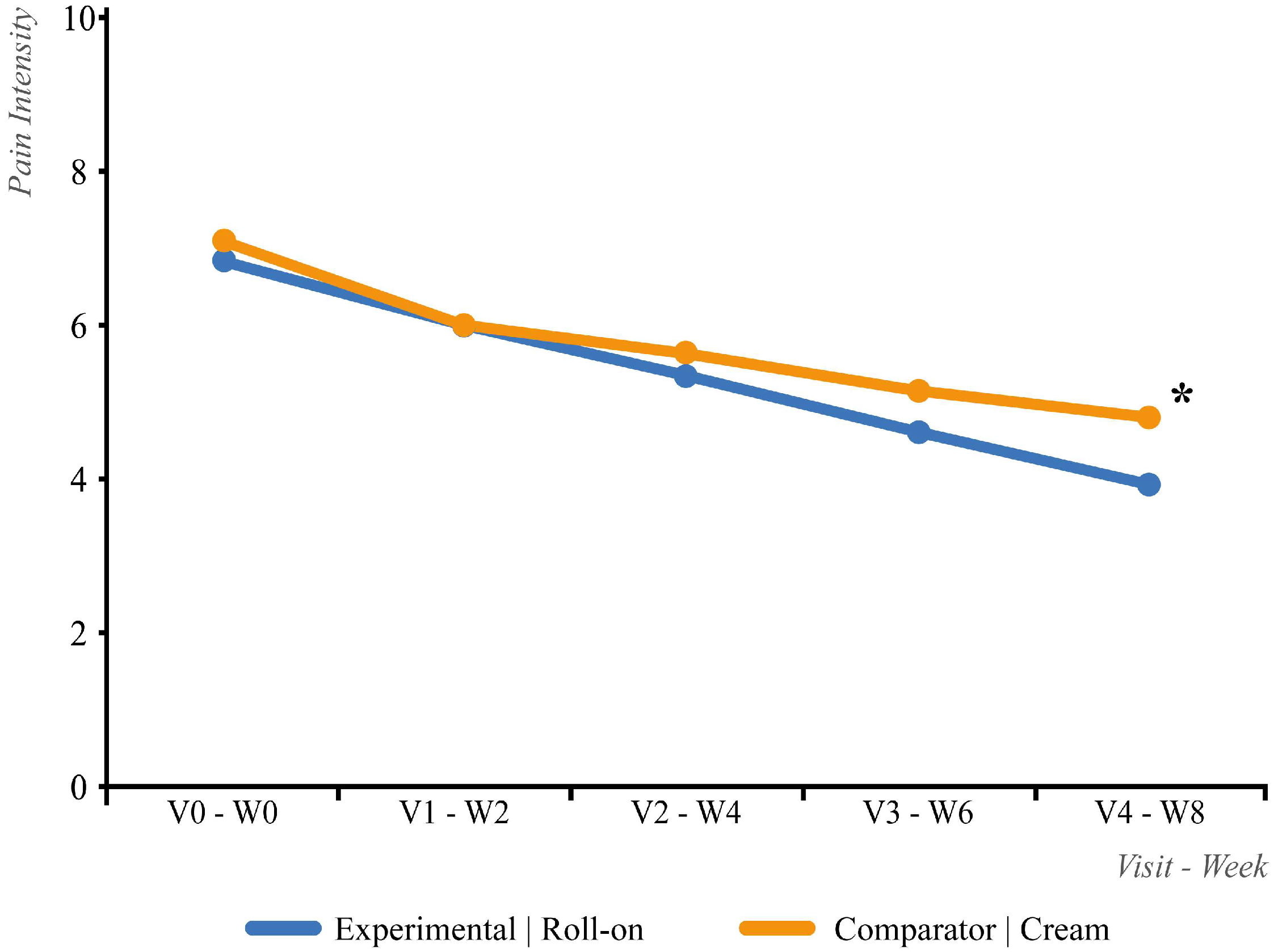
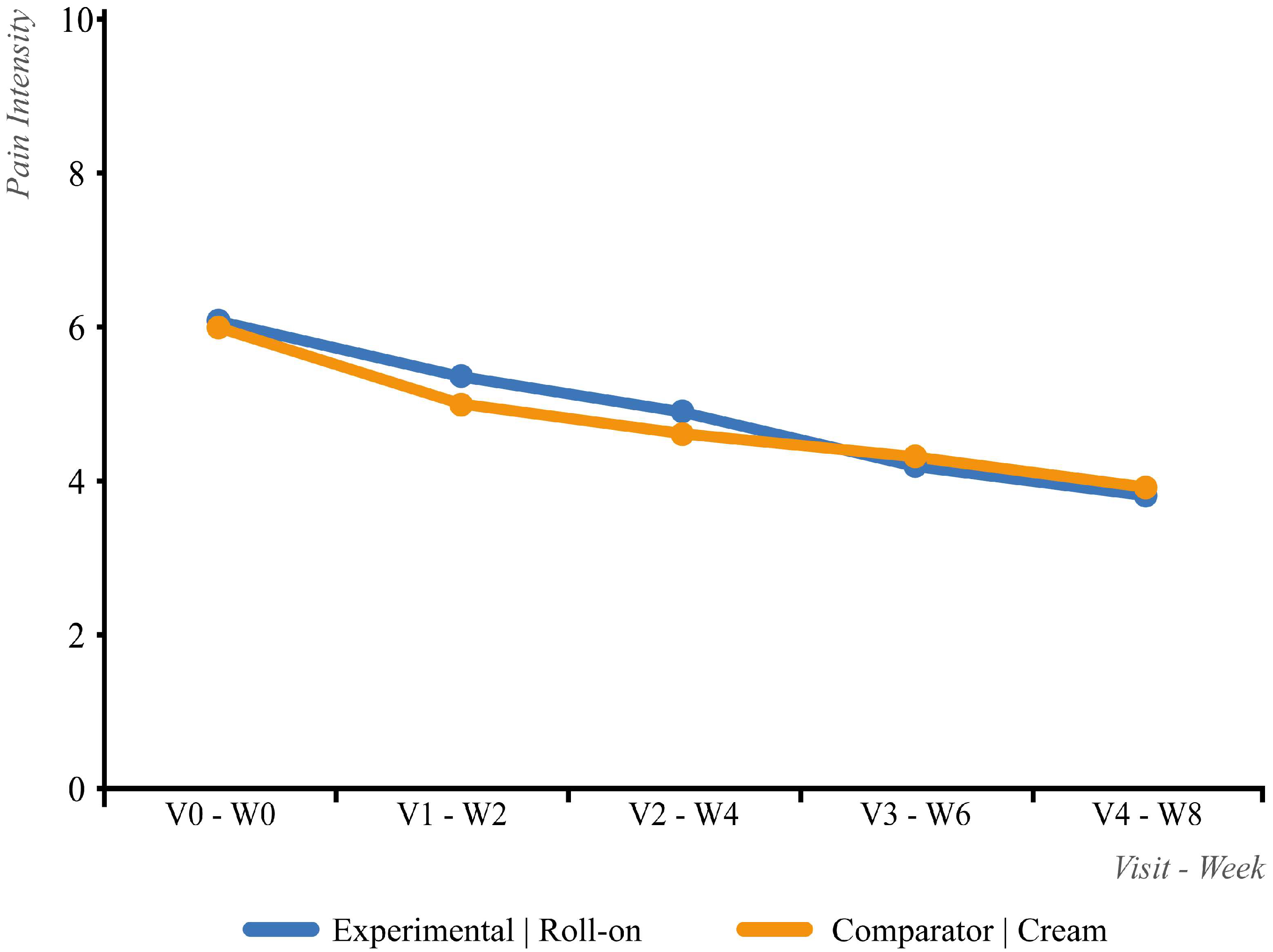
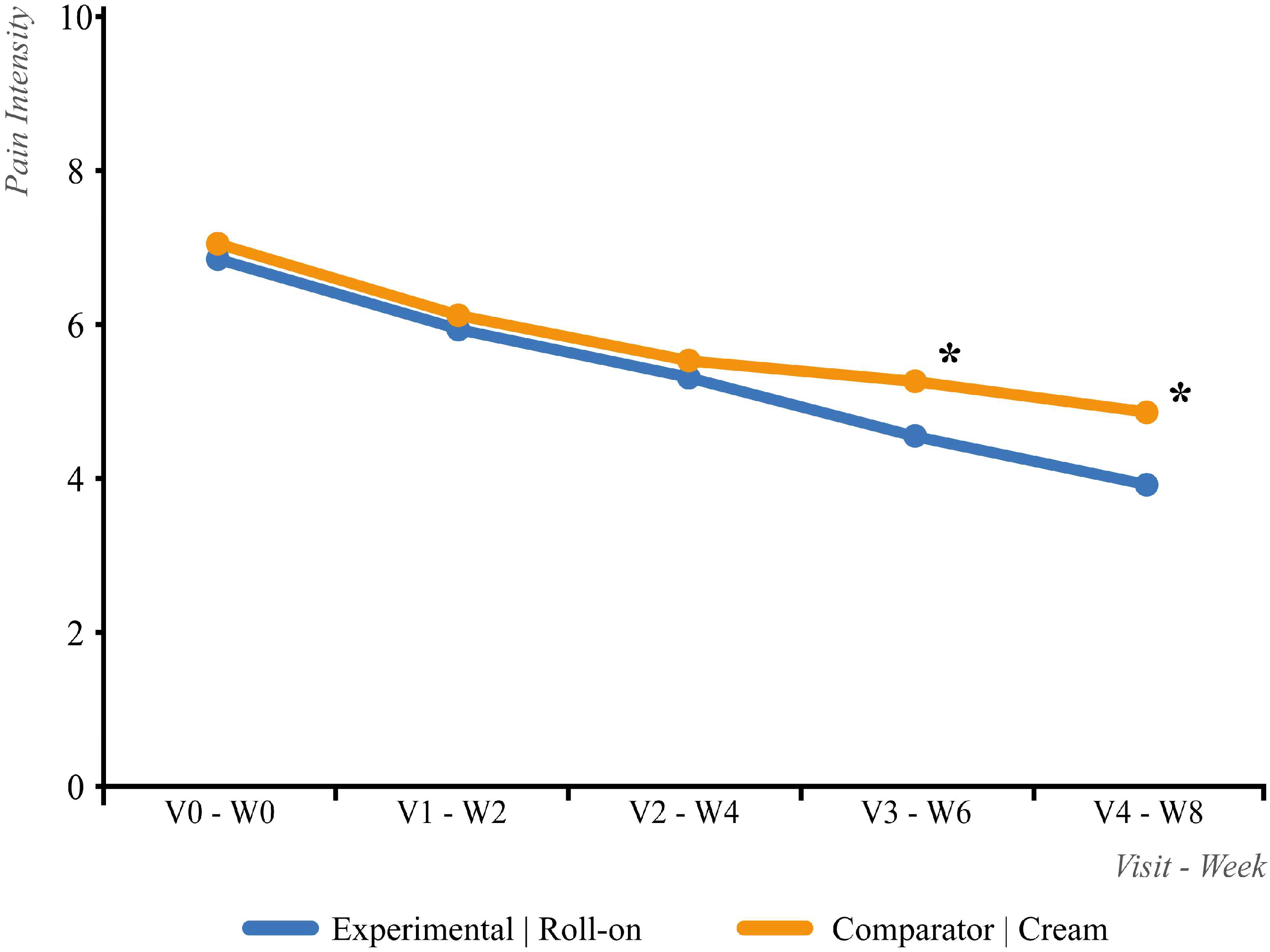
3.2. Efficacy
3.3. Quality of Life (EQ-5D)
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.; Treede, R.D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.; Desai, K.M.; Cantone, R.E. Peripheral Neuropathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2020, 102, 732–739. [Google Scholar] [PubMed]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Ahmadi, R.; Kuner, R.; Weidner, N.; Keßler, J.; Bendszus, M.; Krieg, S.M. The Diagnosis and Treatment of Neuropathic Pain. Dtsch. Ärzteblatt Int. 2024, 121, 825–832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef]
- Devigili, G.; Lombardi, R.; Lauria, G.; Cazzato, D. The Evolving Landscape of Small Fiber Neuropathy. Semin. Neurol. 2025, 45, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, K.J.; Braunwald, E.; Wilson, J.D. Principios de Medicina Interna, 13th ed.; Mc Graw-Hill Interamericana de España: Madrid, Spain, 1994. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119, Erratum in Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The Growing Epidemic of Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Foster, D.W.; Kronenberg, H.M. Williams Textbook of Endocrinology, 9th ed.; W B Saunders Company: Philadelphia, PA, USA, 1998. [Google Scholar]
- Aguilar, E.; De Lugo, B.; Devesa, J.; Tresguerres, J.A.F.; Esteban, B.M. Tratado de Endocrinología Básica y Clínica; Editorial Síntesis SA: Madrid, Spain, 2000. [Google Scholar]
