Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Quality Assessment
3. Results
3.1. Production of SCFAs in the Gastrointestinal Tract
3.1.1. Cross-Feeding and Production of SCFAs in the Human Intestine
3.1.2. Production of Acetate by the Intestinal Microbiota
3.1.3. Production of Propionate by the Intestinal Microbiota
3.1.4. Production of Butyrate by the Intestinal Microbiota
3.1.5. Cross-Feeding Lays the Basis of Butyrate Production by Intestinal Microbiota
3.2. Absorption of SCFAs in the Intestine and SCFAs Supplements
3.2.1. Absorption of Butyrate
3.2.2. Butyrate Supplements
3.2.3. Absorption of Propionate
3.2.4. Propionate Supplements
3.2.5. Absorption of Acetate
3.2.6. Acetate Supplements
4. Implications of SCFAs in Human Gastrointestinal and Metabolic Health
4.1. Gastrointestinal Diseases
4.1.1. Inflammatory Bowel Disease
4.1.2. Colorectal Cancer
4.1.3. Disorders of the Gut-Brain Axis
4.2. Metabolic Diseases
4.2.1. Obesity
4.2.2. Type 2 Diabetes
4.2.3. Metabolic Dysfunction–Associated Steatotic Liver Disease
4.3. Therapeutic Implications
4.3.1. Fecal Microbiota Transplantation
4.3.2. Dietary Intervention
4.3.3. Prebiotic and Probiotic Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | 5’ Adenosine Monophosphate-Activated Protein Kinase |
| AOM | Azoxymethane |
| APP | Amyloid Precursor Protein, Precursor Protein 695 |
| Aβ | Amyloid Β |
| BAD | Bile Acid Diarrhea |
| BAT | Brown Adipose Tissue |
| BCoAT | Butyryl-Coa:Acetate Coatransferase |
| BCRP | Breast Cancer Resistance Protein |
| C2 | Acetate |
| C3 | Propionate |
| C4 | Butyrate |
| CaBu | Calcium Butyrate |
| CD | Crohn’s Disease |
| CRC | Colorectal Cancer |
| DGBI | Disorder Of Gut-Brain Interaction |
| DSS | Dextran Sodium Sulfate |
| F. prausnitzii | Faecalibacterium prausnitzii |
| FAD | Familial Alzheimer’s Disease |
| FAP | Familial Adenomatous Polyposis |
| FGF | Fibroblast Growth Factor |
| FMT | Fecal Microbiota Transplantation |
| FOS | Fructooligosaccharides |
| FXR | Farnesoid X Receptor |
| GLP-1/2 | Glucagon-Like Peptide-1/2 |
| GLUT4 | Glucose Transporter Type 4 |
| GOS | Galactooligosaccharides |
| GPCRs = GPR41-GPR43-109a | G-Protein-Coupled-Receptors 41-43-109a |
| Gpr43(−/−) | GPR43-Deficient |
| HbA1c | Glycated Hemoglobin |
| HDACs | Histone Deacetylases |
| HFD | High-Fat Diet |
| HOMA-IR | Homeostatic Model Assessment Of Insulin Resistance |
| IBD | Inflammatory Bowel Diseases |
| IBS, IBS-C, IBS-D, IBS-M | Irritable Bowel Syndrome, Constipation, Diarrhea, Mixed Stool |
| IGN | Intestinal Gluconeogenesis |
| IL-6,10,17,18 | (Cytokine) Interleukine-6-10-17-18 |
| LPS | Lipopolysaccharides |
| MAFLD | Metabolic-Associated Fatty Liver Disease |
| MASLD | Metabolic-Dysfunction Associated Steatotic Liver Disease |
| MCT1-4 | Monocarboxylate Transporters 1-4 |
| MPTP | 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine |
| MS | Multiple Sclerosis |
| MSI-h | Microsatellite Instability-High |
| MYD88/NF-κB | TLR4/Myeloid Differentiation Primary Response 88 |
| NaBu | Sodium Butyrate |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| Ngn3 | Neurogenin-3 |
| NLRP3 | Nod-Like Receptor Family Pyrin Domain Containing 3 |
| NOD | Nonobese Diabetic |
| PD | Parkinson’s Disease |
| PGC-1α | Peroxisome Coactivator-1 Alpha |
| PKM2 | Pyruvate Kinase Muscle Isozyme 2 |
| PPARγ | Peroxisome Proliferator-Activated Receptor-γ |
| PSC | Primary Sclerosing Cholangitis |
| PSEN1 | Presenilin-1 |
| PYY | Peptide YY |
| rCDI | Recurrent Clostridioides Difficile Infection |
| RCT | Randomized Controlled Trial |
| SCFAs | Short-Chain Fatty Acids |
| SLAB51 | A Mixture Of Lactic Acid Bacteria And Bifidobacteria |
| SMCT1 | Sodium-Coupled Monocarboxylate Transporter 1 |
| SRY | Sex Determining Region Y |
| SRY Sox2 | (Sex Determining Region Y)-Box 2 |
| T1/2D | Type 1/2 Diabetes |
| T2DM | Type 2 Diabetes Mellitus |
| Th1- Th17 | T-Helper 1 Cells- T-Helper 17 Cells |
| TLRs- TLR4- TLR2 | Toll-Like Receptors-4-2 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| Treg | Regulatory T Cells |
| UC | Ulcerative Colitis |
| ZO-1 | Zonula Occludens-1 |
| 5XFAD | Transgenic Mice Overexpress Mutant Human Amyloid Beta |
References
- Musso, G.; Gambino, R.; Cassader, M. Gut Microbiota as a Regulator of Energy Homeostasis and Ectopic Fat Deposition: Mechanisms and Implications for Metabolic Disorders. Curr. Opin. Lipidol. 2010, 21, 76–83. [Google Scholar] [CrossRef]
- Høverstad, T. Studies of Short-Chain Fatty Acid Absorption in Man. Scand. J. Gastroenterol. 1986, 21, 257–260. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of Short-Chain Fatty Acid Production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the Gut to the Peripheral Tissues: The Multiple Effects of Butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van De Wiele, T. Propionate as a Health-Promoting Microbial Metabolite in the Human Gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Culp, E.J.; Goodman, A.L. Cross-Feeding in the Gut Microbiome: Ecology and Mechanisms. Cell Host Microbe 2023, 31, 485–499. [Google Scholar] [CrossRef]
- Germerodt, S.; Bohl, K.; Lück, A.; Pande, S.; Schröter, A.; Kaleta, C.; Schuster, S.; Kost, C. Pervasive Selection for Cooperative Cross-Feeding in Bacterial Communities. PLoS Comput. Biol. 2016, 12, e1004986. [Google Scholar] [CrossRef]
- Rasouli-Saravani, A.; Jahankhani, K.; Moradi, S.; Gorgani, M.; Shafaghat, Z.; Mirsanei, Z.; Mehmandar, A.; Mirzaei, R. Role of Microbiota Short-Chain Fatty Acids in the Pathogenesis of Autoimmune Diseases. Biomed. Pharmacother. 2023, 162, 114620. [Google Scholar] [CrossRef]
- Hosmer, J.; McEwan, A.G.; Kappler, U. Bacterial Acetate Metabolism and Its Influence on Human Epithelia. Emerg. Top. Life Sci. 2023, 8, 1–13. [Google Scholar]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Schug, Z.T.; Voorde, J.V.; Gottlieb, E. The Metabolic Fate of Acetate in Cancer. Nat. Rev. Cancer 2016, 16, 708–717. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Remely, M.; Hippe, B.; Geretschlaeger, I.; Stegmayer, S.; Hoefinger, I.; Haslberger, A. Increased Gut Microbiota Diversity and Abundance of Faecalibacterium Prausnitzii and Akkermansia after Fasting: A Pilot Study. Wien. Klin. Wochenschr. 2015, 127, 394–398. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Duncan, S.H. Growth Requirements and Fermentation Products of Fusobacterium Prausnitzii, and a Proposal to Reclassify It as Faecalibacterium Prausnitzii Gen. Nov., Comb. Nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Enumeration of Amino Acid Fermenting Bacteria in the Human Large Intestine: Effects of pH and Starch on Peptide Metabolism and Dissimilation of Amino Acids. FEMS Microbiol. Ecol. 1998, 25, 355–368. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Jan, G.; Belzacq, A.-S.; Haouzi, D.; Rouault, A.; Kroemer, G.; Brenner, C. Propionibacteria Induce Apoptosis of Colorectal Carcinoma Cells via Short-Chain Fatty Acids Acting on Mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted Distribution of the Butyrate Kinase Pathway among Butyrate-Producing Bacteria from the Human Colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an Anti-Inflammatory Protein from Faecalibacterium prausnitzii, a Commensal Bacterium Deficient in Crohn’s Disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Oliphant, K.; Parreira, V.R.; Cochrane, K.; Allen-Vercoe, E. Drivers of Human Gut Microbial Community Assembly: Coadaptation, Determinism and Stochasticity. ISME J. 2019, 13, 3080–3092. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; De Waard, P.; Plugge, C.M.; De Vos, W.M. Production of Butyrate from Lysine and the Amadori Product Fructoselysine by a Human Gut Commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef]
- Buckel, W. Unusual Enzymes Involved in Five Pathways of Glutamate Fermentation. Appl. Microbiol. Biotechnol. 2001, 57, 263–273. [Google Scholar] [CrossRef]
- Potrykus, J.; White, R.L.; Bearne, S.L. Proteomic Investigation of Amino Acid Catabolism in the Indigenous Gut Anaerobe Fusobacterium varium. Proteomics 2008, 8, 2691–2703. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Cross-Feeding between Bifidobacteria and Butyrate-Producing Colon Bacteria Explains Bifdobacterial Competitiveness, Butyrate Production, and Gas Production. Int. J. Food Microbiol. 2011, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Holtrop, G.; Anderson, S.E.; Lobley, G.E.; Flint, H.J. Impact of pH on Lactate Formation and Utilization by Human Fecal Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 6526–6533. [Google Scholar] [CrossRef]
- Molis, C.; Flourié, B.; Ouarne, F.; Gailing, M.; Lartigue, S.; Guibert, A.; Bornet, F.; Galmiche, J. Digestion, Excretion, and Energy Value of Fructooligosaccharides in Healthy Humans. Am. J. Clin. Nutr. 1996, 64, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two Routes of Metabolic Cross-Feeding between Bifidobacterium Adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef]
- Falony, G.; Vlachou, A.; Verbrugghe, K.; Vuyst, L.D. Cross-Feeding between Bifidobacterium Longum BB536 and Acetate-Converting, Butyrate-Producing Colon Bacteria during Growth on Oligofructose. Appl. Environ. Microbiol. 2006, 72, 7835–7841. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the Effects of Diet on Bacterial Metabolism in the Large Intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Fujiyama, Y.; Mitsuyama, K.; Araki, Y.; Ishii, T.; Nakamura, T.; Hitomi, Y.; Agata, K.; Saiki, T.; Andoh, A.; et al. Increased Growth of Bifidobacterium and Eubacterium by Germinated Barley Foodstuff, Accompanied by Enhanced Butyrate Production in Healthy Volunteers. Int. J. Mol. Med. 1999, 3, 175–184. [Google Scholar] [CrossRef]
- Chassard, C.; Bernalier-Donadille, A. H2 and Acetate Transfers during Xylan Fermentation between a Butyrate-Producing Xylanolytic Species and Hydrogenotrophic Microorganisms from the Human Gut. FEMS Microbiol. Lett. 2006, 254, 116–122. [Google Scholar] [CrossRef]
- Velázquez, O.C.; Lederer, H.M.; Rombeau, J.L. Butyrate and the Colonocyte. Production, Absorption, Metabolism, and Therapeutic Implications. Adv. Exp. Med. Biol. 1997, 427, 123–134. [Google Scholar] [PubMed]
- Garcia, C.K.; Goldstein, J.L.; Pathak, R.K.; Anderson, R.G.; Brown, M.S. Molecular Characterization of a Membrane Transporter for Lactate, Pyruvate, and Other Monocarboxylates: Implications for the Cori Cycle. Cell 1994, 76, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2018, 8, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Coady, M.J.; Chang, M.-H.; Charron, F.M.; Plata, C.; Wallendorff, B.; Sah, J.F.; Markowitz, S.D.; Romero, M.F.; Lapointe, J.-Y. The Human Tumour Suppressor Gene SLC5A8 Expresses a Na+-Monocarboxylate Cotransporter. J. Physiol. 2004, 557, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Blachier, F.; Darcy-Vrillon, B.; de Coppet, P.; Bourreille, A.; Segain, J.-P. Butyrate Utilization by the Colonic Mucosa in Inflammatory Bowel Diseases: A Transport Deficiency. Inflamm. Bowel Dis. 2010, 16, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Wood, I.S.; Ellis, A.; Shirazi-Beechey, S.P. Molecular Changes in the Expression of Human Colonic Nutrient Transporters during the Transition from Normality to Malignancy. Br. J. Cancer 2002, 86, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Ricci, J.-E.; Pouysségur, J. Tumor Hypoxia and Metabolism—Towards Novel Anticancer Approaches. Ann. Endocrinol. 2013, 74, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.P.; Schulze, A. Targeting Cancer Metabolism—Aiming at a Tumour’s Sweet-Spot. Drug Discov. Today 2012, 17, 232–241. [Google Scholar] [CrossRef]
- Marchiq, I.; Pouysségur, J. Hypoxia, Cancer Metabolism and the Therapeutic Benefit of Targeting Lactate/H(+) Symporters. J. Mol. Med. 2016, 94, 155–171. [Google Scholar] [CrossRef]
- Bolden, J.E.; Peart, M.J.; Johnstone, R.W. Anticancer Activities of Histone Deacetylase Inhibitors. Nat. Rev. Drug Discov. 2006, 5, 769–784. [Google Scholar] [CrossRef]
- Fishbein, W.N. Lactate Transporter Defect: A New Disease of Muscle. Science 1986, 234, 1254–1256. [Google Scholar] [CrossRef]
- Gupta, N.; Martin, P.M.; Prasad, P.D.; Ganapathy, V. SLC5A8 (SMCT1)-Mediated Transport of Butyrate Forms the Basis for the Tumor Suppressive Function of the Transporter. Life Sci. 2006, 78, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.G.; Vehr, A.-K.; Martin, I.V.; Gassler, N.; Rath, T.; Roeb, E.; Schmitt, J.; Trautwein, C.; Geier, A. Downregulation of Breast Cancer Resistance Protein in Colon Adenomas Reduces Cellular Xenobiotic Resistance and Leads to Accumulation of a Food-Derived Carcinogen. Int. J. Cancer 2011, 129, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Gregório, I.; Martel, F. The Short-Chain Fatty Acid Butyrate Is a Substrate of Breast Cancer Resistance Protein. Am. J. Physiol. Cell Physiol. 2011, 301, C984–C994. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, S.; Deng, B.; Tan, C.; Deng, J.; Zhu, G.; Yin, Y.; Ren, W. Implication of G Protein-Coupled Receptor 43 in Intestinal Inflammation: A Mini-Review. Front. Immunol. 2018, 9, 1434. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Balogh, A.; Markó, L.; Bartolomaeus, H.; Müller, D.N. The Role of Sodium in Modulating Immune Cell Function. Nat. Rev. Nephrol. 2019, 15, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Gaschott, T.; Stein, J. Short-Chain Fatty Acids and Colon Cancer Cells: The Vitamin D Receptor—Butyrate Connection. In Vitamin D Analogs in Cancer Prevention and Therapy; Reichrath, J., Tilgen, W., Friedrich, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 164, pp. 247–257. ISBN 978-3-642-62435-3. [Google Scholar]
- Jantsch, J.; Schatz, V.; Friedrich, D.; Schröder, A.; Kopp, C.; Siegert, I.; Maronna, A.; Wendelborn, D.; Linz, P.; Binger, K.J.; et al. Cutaneous Na+ Storage Strengthens the Antimicrobial Barrier Function of the Skin and Boosts Macrophage-Driven Host Defense. Cell Metab. 2015, 21, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Byles, V.; Covarrubias, A.J.; Ben-Sahra, I.; Lamming, D.W.; Sabatini, D.M.; Manning, B.D.; Horng, T. The TSC-mTOR Pathway Regulates Macrophage Polarization. Nat. Commun. 2013, 4, 2834. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s Role in Human Health and the Current Progress towards Its Clinical Application to Treat Gastrointestinal Disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Rhodes, J.M. Nutrition and Gut Health: The Impact of Specific Dietary Components—It’s Not Just Five-a-Day. Proc. Nutr. Soc. 2021, 80, 9–18. [Google Scholar] [CrossRef]
- Kannampalli, P.; Shaker, R.; Sengupta, J.N. Colonic Butyrate- Algesic or Analgesic?: Colonic Butyrate Function. Neurogastroenterol. Motil. 2011, 23, 975–979. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota Changes Induced by Microencapsulated Sodium Butyrate in Patients with Inflammatory Bowel Disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Anshory, M.; Effendi, R.M.R.A.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Nijsten, T.E.C.; Nouwen, J.L.; Thio, H.B. Butyrate Properties in Immune-Related Diseases: Friend or Foe? Fermentation 2023, 9, 205. [Google Scholar] [CrossRef]
- Guillemot, F.; Colombel, J.F.; Neut, C.; Verplanck, N.; Lecomte, M.; Romond, C.; Paris, J.C.; Cortot, A. Treatment of Diversion Colitis by Short-Chain Fatty Acids: Prospective and Double-Blind Study. Dis. Colon Rectum 1991, 34, 861–864. [Google Scholar] [CrossRef]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of Butyrate Enemas on the Colonic Mucosa in Distal Ulcerative Colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef]
- Steinhart, A.H.; Brzezinski, A.; Baker, J.P. Treatment of Refractory Ulcerative Proctosigmoiditis with Butyrate Enemas. Am. J. Gastroenterol. 1994, 89, 179–183. [Google Scholar]
- Vernia, P.; Marcheggiano, A.; Caprilli, R.; Frieri, G.; Corrao, G.; Valpiani, D.; DI Paolo, M.C.; Paoluzi, P.; Torsoli, A. Short-chain Fatty Acid Topical Treatment in Distal Ulcerative Colitis. Aliment. Pharmacol. Ther. 1995, 9, 309–313. [Google Scholar] [CrossRef]
- Steinhart, A.H.; Hiruki, T.; Brzezinski, A.; Baker, J.P. Treatment of Left-Sided Ulcerative Colitis with Butyrate Enemas: A Controlled Trial. Aliment. Pharmacol. Ther. 1996, 10, 729–736. [Google Scholar] [CrossRef]
- Scheppach, W.; Christl, S.U.; Bartram, H.P.; Richter, F.; Kasper, H. Effects of Short-Chain Fatty Acids on the Inflamed Colonic Mucosa. Scand. J. Gastroenterol. Suppl. 1997, 32, 53–57. [Google Scholar] [CrossRef]
- Pinto, A.; Fidalgo, P.; Cravo, M.; Midões, J.; Chaves, P.; Rosa, J.; Birto, M.D.A.; Leitão, C.N. Short Chain Fatty Acids Are Effective in Short-Term Treatment of Chronic Radiation Proctitis: Randomized, Double-Blind, Controlled Trial. Dis. Colon Rectum 1999, 42, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Monteleone, G.; Grandinetti, G.; Villotti, G.; Di Giulio, E.; Frieri, G.; Marcheggiano, A.; Pallone, F.; Caprilli, R.; Torsoli, A. Combined Oral Sodium Butyrate and Mesalazine Treatment Compared to Oral Mesalazine Alone in Ulcerative Colitis: Randomized, Double-Blind, Placebo-Controlled Pilot Study. Dig. Dis. Sci. 2000, 45, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Fracasso, P.; Casale, V.; Villotti, G.; Marcheggiano, A.; Stigliano, V.; Pinnaro, P.; Bagnardi, V.; Caprilli, R. Topical Butyrate for Acute Radiation Proctitis: Randomised, Crossover Trial. Lancet 2000, 356, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Lührs, H.; Gerke, T.; Müller, J.G.; Melcher, R.; Schauber, J.; Boxberge, F.; Scheppach, W.; Menzel, T. Butyrate Inhibits NF-kappaB Activation in Lamina Propria Macrophages of Patients with Ulcerative Colitis. Scand. J. Gastroenterol. 2002, 37, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Annese, V.; Bresci, G.; D’albasio, G.; D’incà, R.; Giaccari, S.; Ingrosso, M.; Mansi, C.; Riegler, G.; Valpiani, D.; et al. Topical Butyrate Improves Efficacy of 5-ASA in Refractory Distal Ulcerative Colitis: Results of a Multicentre Trial. Eur. J. Clin. Investig. 2003, 33, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral Butyrate for Mildly to Moderately Active Crohn’s Disease. Aliment. Pharmacol. Ther. 2005, 22, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Assisi, R.F.; GISDI Study Group Combined Butyric Acid/Mesalazine Treatment in Ulcerative Colitis with Mild-Moderate Activity. Results of a Multicentre Pilot Study. Minerva Gastroenterol. E Dietol. 2008, 54, 231–238. [Google Scholar]