- Gregg, E.W.; Pratt, A.; Owens, A.; Barron, E.; Dunbar-Rees, R.; Slade, E.T.; Hafezparast, N.; Bakhai, C.; Chappell, P.; Cornelius, V.; et al. The burden of diabetes-associated multiple long-term conditions on years of life spent and lost. Nat. Med. 2024, 30, 2830–2837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, P.; Larson, J. Management of painful diabetic neuropathy. Drugs Aging 2001, 18, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Oputa, R.N.; Oputa, P.U. Chronic Complications of Diabetes Mellitus. West Afr. J. Med. 2024, 41, 904–908. [Google Scholar] [PubMed]
- Dillon, B.R.; Ang, L.; Pop-Busui, R. Spectrum of Diabetic Neuropathy: New Insights in Diagnosis and Treatment. Annu. Rev. Med. 2024, 75, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Hillman, N. Neuropatía diabética periférica. Medicine 2000, 19, 67–76. [Google Scholar] [CrossRef]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef]
- Alles, S.R.; Smith, P.A. Etiology and pharmacology of neuropathic pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X. Neuropathic pain: Evidence based recommendations. Presse Med. 2024, 53, 104232. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Bouhassira, D.; Attal, N. French guidelines for neuropathic pain: An update and commentary. Rev. Neurol. 2021, 177, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.M.; Ciotu, C.I.; Szallasi, A. The Mysteries of Capsaicin-Sensitive Afferents. Front. Physiol. 2020, 11, 554195. [Google Scholar] [CrossRef]
- Scheffler, N.M.; Sheitel, P.L.; Lipton, M.N. Treatment of painful diabetic neuropathy with capsaicin 0.075%. J. Am. Podiatr. Med. Assoc. 1991, 81, 288–293. [Google Scholar] [CrossRef]