- Vanhoutvin, S.A.L.W.; Troost, F.J.; Kilkens, T.O.C.; Lindsey, P.J.; Hamer, H.M.; Jonkers, D.M.A.E.; Venema, K.; Brummer, R.J.M. The Effects of Butyrate Enemas on Visceral Perception in Healthy Volunteers. Neurogastroenterol. Motil. 2009, 21, 952-e76. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.A.E.; Vanhoutvin, S.A.L.W.; Troost, F.J.; Rijkers, G.; de Bruïne, A.; Bast, A.; Venema, K.; Brummer, R.J.M. Effect of Butyrate Enemas on Inflammation and Antioxidant Status in the Colonic Mucosa of Patients with Ulcerative Colitis in Remission. Clin. Nutr. 2010, 29, 738–744. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Krokowicz, L.; Stojcev, Z.; Kaczmarek, B.F.; Kaczmarek, E.; Maik, J.; Marciniak, R.; Krokowicz, P.; Walkowiak, J.; Drews, M. Microencapsulated Sodium Butyrate Reduces the Frequency of Abdominal Pain in Patients with Irritable Bowel Syndrome. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2013, 15, 204–209. [Google Scholar] [CrossRef]
- Krokowicz, L.; Stojcev, Z.; Kaczmarek, B.F.; Kociemba, W.; Kaczmarek, E.; Walkowiak, J.; Krokowicz, P.; Drews, M.; Banasiewicz, T. Microencapsulated Sodium Butyrate Administered to Patients with Diverticulosis Decreases Incidence of Diverticulitis—A Prospective Randomized Study. Int. J. Color. Dis. 2014, 29, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Krokowicz, L.; Kaczmarek, B.F.; Krokowicz, P.; Stojcev, Z.; Mackiewicz, J.; Walkowiak, J.; Drews, M.; Banasiewicz, T. Sodium Butyrate and Short Chain Fatty Acids in Prevention of Travellers’ Diarrhoea: A Randomized Prospective Study. Travel. Med. Infect. Dis. 2014, 12, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Magli, A.; Rancati, T.; Fiorino, C.; Valvo, F.; Fellin, G.; Ricardi, U.; Munoz, F.; Cosentino, D.; Cazzaniga, L.F.; et al. Daily Sodium Butyrate Enema for the Prevention of Radiation Proctitis in Prostate Cancer Patients Undergoing Radical Radiation Therapy: Results of a Multicenter Randomized Placebo-Controlled Dose-Finding Phase 2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 518–524. [Google Scholar] [CrossRef]
- Luceri, C.; Femia, A.P.; Fazi, M.; Di Martino, C.; Zolfanelli, F.; Dolara, P.; Tonelli, F. Effect of Butyrate Enemas on Gene Expression Profiles and Endoscopic/Histopathological Scores of Diverted Colorectal Mucosa: A Randomized Trial. Dig. Liver Dis. 2016, 48, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Jafarabadi, M.; Hedayati, M.; Ghavami, A.; Alipour, S.; Alamdari, N.; Barati, M.; Ostadrahimi, A. Effect of Butyrate and Inulin Supplementation on Glycemic Status, Lipid Profile and Glucagon-Like Peptide 1 Level in Patients with Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017, 49, 886–891. [Google Scholar] [CrossRef]
- Vernero, M.; De Blasio, F.; Ribaldone, D.G.; Bugianesi, E.; Pellicano, R.; Saracco, G.M.; Astegiano, M.; Caviglia, G.P. The Usefulness of Microencapsulated Sodium Butyrate Add-On Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 2020, 9, 3941. [Google Scholar] [CrossRef]
- De Groot, P.F.; Nikolic, T.; Imangaliyev, S.; Bekkering, S.; Duinkerken, G.; Keij, F.M.; Herrema, H.; Winkelmeijer, M.; Kroon, J.; Levin, E.; et al. Oral Butyrate Does Not Affect Innate Immunity and Islet Autoimmunity in Individuals with Longstanding Type 1 Diabetes: A Randomised Controlled Trial. Diabetologia 2020, 63, 597–610. [Google Scholar] [CrossRef]
- Coppola, S.; Nocerino, R.; Paparo, L.; Bedogni, G.; Calignano, A.; Di Scala, C.; De Giovanni Di Santa Severina, A.F.; De Filippis, F.; Ercolini, D.; Berni Canani, R. Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244912. [Google Scholar] [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases—Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [Google Scholar] [CrossRef]
- Khosravi, Z.; Hadi, A.; Tutunchi, H.; Asghari-Jafarabadi, M.; Naeinie, F.; Roshanravan, N.; Ostadrahimi, A.; Fadel, A. The Effects of Butyrate Supplementation on Glycemic Control, Lipid Profile, Blood Pressure, Nitric Oxide Level and Glutathione Peroxidase Activity in Type 2 Diabetic Patients: A Randomized Triple -Blind, Placebo-Controlled Trial. Clin. Nutr. ESPEN 2022, 49, 79–85. [Google Scholar] [CrossRef]
- Qaisar, R.; Karim, A.; Muhammad, T.; Ahmad, F. Butyrate Supplementation Reduces Sarcopenia by Repairing Neuromuscular Junction in Patients with Chronic Obstructive Pulmonary Disease. Respir. Med. 2024, 222, 107510. [Google Scholar] [CrossRef]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Investig. Dermatol. 2017, 137, 855–864. [Google Scholar] [CrossRef]
- Stacey, S.K.; McEleney, M. Topical Corticosteroids: Choice and Application. Am. Fam. Physician 2021, 103, 337–343. [Google Scholar]
- Delzenne, N.M.; Williams, C.M. Prebiotics and Lipid Metabolism. Curr. Opin. Lipidol. 2002, 13, 61–67. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Intraruminal Infusion of Propionate Alters Feeding Behavior and Decreases Energy Intake of Lactating Dairy Cows. J. Nutr. 2003, 133, 1094–1099. [Google Scholar] [CrossRef]
- Li, C.J.; Elsasser, T.H. Butyrate-Induced Apoptosis and Cell Cycle Arrest in Bovine Kidney Epithelial Cells: Involvement of Caspase and Proteasome Pathways1. J. Anim. Sci. 2005, 83, 89–97. [Google Scholar] [CrossRef]
- Kamp, F.; Hamilton, J.A. How Fatty Acids of Different Chain Length Enter and Leave Cells by Free Diffusion. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 149–159. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, H.W.J.; Naylor, C.P.E.; MacFarlane, G.T. Short Chain Fatty Acids in Human Large Intestine, Portal, Hepatic and Venous Blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-Chain Fatty Acids Induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the mTOR–S6K Pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Eilers, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Malkova, D.; Polyviou, T.; Rizou, E.; Gerasimidis, K.; Chambers, E.S.; Preston, T.; Tedford, M.C.; Frost, G.; Morrison, D.J. Moderate Intensity Exercise Training Combined with Inulin-Propionate Ester Supplementation Increases Whole Body Resting Fat Oxidation in Overweight Women. Metabolism 2020, 104, 154043. [Google Scholar] [CrossRef]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate Attenuates Atherosclerosis by Immune-Dependent Regulation of Intestinal Cholesterol Metabolism. Eur. Heart J. 2021, 43, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Offei, B.; Vandecruys, P.; De Graeve, S.; Foulquié-Moreno, M.R.; Thevelein, J.M. Unique Genetic Basis of the Distinct Antibiotic Potency of High Acetic Acid Production in the Probiotic Yeast Saccharomyces cerevisiae Var. boulardii. Genome Res. 2019, 29, 1478–1494. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic Acid Upregulates the Expression of Genes for Fatty Acid Oxidation Enzymes in Liver to Suppress Body Fat Accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (Ed.) Evaluation of Certain Food Additives: Fifty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-120896-3. [Google Scholar]
- Freeland, K.R.; Wolever, T.M.S. Acute Effects of Intravenous and Rectal Acetate on Glucagon-like Peptide-1, Peptide YY, Ghrelin, Adiponectin and Tumour Necrosis Factor-α. Br. J. Nutr. 2010, 103, 460–466. [Google Scholar] [CrossRef]
- Fernandes, J.; Vogt, J.; Wolever, T.M. Intravenous Acetate Elicits a Greater Free Fatty Acid Rebound in Normal than Hyperinsulinaemic Humans. Eur. J. Clin. Nutr. 2012, 66, 1029–1034. [Google Scholar] [CrossRef]
- Van Der Beek, C.M.; Canfora, E.E.; Lenaerts, K.; Troost, F.J.; Olde Damink, S.W.M.; Holst, J.J.; Masclee, A.A.M.; Dejong, C.H.C.; Blaak, E.E. Distal, Not Proximal, Colonic Acetate Infusions Promote Fat Oxidation and Improve Metabolic Markers in Overweight/Obese Men. Clin. Sci. 2016, 130, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic Infusions of Short-Chain Fatty Acid Mixtures Promote Energy Metabolism in Overweight/Obese Men: A Randomized Crossover Trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota Metabolite Short Chain Fatty Acids, GPCR, and Inflammatory Bowel Diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blad, C.C.; Tang, C.; Offermanns, S. G Protein-Coupled Receptors for Energy Metabolites as New Therapeutic Targets. Nat. Rev. Drug Discov. 2012, 11, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Candido, E.P.; Reeves, R.; Davie, J.R. Sodium Butyrate Inhibits Histone Deacetylation in Cultured Cells. Cell 1978, 14, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sealy, L.; Chalkley, R. The Effect of Sodium Butyrate on Histone Modification. Cell 1978, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg Effect Dictates the Mechanism of Butyrate-Mediated Histone Acetylation and Cell Proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, Neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Facchin, S.; Patuzzi, I.; Ford, A.C.; Massimi, D.; Valle, G.; Sattin, E.; Simionati, B.; Bertazzo, E.; Zingone, F.; et al. A Specific Microbiota Signature Is Associated to Various Degrees of Ulcerative Colitis as Assessed by a Machine Learning Approach. Gut Microbes 2022, 14, 2028366. [Google Scholar] [CrossRef] [PubMed]
- Segain, J.; de la Bletiere, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.; Galmiche, J. Butyrate Inhibits Inflammatory Responses through NFκB Inhibition: Implications for Crohn’s Disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A Cross-Talk between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, X.; Yang, L.; Wang, H.; Sun, Y.; Liu, N. Association between Intestinal Microbiota and Inflammatory Bowel Disease. Anim. Model. Exp. Med. 2022, 5, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The Interaction among Gut Microbes, the Intestinal Barrier and Short Chain Fatty Acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, J.J.; Tillinger, W.; Hofer, J.; Hölzl, M.A.; Heinzl, H.; Saemann, M.D.; Zlabinger, G.J. Impaired Anti-Inflammatory Efficacy of n-Butyrate in Patients with IBD. Eur. J. Clin. Investig. 2011, 41, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-L.; Ni, W.-W.; Zhang, Q.-M.; Li, Y.; Zhang, X.; Wu, H.-Y.; Du, P.; Hou, J.-C.; Zhang, Y. Effect of Cinnamon Essential Oil on Gut Microbiota in the Mouse Model of Dextran Sodium Sulfate-Induced Colitis. Microbiol. Immunol. 2020, 64, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sefat, N.A.K.; Mohammadi, M.M.; Hadjati, J.; Talebi, S.; Ajami, M.; Daneshvar, H. Sodium Butyrate as a Histone Deacetylase Inhibitor Affects Toll-Like Receptor 4 Expression in Colorectal Cancer Cell Lines. Immunol. Investig. 2019, 48, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Tews, H.C.; Elger, T.; Gunawan, S.; Fererberger, T.; Sommersberger, S.; Loibl, J.; Huss, M.; Liebisch, G.; Müller, M.; Kandulski, A.; et al. Fecal Short Chain Fatty Acids and Urinary 3-Indoxyl Sulfate Do Not Discriminate between Patients with Crohn’s Disease and Ulcerative Colitis and Are Not of Diagnostic Utility for Predicting Disease Severity. Lipids Health Dis. 2023, 22, 164. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota Metabolite Butyrate Constrains Neutrophil Functions and Ameliorates Mucosal Inflammation in Inflammatory Bowel Disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium Prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-Onset Colorectal Cancer: Initial Clues and Current Views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.-H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef]
- Oh, H.; Kim, H.; Lee, D.H.; Lee, A.; Giovannucci, E.L.; Kang, S.-S.; Keum, N. Different Dietary Fibre Sources and Risks of Colorectal Cancer and Adenoma: A Dose-Response Meta-Analysis of Prospective Studies. Br. J. Nutr. 2019, 122, 605–615. [Google Scholar] [CrossRef]
- Gianfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M.; Nucci, D.; Realdon, S. Is Dietary Fibre Truly Protective against Colon Cancer? A Systematic Review and Meta-Analysis. Int. J. Food Sci. Nutr. 2018, 69, 904–915. [Google Scholar] [CrossRef]
- Alvandi, E.; Wong, W.K.M.; Joglekar, M.V.; Spring, K.J.; Hardikar, A.A. Short-Chain Fatty Acid Concentrations in the Incidence and Risk-Stratification of Colorectal Cancer: A Systematic Review and Meta-Analysis. BMC Med. 2022, 20, 323. [Google Scholar] [CrossRef] [PubMed]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The Microbiome and Human Cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of Short-Chain Fatty Acids in Colonic Inflammation, Carcinogenesis, and Mucosal Protection and Healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.; Greathouse, K.L. Targeting Dietary and Microbial Tryptophan-Indole Metabolism as Therapeutic Approaches to Colon Cancer. Nutrients 2021, 13, 1189. [Google Scholar] [CrossRef]
- Li, Q.; Cao, L.; Tian, Y.; Zhang, P.; Ding, C.; Lu, W.; Jia, C.; Shao, C.; Liu, W.; Wang, D.; et al. Butyrate Suppresses the Proliferation of Colorectal Cancer Cells via Targeting Pyruvate Kinase M2 and Metabolic Reprogramming. Mol. Cell Proteom. 2018, 17, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.D. Diet, Microorganisms and Their Metabolites, and Colon Cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The Effects of Short-Chain Fatty Acids on Human Colon Cancer Cell Phenotype Are Associated with Histone Hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.J. Metabolism: Warburg behind the Butyrate Paradox? Nat. Rev. Cancer 2012, 12, 798. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Liu, J.; Wu, X.; Chen, L.; Ma, L.; Wu, P. Histone Deacetylase Inhibitor Sodium Butyrate Suppresses DNA Double Strand Break Repair Induced by Etoposide More Effectively in MCF-7 Cells than in HEK293 Cells. BMC Biochem. 2015, 16, 2. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Wang, J. Small Molecules in the Big Picture of Gut Microbiome-Host Cross-Talk. EBioMedicine 2022, 81, 104085. [Google Scholar] [CrossRef]
- Mowat, C.; Dhatt, J.; Bhatti, I.; Hamie, A.; Baker, K. Short Chain Fatty Acids Prime Colorectal Cancer Cells to Activate Antitumor Immunity. Front. Immunol. 2023, 14, 1190810. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium Nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-Regulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef]
- Stoilov, L.; Darroudi, F.; Meschini, R.; van der Schans, G.; Mullenders, L.H.; Natarajan, A.T. Inhibition of Repair of X-Ray-Induced DNA Double-Strand Breaks in Human Lymphocytes Exposed to Sodium Butyrate. Int. J. Radiat. Biol. 2000, 76, 1485–1491. [Google Scholar] [CrossRef]
- Toyooka, T.; Ibuki, Y. Histone Deacetylase Inhibitor Sodium Butyrate Enhances the Cell Killing Effect of Psoralen plus UVA by Attenuating Nucleotide Excision Repair. Cancer Res. 2009, 69, 3492–3500. [Google Scholar] [CrossRef] [PubMed]
- Koprinarova, M.; Botev, P.; Russev, G. Histone Deacetylase Inhibitor Sodium Butyrate Enhances Cellular Radiosensitivity by Inhibiting Both DNA Nonhomologous End Joining and Homologous Recombination. DNA Repair 2011, 10, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Robert, T.; Vanoli, F.; Chiolo, I.; Shubassi, G.; Bernstein, K.A.; Rothstein, R.; Botrugno, O.A.; Parazzoli, D.; Oldani, A.; Minucci, S.; et al. HDACs Link the DNA Damage Response, Processing of Double-Strand Breaks and Autophagy. Nature 2011, 471, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial Short-Chain Fatty Acids Modulate CD8+ T Cell Responses and Improve Adoptive Immunotherapy for Cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; Mosley, S.R.; Namdar, A.; Schiller, D.; Baker, K. Anti-Tumor Immunity in Mismatch Repair-Deficient Colorectal Cancers Requires Type I IFN-Driven CCL5 and CXCL10. J. Exp. Med. 2021, 218, e20210108. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.; Esposito, F.; Thomson, T.M.; Maurel, J. The Tumor Microenvironment in Colorectal Cancer Therapy. Cancers 2019, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Gebhardt, T.; Mackay, L.K. Tissue-Resident Memory T Cells in Cancer Immunosurveillance. Trends Immunol. 2019, 40, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, Q.; Sun, L.; Ye, Y.; Ji, G. Short-Chain Fatty Acids Administration Is Protective in Colitis-Associated Colorectal Cancer Development. J. Nutr. Biochem. 2018, 57, 103–109. [Google Scholar] [CrossRef]
- Gracie, D.J.; Hamlin, P.J.; Ford, A.C. The Influence of the Brain-Gut Axis in Inflammatory Bowel Disease and Possible Implications for Treatment. Lancet Gastroenterol. Hepatol. 2019, 4, 632–642. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling Inflammation across the Gut-Brain Axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Emmi, A.; Sandre, M.; Russo, F.P.; Tombesi, G.; Garrì, F.; Campagnolo, M.; Carecchio, M.; Biundo, R.; Spolverato, G.; Macchi, V.; et al. Duodenal Alpha-Synuclein Pathology and Enteric Gliosis in Advanced Parkinson’s Disease. Mov. Disord. 2023, 38, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Liu, X. The Microbiota-Gut-Brain Axis and Neurodevelopmental Disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The Microbiota-Gut-Brain Axis in Sleep Disorders. Sleep. Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef]
- Castelli, V.; d’Angelo, M.; Quintiliani, M.; Benedetti, E.; Cifone, M.G.; Cimini, A. The Emerging Role of Probiotics in Neurodegenerative Diseases: New Hope for Parkinson’s Disease? Neural Regen. Res. 2021, 16, 628–634. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is Alpha-Synuclein in the Colon a Biomarker for Premotor Parkinson’s Disease? Evidence from 3 Cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef]
- Brudek, T. Inflammatory Bowel Diseases and Parkinson’s Disease. J. Park. Dis. 2019, 9, S331–S344. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Haikal, C.; Li, W.; Li, J.-Y. Gut Inflammation in Association with Pathogenesis of Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 218. [Google Scholar] [CrossRef]