- Tandan, R.; Lewis, G.A.; Krusinski, P.B.; Badger, G.B.; Fries, T.J. Topical capsaicin in painful diabetic neuropathy. Controlled study with long-term follow-up. Diabetes Care 1992, 15, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Capsaicin Study Group. Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Diabetes Care 1992, 15, 159–165. [Google Scholar] [CrossRef]
- Biesbroeck, R.; Bril, V.; Hollander, P.; Kabadi, U.; Schwartz, S.; Singh, S.P.; Ward, W.K.; Bernstein, J.E. A double-blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Adv. Ther. 1995, 12, 111–120. [Google Scholar] [PubMed]
- Derry, S.; Lloyd, R.; Moore, R.A.; McQuay, H.J. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2009, 4, CD007393. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Rice, A.S.C.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013, 2, CD007393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Po, A.L.W. The effectiveness of topically applied capsaicin. A meta-analysis. Eur. J. Clin. Pharmacol. 1994, 46, 517–522. [Google Scholar] [CrossRef]
- Ellison, N.; Loprinzi, C.L.; Kugler, J.; Hatfield, A.K.; Miser, A.; Sloan, J.A.; Wender, D.B.; Rowland, K.M.; Molina, R.; Cascino, T.L.; et al. Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J. Clin. Oncol. 1997, 15, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- McCleane, G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: A randomized, double-blind, placebo-controlled study. Br. J. Clin. Pharmacol. 2000, 49, 574–579. [Google Scholar] [CrossRef]
- Mason, L.; Moore, R.A.; Derry, S.; Edwards, J.E.; McQuay, H.J. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ 2004, 328, 991. [Google Scholar] [CrossRef]