- Mertsalmi, T.H.; Pekkonen, E.; Scheperjans, F. Antibiotic Exposure and Risk of Parkinson’s Disease in Finland: A Nationwide Case-Control Study. Mov. Disord. 2020, 35, 431–442. [Google Scholar] [CrossRef]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-Seeded α-Synuclein Fibrils Promote Gut Dysfunction and Brain Pathology Specifically in Aged Mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. A Timeline for Parkinson’s Disease. Park. Relat. Disord. 2010, 16, 79–84. [Google Scholar] [CrossRef]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; des Varannes, S.B.; Naveilhan, P.; Nguyen, J.-M.; et al. Colonic Inflammation in Parkinson’s Disease. Neurobiol. Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef]
- Chalazonitis, A.; Rao, M. Enteric Nervous System Manifestations of Neurodegenerative Disease. Brain Res. 2018, 1693, 207–213. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Lynch, C.M.K.; Lee, Y.S.; O’Driscoll, C.M.; Clarke, G.; Cryan, J.F.; Aburto, M.R. The Gut Microbiota Is Important for the Maintenance of Blood–Cerebrospinal Fluid Barrier Integrity. Eur. J. Neurosci. 2023, 57, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, C.-C.; Chiang, H.-L.; Liou, J.-M.; Chang, C.-M.; Lu, T.-P.; Chuang, E.Y.; Tai, Y.-C.; Cheng, C.; Lin, H.-Y.; et al. Altered Gut Microbiota and Inflammatory Cytokine Responses in Patients with Parkinson’s Disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-Microglia Connections via the Gut-Brain Axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut Bacterial Profiles in Parkinson’s Disease: A Systematic Review. CNS Neurosci. Ther. 2022, 29, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Trinder, M.; Daisley, B.A.; Dube, J.S.; Reid, G. Drosophila Melanogaster as a High-Throughput Model for Host-Microbiota Interactions. Front. Microbiol. 2017, 8, 751. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of Microbiota on Central Nervous System and Neurological Diseases: The Gut-Brain Axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial Genes, Brain & Behaviour—Epigenetic Regulation of the Gut-Brain Axis. Genes. Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef]
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of Diet and the Gut Microbiome on Epigenetic Modulation in Cancer and Other Diseases. Clin. Epigenet. 2015, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-Gut-Brain Axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Meng, L.; Shen, L. Multiple Roles of Short-Chain Fatty Acids in Alzheimer Disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef] [PubMed]
- St Laurent, R.; O’Brien, L.M.; Ahmad, S.T. Sodium Butyrate Improves Locomotor Impairment and Early Mortality in a Rotenone-Induced Drosophila Model of Parkinson’s Disease. Neuroscience 2013, 246, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short Chain Fatty Acids and Gut Microbiota Differ between Patients with Parkinson’s Disease and Age-Matched Controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Manfready, R.A.; Forsyth, C.B.; Voigt, R.M.; Hall, D.A.; Goetz, C.G.; Keshavarzian, A. Gut-Brain Communication in Parkinson’s Disease: Enteroendocrine Regulation by GLP-1. Curr. Neurol. Neurosci. Rep. 2022, 22, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, S.; Mohajeri, M.H. Changes of Colonic Bacterial Composition in Parkinson’s Disease and Other Neurodegenerative Diseases. Nutrients 2018, 10, 708. [Google Scholar] [CrossRef]
- Xie, A.; Ensink, E.; Li, P.; Gordevičius, J.; Marshall, L.L.; George, S.; Pospisilik, J.A.; Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov. Disord. 2022, 37, 1644–1653. [Google Scholar] [CrossRef]
- Kong, Y.; Jiang, B.; Luo, X. Gut Microbiota Influences Alzheimer’s Disease Pathogenesis by Regulating Acetate in Drosophila Model. Future Microbiol. 2018, 13, 1117–1128. [Google Scholar] [CrossRef]
- Fernandez-Real, J.-M.; Serino, M.; Blasco, G.; Puig, J.; Daunis-i-Estadella, J.; Ricart, W.; Burcelin, R.; Fernández-Aranda, F.; Portero-Otin, M. Gut Microbiota Interacts with Brain Microstructure and Function. J. Clin. Endocrinol. Metab. 2015, 100, 4505–4513. [Google Scholar] [CrossRef]
- Müller, B.; Rasmusson, A.J.; Just, D.; Jayarathna, S.; Moazzami, A.; Novicic, Z.K.; Cunningham, J.L. Fecal Short-Chain Fatty Acid Ratios as Related to Gastrointestinal and Depressive Symptoms in Young Adults. Psychosom. Med. 2021, 83, 693–699. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Abeta and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and Probiotics Attenuate the Development of Alzheimer’s Disease in Transgenic Mice: Role of Microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal Beta-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan Oligosaccharide Attenuates Cognitive and Behavioral Disorders in the 5xFAD Alzheimer’s Disease Mouse Model via Regulating the Gut Microbiota-Brain Axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, J.H.; Shin, J.; Kim, J.-S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive Function Improvement after Fecal Microbiota Transplantation in Alzheimer’s Dementia Patient: A Case Report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.M.; Ibrahim, N.M. Multi-Strain Probiotics (Hexbio) Containing MCP BCMC Strains Improved Constipation and Gut Motility in Parkinson’s Disease: A Randomised Controlled Trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor Gastrointestinal Disorders in Older Patients with Parkinson’s Disease: Is There Hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, X.; Miao, Y.; Han, Y.; Wei, J.; Chen, T. Therapeutic Effect of GLP-1 Engineered Strain on Mice Model of Alzheimer’s Disease and Parkinson’s Disease. AMB Express 2020, 10, 80. [Google Scholar] [CrossRef]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of Probiotics for the Treatment of Constipation in Parkinson’s Disease Patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Taghizadeh, M.; Kakhaki, R.D.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and Metabolic Response to Probiotic Administration in People with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.-Y.; Chong, K.K.; A Manap, M.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Suescun, J.; Jiang, Z.-D.; Brown, E.L.; Essigmann, H.T.; Alexander, A.S.; DuPont, A.W.; Iqbal, T.; Utay, N.S.; Newmark, M.; et al. Fecal Microbiota Transplantation in Parkinson’s Disease—A Randomized Repeat-Dose, Placebo-Controlled Clinical Pilot Study. Front. Neurol. 2023, 14, 1104759. [Google Scholar] [CrossRef]
- Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective Effects of Bifidobacterium Breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients 2022, 14, 4678. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Boktor, J.C.; Cantu-Jungles, T.M.; Thron, T.; Zhang, M.; Bostick, J.W.; Khazaei, T.; Chilakala, S.; Morais, L.H.; et al. A Prebiotic Diet Modulates Microglial States and Motor Deficits in α-Synuclein Overexpressing Mice. Elife 2022, 11, e81453. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic Acid Prebiotic plus Lacticaseibacillus Rhamnosus GG Probiotic as a Novel Synbiotic Promoted Their Separate Neuroprotection against Parkinson’s Disease. Food Res. Int. 2022, 155, 111067. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, F.; Liu, Y.; Ma, T.; Jin, H.; Quan, K.; Leng, B.; Zhao, J.; Yuan, X.; Li, Z.; et al. Probiotics Synergized with Conventional Regimen in Managing Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian Guidelines for the Management of Irritable Bowel Syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional Bowel Disorders with Diarrhoea: Clinical Guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered Profiles of Intestinal Microbiota and Organic Acids May Be the Origin of Symptoms in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2010, 22, 512-e115. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jia, Q.; Song, L.; Duan, L. Alterations in Fecal Short-Chain Fatty Acids in Patients with Irritable Bowel Syndrome. Medicine 2019, 98, e14513. [Google Scholar] [CrossRef] [PubMed]
- Gargari, G.; Taverniti, V.; Gardana, C.; Cremon, C.; Canducci, F.; Pagano, I.; Barbaro, M.R.; Bellacosa, L.; Castellazzi, A.M.; Valsecchi, C.; et al. Fecal Clostridiales Distribution and Short-Chain Fatty Acids Reflect Bowel Habits in Irritable Bowel Syndrome. Environ. Microbiol. 2018, 20, 3201–3213. [Google Scholar] [CrossRef] [PubMed]
- Treem, W.R.; Ahsan, N.; Kastoff, G.; Hyams, J.S. Fecal Short-Chain Fatty Acids in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: In Vitro Studies of Carbohydrate Fermentation. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 280–286. [Google Scholar] [CrossRef]
- Fredericks, E.; Theunissen, R.; Roux, S. Short Chain Fatty Acids and Monocarboxylate Transporters in Irritable Bowel Syndrome. Turk. J. Gastroenterol. 2020, 31, 840–847. [Google Scholar] [CrossRef]