- Armitage, P.; Berry, G. Estadística Para la Investigación Biomédica. Tabla A6. Números Aleatorios, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 543–546. [Google Scholar]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; A Berlin, J.; et al. CONSORT 2025 Statement: Updated guideline for reporting randomised trials. BMJ 2025, 388, e081123. [Google Scholar] [CrossRef] [PubMed]
- Emshoff, R.; Bertram, S.; Emshoff, I. Clinically important difference thresholds of the visual analog scale: A conceptual model for identifying meaningful intraindividual changes for pain intensity. Pain 2011, 152, 2277–2282. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Rowbotham, M.C. What is a “clinically meaningful” reduction in pain? Pain 2001, 94, 131–132. [Google Scholar] [CrossRef]
- Simpson, D.M.; Robinson-Papp, J.; Van, J.; Stoker, M.; Jacobs, H.; Snijder, R.J.; Schregardus, D.S.; Long, S.K.; Lambourg, B.; Katz, N. Capsaicin 8% patch in painful diabetic peripheral neuropathy: A randomized, double-blind, placebo-controlled study. J. Pain 2017, 18, 42–53. [Google Scholar] [CrossRef]
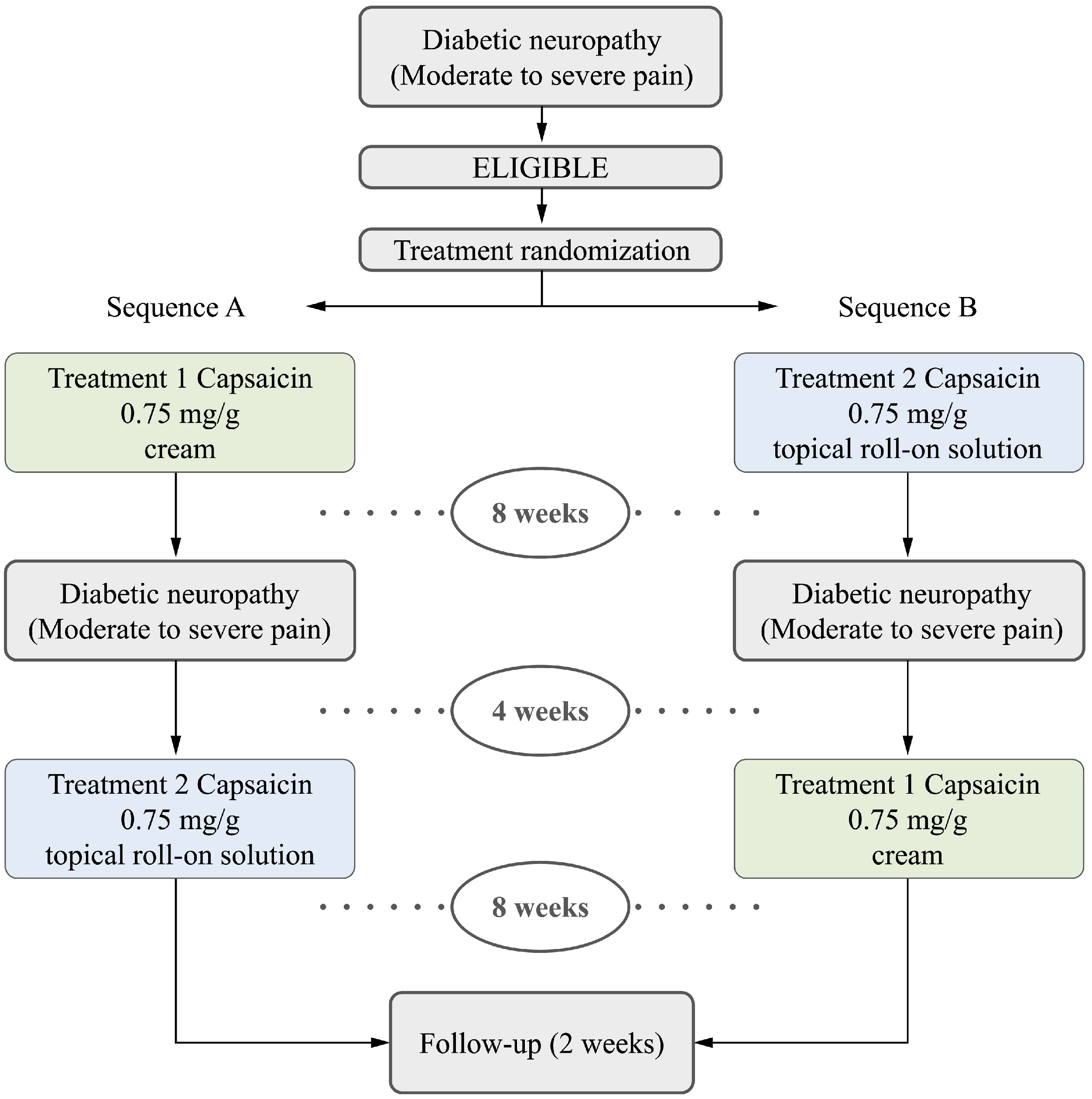
| Inclusion Criteria: |
| 1. Written or witnessed oral informed consent obtained prior to protocol-specific procedures. |
| 2. Male or non-pregnant, non-lactating female ≥18 years of age. |
| 3. Patients diagnosed with type I or II diabetes mellitus, treated or untreated. |
| 4. Patients diagnosed with painful diabetic peripheral neuropathy, treated or untreated. |
| 5. Painful diabetic neuropathy of at least 3 months’ duration with moderate to severe pain ≥4 on the visual analogue pain scale during the screening phase. |
| 6. Pain must have been experienced daily, must have interfered with daily activities or sleep, and must not be attributable to psychological causes. |