- Undseth, R.; Jakobsdottir, G.; Nyman, M.; Berstad, A.; Valeur, J. Low Serum Levels of Short-Chain Fatty Acids after Lactulose Ingestion May Indicate Impaired Colonic Fermentation in Patients with Irritable Bowel Syndrome. Clin. Exp. Gastroenterol. 2015, 8, 303–308. [Google Scholar] [CrossRef]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional Dysbiosis within the Gut Microbiota of Patients with Constipated-Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of Butyrate- and Methane-Producing Microorganisms in Patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wei, H.; Zhou, A.; Xiao, X.; Xie, X.; Tang, B.; Lin, H.; Tang, L.; Meng, R.; Yuan, X.; et al. The Gut Microbiota Participates in the Effect of Linaclotide in Patients with Irritable Bowel Syndrome with Constipation (IBS-C): A Multicenter, Prospective, Pre-Post Study. J. Transl. Med. 2024, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Rudi, K.; Hestad, K. Faecal Short-Chain Fatty Acids—A Diagnostic Biomarker for Irritable Bowel Syndrome? BMC Gastroenterol. 2016, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, e1–e10. [Google Scholar] [CrossRef]
- Zaki, M.H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.-D. The NLRP3 Inflammasome Protects against Loss of Epithelial Integrity and Mortality during Experimental Colitis. Immunity 2010, 32, 379–391. [Google Scholar] [CrossRef]
- Hirota, S.A.; Ng, J.; Lueng, A.; Khajah, M.; Parhar, K.; Li, Y.; Lam, V.; Potentier, M.S.; Ng, K.; Bawa, M.; et al. NLRP3 Inflammasome Plays a Key Role in the Regulation of Intestinal Homeostasis. Inflamm. Bowel Dis. 2011, 17, 1359–1372. [Google Scholar] [CrossRef]
- Dunsmore, G.; Koleva, P.; Ghobakhloo, N.; Sutton, R.; Ambrosio, L.; Meng, X.; Hotte, N.; Nguyen, V.; Madsen, K.L.; Dieleman, L.A.; et al. Lower Abundance and Impaired Function of CD71+ Erythroid Cells in Inflammatory Bowel Disease Patients during Pregnancy. J. Crohns Colitis 2019, 13, 230–244. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote Th1 Cell IL-10 Production to Maintain Intestinal Homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The Regulation of Intestinal Mucin MUC2 Expression by Short-Chain Fatty Acids: Implications for Epithelial Protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, H.; Iwashita, J.; Kuwajima, A.; Abe, T. The Short Chain Fatty Acid, Butyrate, Stimulates MUC2 Mucin Production in the Human Colon Cancer Cell Line, LS174T. Biochem. Biophys. Res. Commun. 2007, 356, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Augenlicht, L.; Shi, L.; Mariadason, J.; Laboisse, C.; Velcich, A. Repression of MUC2 Gene Expression by Butyrate, a Physiological Regulator of Intestinal Cell Maturation. Oncogene 2003, 22, 4983–4992. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.M.; Koetsier, M.A.; van Deventer, S.J.H.; van Tol, E.A.F. Short Chain Fatty Acids Stimulate Epithelial Mucin 2 Expression through Differential Effects on Prostaglandin E(1) and E(2) Production by Intestinal Myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Finnie, I.A.; Dwarakanath, A.D.; Taylor, B.A.; Rhodes, J.M. Colonic Mucin Synthesis Is Increased by Sodium Butyrate. Gut 1995, 36, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Wu, X.; Wang, K.; Wang, W.; Wang, Y.; Li, Z.; Liu, J.; Li, L.; Peng, L. Sodium Butyrate Promotes Reassembly of Tight Junctions in Caco-2 Monolayers Involving Inhibition of MLCK/MLC2 Pathway and Phosphorylation of PKCβ2. Int. J. Mol. Sci. 2016, 17, 1696. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Ha, S.E.; Wei, L.; Singh, R.; Zogg, H.; Clemmensen, B.; Heredia, D.J.; Gould, T.W.; Sanders, K.M.; Ro, S. Colonic Motility Is Improved by the Activation of 5-HT2B Receptors on Interstitial Cells of Cajal in Diabetic Mice. Gastroenterology 2021, 161, 608–622.e7. [Google Scholar] [CrossRef] [PubMed]
- Tharayil, V.S.; Wouters, M.M.; Stanich, J.E.; Roeder, J.L.; Lei, S.; Beyder, A.; Gomez-Pinilla, P.J.; Gershon, M.D.; Maroteaux, L.; Gibbons, S.J.; et al. Lack of Serotonin 5-HT2B Receptor Alters Proliferation and Network Volume of Interstitial Cells of Cajal In Vivo. Neurogastroenterol. Motil. 2010, 22, 462-e110. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Gibbons, S.J.; Roeder, J.L.; Distad, M.; Ou, Y.; Strege, P.R.; Szurszewski, J.H.; Farrugia, G. Exogenous Serotonin Regulates Proliferation of Interstitial Cells of Cajal in Mouse Jejunum through 5-HT2B Receptors. Gastroenterology 2007, 133, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Karaki, S.; Kuwahara, A. Short-Chain Fatty Acids Decrease the Frequency of Spontaneous Contractions of Longitudinal Muscle via Enteric Nerves in Rat Distal Colon. Jpn. J. Physiol. 2004, 54, 483–493. [Google Scholar] [CrossRef]
- Grider, J.R.; Kuemmerle, J.F.; Jin, J.G. 5-HT Released by Mucosal Stimuli Initiates Peristalsis by Activating 5-HT4/5-HT1p Receptors on Sensory CGRP Neurons. Am. J. Physiol. 1996, 270, G778–G782. [Google Scholar] [CrossRef] [PubMed]
- Foxx-Orenstein, A.E.; Kuemmerle, J.F.; Grider, J.R. Distinct 5-HT Receptors Mediate the Peristaltic Reflex Induced by Mucosal Stimuli in Human and Guinea Pig Intestine. Gastroenterology 1996, 111, 1281–1290. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.-B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal Microbiota Shapes Gut Physiology and Regulates Enteric Neurons and Glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-Chain Fatty Acids Regulate the Enteric Neurons and Control Gastrointestinal Motility in Rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Suply, E.; de Vries, P.; Soret, R.; Cossais, F.; Neunlist, M. Butyrate Enemas Enhance Both Cholinergic and Nitrergic Phenotype of Myenteric Neurons and Neuromuscular Transmission in Newborn Rat Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1373–G1380. [Google Scholar] [CrossRef] [PubMed]
- Shaidullov, I.F.; Sorokina, D.M.; Sitdikov, F.G.; Hermann, A.; Abdulkhakov, S.R.; Sitdikova, G.F. Short Chain Fatty Acids and Colon Motility in a Mouse Model of Irritable Bowel Syndrome. BMC Gastroenterol. 2021, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Tan, W.; Ren, H.; Yan, L.; Wang, Y.; Luo, H. The Effects of Short-Chain Fatty Acids on Rat Colonic Hypermotility Induced by Water Avoidance Stress. Drug Des. Devel Ther. 2020, 14, 4671–4684. [Google Scholar] [CrossRef] [PubMed]
- Hurst, N.R.; Kendig, D.M.; Murthy, K.S.; Grider, J.R. The Short Chain Fatty Acids, Butyrate and Propionate, Have Differential Effects on the Motility of the Guinea Pig Colon. Neurogastroenterol. Motil. 2014, 26, 1586–1596. [Google Scholar] [CrossRef]
- Waseem, M.R.; Shin, A.; Siwiec, R.; James-Stevenson, T.; Bohm, M.; Rogers, N.; Wo, J.; Waseem, L.; Gupta, A.; Jarrett, M.; et al. Associations of Fecal Short Chain Fatty Acids with Colonic Transit, Fecal Bile Acid, and Food Intake in Irritable Bowel Syndrome. Clin. Transl. Gastroenterol. 2023, 14, e00541. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The Gut Microbiome and Metabolic Syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Waalen, J. The Genetics of Human Obesity. Transl. Res. 2014, 164, 293–301. [Google Scholar] [CrossRef]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the Diversity and Short-Chain Fatty Acids Production by the Bacterial Community in Overweight and Obese Mexican Children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef]
- Ecklu-Mensah, G.; Choo-Kang, C.; Maseng, M.G.; Donato, S.; Bovet, P.; Viswanathan, B.; Bedu-Addo, K.; Plange-Rhule, J.; Oti Boateng, P.; Forrester, T.E.; et al. Gut Microbiota and Fecal Short Chain Fatty Acids Differ with Adiposity and Country of Origin: The METS-Microbiome Study. Nat. Commun. 2023, 14, 5160. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Tirosh, A.; Calay, E.S.; Tuncman, G.; Claiborn, K.C.; Inouye, K.E.; Eguchi, K.; Alcala, M.; Rathaus, M.; Hollander, K.S.; Ron, I.; et al. The Short-Chain Fatty Acid Propionate Increases Glucagon and FABP4 Production, Impairing Insulin Action in Mice and Humans. Sci. Transl. Med. 2019, 11, eaav0120. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate Mediates a Microbiome-Brain-β Cell Axis Promoting Metabolic Syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.S.; Grześkowiak, Ł.; Franceschini, S.C.C.; Bressan, J.; Ferreira, C.L.L.F.; Peluzio, M.C.G. Higher Level of Faecal SCFA in Women Correlates with Metabolic Syndrome Risk Factors. Br. J. Nutr. 2013, 109, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.; Wolever, T.M.; Jeejeebhoy, K.N. Clinical Significance of Colonic Fermentation. Am. J. Gastroenterol. 1990, 85, 1307–1312. [Google Scholar] [PubMed]
- Reshef, L.; Niv, J.; Shapiro, B. Effect of Propionate on Lipogenesis in Adipose Tissue. J. Lipid Res. 1967, 8, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Brighenti, F.; Royall, D.; Jenkins, A.L.; Jenkins, D.J. Effect of Rectal Infusion of Short Chain Fatty Acids in Human Subjects. Am. J. Gastroenterol. 1989, 84, 1027–1033. [Google Scholar] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-Fat-Diet-Induced Obesity in Mice by Regulating G Protein-Coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Bishop, K.S.; Kao, C.H.J.; Xu, Y.; Glucina, M.P.; Paterson, R.R.M.; Ferguson, L.R. From 2000 years of Ganoderma Lucidum to Recent Developments in Nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of Obesity and Inflammation by Polysaccharide from Sporoderm-Broken Spore of Ganoderma Lucidum via Gut Microbiota Regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef] [PubMed]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.E.; González Hernández, M.A.; Hoebers, N.T.H.; van der Beek, C.M.; Essers, Y.P.G.; Blaak, E.E.; Canfora, E.E. Short-Chain Fatty Acids Differentially Affect Intracellular Lipolysis in a Human White Adipocyte Model. Front. Endocrinol. 2017, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The Impact of Short-Chain Fatty Acids on GLP-1 and PYY Secretion from the Isolated Perfused Rat Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [PubMed]