| 7. Stabilization on pain medication for at least one month prior to starting treatment. The patient must be prepared to remain on the same pain medications at the same doses as those before baseline throughout the study and during the follow-up phase (24 weeks). |
| 8. Intact, non-irritated, dry skin in the painful areas to be treated. |
| 9. Patients able to participate in the trial. |
| Exclusion Criteria: |
| 1. Allergic reactions to capsaicin. |
| 2. Patients with neuropathic pain of an etiology other than diabetes. |
| 3. Patients with peripheral ischemic pain due to diabetic arterial disease. |
| 4. Patients with unstable glycemic control (glycated hemoglobin ≥ 10.5%). |
| 5. Amputation of any part of the lower extremity. |
| 6. Surgery scheduled during the clinical trial. |
| 7. Mild painful diabetic neuropathy (<4 VAS). |
| 8. Other serious conditions: |
|
|
|
|
|
|
| 9. Use of other topical pain medications on the painful areas. |
| 10. History or current substance abuse problem. |
| 11. Pregnant or breastfeeding women. Women of childbearing potential must use effective contraception. |
| 12. Participation in another clinical trial with any non-marketed investigational drug within 90 days prior to enrollment. |
| Variables and Values | Sequence A | Sequence B | Total |
|---|---|---|---|
| BMI (Kg/m2), mean ± SD | 29.8 ± 4.3 | 30.2 ± 6.0 | 30.0 ± 5.2 |
| SBP (mmHg), mean ± SD | 134.2 ± 14.2 | 132.7 ± 13.7 | 133.5 ± 13.9 |
| DBP (mmHg), mean ± SD | 75.0 ± 10.1 | 74.4 ± 9.1 | 74.7 ± 9.6 |
| Period | Visit/Week | Cream | Roll-On | Total |
|---|---|---|---|---|
| First stage | V0/W0 | 83 | 77 | 160 |
| V1/W2 | 80 | 74 | 154 | |
| V2/W4 | 77 | 69 | 146 | |
| V3/W6 | 73 | 69 | 142 | |