- Veprik, A.; Laufer, D.; Weiss, S.; Rubins, N.; Walker, M.D. GPR41 Modulates Insulin Secretion and Gene Expression in Pancreatic β-Cells and Modifies Metabolic Homeostasis in Fed and Fasting States. FASEB J. 2016, 30, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Villa, S.R.; Fuller, M.; Wicksteed, B.; Mackay, C.R.; Alquier, T.; Poitout, V.; Mancebo, H.; Mirmira, R.G.; Gilchrist, A.; et al. An Acetate-Specific GPCR, FFAR2, Regulates Insulin Secretion. Mol. Endocrinol. 2015, 29, 1055–1066. [Google Scholar] [CrossRef]
- Fushimi, T.; Tayama, K.; Fukaya, M.; Kitakoshi, K.; Nakai, N.; Tsukamoto, Y.; Sato, Y. Acetic Acid Feeding Enhances Glycogen Repletion in Liver and Skeletal Muscle of Rats. J. Nutr. 2001, 131, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Z.; Zhang, J.; Ye, X.; Xu, A.; Ye, J.; Jia, W. Sodium Butyrate Stimulates Expression of Fibroblast Growth Factor 21 in Liver by Inhibition of Histone Deacetylase 3. Diabetes 2012, 61, 797–806. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Guan, Y.; Li, X.; Lei, L.; Liu, J.; Yin, L.; Liu, G.; Wang, Z. Acetic Acid Activates the AMP-Activated Protein Kinase Signaling Pathway to Regulate Lipid Metabolism in Bovine Hepatocytes. PLoS ONE 2013, 8, e67880. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H. Biological Function of Acetic Acid-Improvement in Obesity and Glucose Tolerance by Acetic Acid in Type 2 Diabetic Rats. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S171–S175. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Vily-Petit, J.; Soty, M.; Silva, M.; Micoud, M.; Bron, C.; Guérin-Deremaux, L.; Mithieux, G. Improvement of Energy Metabolism Associated with NUTRIOSE® Soluble Fiber, a Dietary Ingredient Exhibiting Prebiotic Properties, Requires Intestinal Gluconeogenesis. Food Res. Int. 2023, 167, 112723. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Zhang, Y.; Li, X. Comprehensive Relationships between Gut Microbiome and Faecal Metabolome in Individuals with Type 2 Diabetes and Its Complications. Endocrine 2019, 66, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Perera, D.; Kleinstein, S.E.; Hanson, B.; Hasturk, H.; Eveloff, R.; Freire, M.; Ramsey, M. Impaired Host Response and the Presence of Acinetobacter Baumannii in the Serum Microbiome of Type-II Diabetic Patients. iScience 2021, 24, 101941. [Google Scholar] [CrossRef]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut Microbial Metabolites Limit the Frequency of Autoimmune T Cells and Protect against Type 1 Diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal Relationships among the Gut Microbiome, Short-Chain Fatty Acids and Metabolic Diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Venugopal, S. Probiotics and Their Role in the Management of Type 2 Diabetes Mellitus (Short-Term Versus Long-Term Effect): A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46741. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Preston, T.; Tedford, C.; Brignardello, J.; Garcia-Perez, I.; Holmes, E.; Wallis, G.A.; Morrison, D.J.; Frost, G.S. Effects of Inulin Propionate Ester Incorporated into Palatable Food Products on Appetite and Resting Energy Expenditure: A Randomised Crossover Study. Nutrients 2019, 11, 861. [Google Scholar] [CrossRef]
- Bouter, K.; Bakker, G.J.; Levin, E.; Hartstra, A.V.; Kootte, R.S.; Udayappan, S.D.; Katiraei, S.; Bahler, L.; Gilijamse, P.W.; Tremaroli, V.; et al. Differential Metabolic Effects of Oral Butyrate Treatment in Lean versus Metabolic Syndrome Subjects. Clin. Transl. Gastroenterol. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Komaroff, A.L. The Microbiome and Risk for Obesity and Diabetes. JAMA 2017, 317, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal Microbiota in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yanagi, K.; Cheng, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Interactions between Gut Microbiota and Non-Alcoholic Liver Disease: The Role of Microbiota-Derived Metabolites. Pharmacol. Res. 2019, 141, 521–529. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Xie, C.; Halegoua-DeMarzio, D. Role of Probiotics in Non-Alcoholic Fatty Liver Disease: Does Gut Microbiota Matter? Nutrients 2019, 11, 2837. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary Cholesterol Drives Fatty Liver-Associated Liver Cancer by Modulating Gut Microbiota and Metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-Producing Bacteria in Gut Microbiome of Human NAFLD as a Putative Link to Systemic T-Cell Activation and Advanced Disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Aragonès, G.; Colom-Pellicer, M.; Aguilar, C.; Guiu-Jurado, E.; Martínez, S.; Sabench, F.; Porras, J.A.; Riesco, D.; Del Castillo, D.; Richart, C.; et al. Circulating Microbiota-Derived Metabolites: A “liquid Biopsy? Int. J. Obes. 2020, 44, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Adler, G.K.; Hornik, E.S.; Murray, G.; Bhandari, S.; Yadav, Y.; Heydarpour, M.; Basu, R.; Garg, R.; Tirosh, A. Acute Effects of the Food Preservative Propionic Acid on Glucose Metabolism in Humans. BMJ Open Diabetes Res. Care 2021, 9, e002336. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Chen, X.; Zhao, Z.; Liao, Y.; Zhou, T.; Xiang, Q. A Potential Link between Plasma Short-Chain Fatty Acids, TNF-α Level and Disease Progression in Non-Alcoholic Fatty Liver Disease: A Retrospective Study. Exp. Ther. Med. 2022, 24, 598. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut Microbiota Impact on the Peripheral Immune Response in Non-Alcoholic Fatty Liver Disease Related Hepatocellular Carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Hung, W.-C.; Hung, W.-W.; Lee, Y.-J.; Chen, Y.-C.; Lee, C.-Y.; Tsai, Y.-C.; Dai, C.-Y. Circulating Short-Chain Fatty Acids and Non-Alcoholic Fatty Liver Disease Severity in Patients with Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1712. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Nogal, A.; Louca, P.; Zhang, X.; Wells, P.M.; Steves, C.J.; Spector, T.D.; Falchi, M.; Valdes, A.M.; Menni, C. Circulating Levels of the Short-Chain Fatty Acid Acetate Mediate the Effect of the Gut Microbiome on Visceral Fat. Front. Microbiol. 2021, 12, 711359. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Thing, M.; Werge, M.P.; Kimer, N.; Hetland, L.E.; Rashu, E.B.; Nabilou, P.; Junker, A.E.; Galsgaard, E.D.; Bendtsen, F.; Laupsa-Borge, J.; et al. Targeted Metabolomics Reveals Plasma Short-Chain Fatty Acids Are Associated with Metabolic Dysfunction-Associated Steatotic Liver Disease. BMC Gastroenterol. 2024, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Dangana, E.O.; Omolekulo, T.E.; Areola, E.D.; Olaniyi, K.S.; Soladoye, A.O.; Olatunji, L.A. Sodium Acetate Protects against Nicotine-Induced Excess Hepatic Lipid in Male Rats by Suppressing Xanthine Oxidase Activity. Chem. Biol. Interact. 2020, 316, 108929. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.J.; Sellmann, C.; Engstler, A.J.; Ziegenhardt, D.; Bergheim, I. Supplementation of Sodium Butyrate Protects Mice from the Development of Non-Alcoholic Steatohepatitis (NASH). Br. J. Nutr. 2015, 114, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Fan, J.-G. Microbial Metabolites in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2019, 25, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs Alleviated Steatosis and Inflammation in Mice with NASH Induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Wang, Z.-X.; Zhou, D.; Han, Y.; Ma, F.; Hu, Z.; Xin, F.-Z.; Liu, X.-L.; Ren, T.-Y.; Zhang, F.; et al. Sodium Butyrate Supplementation Inhibits Hepatic Steatosis by Stimulating Liver Kinase B1 and Insulin-Induced Gene. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium Butyrate Attenuates High-Fat Diet-Induced Steatohepatitis in Mice by Improving Gut Microbiota and Gastrointestinal Barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Tang, J.; Mo, Q.; Zhong, H.; Zhang, H.; Feng, F. A Bridge for Short-Chain Fatty Acids to Affect Inflammatory Bowel Disease, Type 1 Diabetes, and Non-Alcoholic Fatty Liver Disease Positively: By Changing Gut Barrier. Eur. J. Nutr. 2021, 60, 2317–2330. [Google Scholar] [CrossRef]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.-F.; et al. Fungal Trans-Kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020, 27, 823–829.e3. [Google Scholar] [CrossRef]
- de Groot, P.; Scheithauer, T.; Bakker, G.J.; Prodan, A.; Levin, E.; Khan, M.T.; Herrema, H.; Ackermans, M.; Serlie, M.J.M.; de Brauw, M.; et al. Donor Metabolic Characteristics Drive Effects of Faecal Microbiota Transplantation on Recipient Insulin Sensitivity, Energy Expenditure and Intestinal Transit Time. Gut 2020, 69, 502–512. [Google Scholar] [CrossRef]