| V4/W8 | 73 | 66 | 139 | |
| Second stage | V6/W12 | 72 | 66 | 138 |
| V7/W14 | 72 | 66 | 138 | |
| V8/W16 | 68 | 62 | 130 | |
| V9/W18 | 67 | 64 | 131 | |
| V10/W20 | 67 | 64 | 131 |
| Reasons for Discontinuation | Start with | Global | ||
|---|---|---|---|---|
| Cream | Roll-On | n | % | |
| Adverse Event | 2 | 3 | 5 | 44.83% |
| Absence of symptoms | 1 | 2 | 3 | 10.35% |
| Personal reasons | 2 | 4 | 6 | 20.69% |
| Other pathologies | 1 | 1 | 2 | 6.90% |
| Loss to follow-up | 4 | 1 | 5 | 17.24% |
| Total | 10 | 11 | 21 | 100.00% |
| Visits | Patients with Cream Treatment | Patients with Roll-On Treatment | ||||
|---|---|---|---|---|---|---|
| First Stage | Second Stage | Total | First Stage | Second Stage | Total | |
| V0 + V6 | 83 | 66 | 149 | 77 | 72 | 149 |
| V1 + V7 | 80 | 66 | 146 | 74 | 72 | 146 |
| V2 + V8 | 77 | 62 | 139 | 69 | 68 | 137 |
| V3 + V9 | 73 | 64 | 137 | 69 | 67 | 136 |
| V4 + V10 | 73 | 64 | 137 | 66 | 67 | 133 |
| AE (No/Yes) | Experimental (Roll-On) | Comparator (Cream) | Total |
|---|---|---|---|
| No | 137 (85.6%) | 132 (82.5%) | 122 (76.3%) |
| Yes | 23 (14.4%) | 28 (17.5%) | 38 (23.8%) |
| Total | 160 (100.0%) | 160 (100%) | 160 (100.0%) |
| AE Description | Experimental (Roll-On) | Comparator (Cream) | Total |
|---|---|---|---|
| Erythema | 13 (8.1%) | 14 (8.8%) | 27 (16.9%) |
| Itching/Stinging | 4 (2.5%) | 5 (3.1%) | 9 (5.6%) |
| Burning | 3 (1.9%) | 4 (2.5%) | 7 (4.4%) |
| Pruritus | 2 (1.3%) | 0 | 2 (1.3%) |
| Increasing Pain | 1 (0.6%) | 1 (0.6%) | 2 (1.3%) |
| Edema | 1 (0.6%) | 1 (0.6%) | 2 (1.3%) |
| Pharyngeal Irritation | 1 (0.6%) | 1 (0.6%) | 2 (1.3%) |
| Worse Neuropathic Pain | 1 (0.6%) | 0 | 1 (0.6%) |
| Sneezing | 1 (0.6%) | 0 | 1 (0.6%) |
| Papules | 1 (0.6%) | 0 | 1 (0.6%) |
| Runny Nose | 1 (0.6%) | 0 | 1 (0.6%) |
| Urticaria | 1 (0.6%) | 0 | 1 (0.6%) |
| Bronchitis | 0 | 1 (0.6%) | 1 (0.6%) |
| Intolerance to Study Drug | 0 | 1 (0.6%) | 1 (0.6%) |
| Secondary Lesion | 0 | 1 (0.6%) | 1 (0.6%) |
| Rhinitis | 0 | 1 (0.6%) | 1 (0.6%) |
| Cough | 0 | 1 (0.6%) | 1 (0.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya, T.R.; Abad-Santos, F.; Ortells, J.M.S.; Navarro, J.N.; González-López, P.; Abarca-Olivas, J.; Ruiz, E.B.; Iglesias-García, C.; García, J.F.V. Non-Inferiority Study of Two Capsaicin Formulations for Painful Diabetic Neuropathy. Life 2025, 15, 1507. https://doi.org/10.3390/life15101507
Araya TR, Abad-Santos F, Ortells JMS, Navarro JN, González-López P, Abarca-Olivas J, Ruiz EB, Iglesias-García C, García JFV. Non-Inferiority Study of Two Capsaicin Formulations for Painful Diabetic Neuropathy. Life. 2025; 15(10):1507. https://doi.org/10.3390/life15101507
Chicago/Turabian StyleAraya, Tamara Rodríguez, Francisco Abad-Santos, José Miguel Sempere Ortells, Juan Nieto Navarro, Pablo González-López, Javier Abarca-Olivas, Elena Baño Ruiz, Carlos Iglesias-García, and José Fernando Villalba García. 2025. "Non-Inferiority Study of Two Capsaicin Formulations for Painful Diabetic Neuropathy" Life 15, no. 10: 1507. https://doi.org/10.3390/life15101507
APA StyleAraya, T. R., Abad-Santos, F., Ortells, J. M. S., Navarro, J. N., González-López, P., Abarca-Olivas, J., Ruiz, E. B., Iglesias-García, C., & García, J. F. V. (2025). Non-Inferiority Study of Two Capsaicin Formulations for Painful Diabetic Neuropathy. Life, 15(10), 1507. https://doi.org/10.3390/life15101507