- Peery, A.F.; Kelly, C.R.; Kao, D.; Vaughn, B.P.; Lebwohl, B.; Singh, S.; Imdad, A.; Altayar, O. AGA Clinical Practice Guideline on Fecal Microbiota–Based Therapies for Select Gastrointestinal Diseases. Gastroenterology 2024, 166, 409–434. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.J.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The Use of Faecal Microbiota Transplant as Treatment for Recurrent or Refractory Clostridium Difficile Infection and Other Potential Indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) Guidelines. Gut 2018, 67, 1920–1941. [Google Scholar] [CrossRef] [PubMed]
- Gweon, T.-G.; Lee, Y.J.; Kim, K.O.; Yim, S.K.; Soh, J.S.; Kim, S.Y.; Park, J.J.; Shin, S.Y.; Lee, T.H.; Choi, C.H.; et al. Clinical Practice Guidelines for Fecal Microbiota Transplantation in Korea. J. Neurogastroenterol. Motil. 2022, 28, 28–42. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Barberio, B.; Facchin, S.; Mele, E.; D’Incà, R.; Sturniolo, G.C.; Farinati, F.; Zingone, F.; Quagliariello, A.; Ghisa, M.; Massimi, D.; et al. Faecal Microbiota Transplantation in Clostridioides Difficile Infection: Real-Life Experience from an Academic Italian Hospital. Ther. Adv. Gastroenterol. 2020, 13, 1756284820934315. [Google Scholar] [CrossRef]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective Effects of Fecal Microbiota Transplantation on MPTP-Induced Parkinson’s Disease Mice: Gut Microbiota, Glial Reaction and TLR4/TNF-α Signaling Pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Smits, L.P.; Bouter, K.E.C.; de Vos, W.M.; Borody, T.J.; Nieuwdorp, M. Therapeutic Potential of Fecal Microbiota Transplantation. Gastroenterology 2013, 145, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, J.-Y.; Li, Q.-H.; Su, H.; Sun, X. Lactobacillus Rhamnosus GG Induced Protective Effect on Allergic Airway Inflammation Is Associated with Gut Microbiota. Cell Immunol. 2018, 332, 77–84. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hausken, T.; Hatlebakk, J.G. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). Nutrients 2019, 11, 1415. [Google Scholar] [CrossRef]
- Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Leong, K.S.W.; Tweedie-Cullen, R.Y.; Creagh, C.; Depczynski, M.; Edwards, T.; Vatanen, T.; Thabrew, H.; et al. An Open-Label Pilot Trial of Faecal Microbiome Transfer to Restore the Gut Microbiome in Anorexia Nervosa: Protocol. BMJ Open 2023, 13, e070616. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Støving, R.K.; Ibraim, S.B.; Hyötyläinen, T.; Thirion, F.; Arora, T.; Lyu, L.; Stankevic, E.; Hansen, T.H.; Déchelotte, P.; et al. The Gut Microbiota Contributes to the Pathogenesis of Anorexia Nervosa in Humans and Mice. Nat. Microbiol. 2023, 8, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, L.; Wang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Cell Infect. Microbiol. 2022, 12, 827395. [Google Scholar] [CrossRef]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Dorsaz, S.; Charretier, Y.; Girard, M.; Gaïa, N.; Leo, S.; Schrenzel, J.; Harbarth, S.; Huttner, B.; Lazarevic, V. Changes in Microbiota Profiles after Prolonged Frozen Storage of Stool Suspensions. Front. Cell Infect. Microbiol. 2020, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Kocsis, B.; Sipos, D.; Kása, P.; Vigvári, S.; Pál, S.; Dembrovszky, F.; Farkas, K.; Péterfi, Z. How to Apply FMT More Effectively, Conveniently and Flexible—A Comparison of FMT Methods. Front. Cell. Infect. Microbiol. 2021, 11, 657320. [Google Scholar] [CrossRef]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef]
- Joseph, J.; Depp, C.; Shih, P.-A.B.; Cadenhead, K.S.; Schmid-Schönbein, G. Modified Mediterranean Diet for Enrichment of Short Chain Fatty Acids: Potential Adjunctive Therapeutic to Target Immune and Metabolic Dysfunction in Schizophrenia? Front. Neurosci. 2017, 11, 155. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of Microbiota from Drug-Free Patients with Schizophrenia Causes Schizophrenia-like Abnormal Behaviors and Dysregulated Kynurenine Metabolism in Mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Osaki, H.; Jodai, Y.; Koyama, K.; Omori, T.; Horiguchi, N.; Kamano, T.; Funasaka, K.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; et al. Clinical Response and Changes in the Fecal Microbiota and Metabolite Levels after Fecal Microbiota Transplantation in Patients with Inflammatory Bowel Disease and Recurrent Clostridioides Difficile Infection. Fujita Med. J. 2021, 7, 87–98. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Theriot, C.M.; Rao, K.; Chang, Y.-M.; Freeman, A.E.; Kao, J.Y.; Young, V.B. Restoration of Short Chain Fatty Acid and Bile Acid Metabolism Following Fecal Microbiota Transplantation in Patients with Recurrent Clostridium Difficile Infection. Anaerobe 2018, 53, 64–73. [Google Scholar] [CrossRef]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Gunnar Hatlebakk, J. Changes in Fecal Short-Chain Fatty Acids Following Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2021, 33, e13983. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Shock, T.; Badang, L.; Ferguson, B.; Martinez-Guryn, K. The Interplay between Diet, Gut Microbes, and Host Epigenetics in Health and Disease. J. Nutr. Biochem. 2021, 95, 108631. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A Fiber-Deprived Diet Causes Cognitive Impairment and Hippocampal Microglia-Mediated Synaptic Loss through the Gut Microbiota and Metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef]
- Adolph, T.E.; Zhang, J. Diet Fuelling Inflammatory Bowel Diseases: Preclinical and Clinical Concepts. Gut 2022, 71, 2574–2586. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Jacka, F.N.; Pizzinga, J.; Itsiopoulos, C. A Modified Mediterranean Dietary Intervention for Adults with Major Depression: Dietary Protocol and Feasibility Data from the SMILES Trial. Nutr. Neurosci. 2018, 21, 487–501. [Google Scholar] [CrossRef]
- Pylkas, A.M.; Juneja, L.R.; Slavin, J.L. Comparison of Different Fibers for In Vitro Production of Short Chain Fatty Acids by Intestinal Microflora. J. Med. Food 2005, 8, 113–116. [Google Scholar] [CrossRef]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Bonazzi, E.; Bretin, A.; Vigué, L.; Hao, F.; Patterson, A.D.; Gewirtz, A.T.; Chassaing, B. Individualized Microbiotas Dictate the Impact of Dietary Fiber on Colitis Sensitivity. Microbiome 2024, 12, 5. [Google Scholar] [CrossRef]
- Gudan, A.; Skonieczna-Żydecka, K.; Palma, J.; Drozd, A.; Stachowska, E. Effects of Dietary Components on Intestinal Short-Chain Fatty Acids (SCFAs) Synthesis in Healthy Adult Persons Following a Ketogenic Diet. Rocz. Panstw. Zakl. Hig. 2022, 73, 51–69. [Google Scholar] [CrossRef]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-Chain Fatty Acids Are Key Mediators of the Favorable Effects of the Mediterranean Diet on Intestinal Barrier Integrity: Data from the Randomized Controlled LIBRE Trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary Fiber and SCFAs in the Regulation of Mucosal Immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- François, I.E.J.A.; Lescroart, O.; Veraverbeke, W.S.; Marzorati, M.; Possemiers, S.; Evenepoel, P.; Hamer, H.; Houben, E.; Windey, K.; Welling, G.W.; et al. Effects of a Wheat Bran Extract Containing Arabinoxylan Oligosaccharides on Gastrointestinal Health Parameters in Healthy Adult Human Volunteers: A Double-Blind, Randomised, Placebo-Controlled, Cross-over Trial. Br. J. Nutr. 2012, 108, 2229–2242. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Johansson-Boll, E.V.; Björck, I.M.E. Increased Gut Hormones and Insulin Sensitivity Index Following a 3-d Intervention with a Barley Kernel-Based Product: A Randomised Cross-over Study in Healthy Middle-Aged Subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High Molecular Weight Barley β-Glucan Alters Gut Microbiota toward Reduced Cardiovascular Disease Risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Farup, P.G.; Valeur, J. Changes in Faecal Short-Chain Fatty Acids after Weight-Loss Interventions in Subjects with Morbid Obesity. Nutrients 2020, 12, 802. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion Mechanisms Mediated by Probiotics and Prebiotics and Their Potential Impact on Human Health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; de Foy, J.-M.P.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Li, R.-Q.; An, J.-X.; Xie, T.-Q.; Han, Z.-Y.; Xu, R.; Fang, Y.; Zhang, X.-Z. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, e2004529. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide Is a Prebiotic That Reduces Desulfovibrio Bacteria, Increases Cecal Short-Chain Fatty Acids, and Is Anti-Inflammatory in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef]
- Holmes, Z.C.; Villa, M.M.; Durand, H.K.; Jiang, S.; Dallow, E.P.; Petrone, B.L.; Silverman, J.D.; Lin, P.-H.; David, L.A. Microbiota Responses to Different Prebiotics Are Conserved within Individuals and Associated with Habitual Fiber Intake. Microbiome 2022, 10, 114. [Google Scholar] [CrossRef]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic Effect of Inulin-Type Fructans on Faecal Microbiota and Short-Chain Fatty Acids in Type 2 Diabetes: A Randomised Controlled Trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Gulseth, H.L.; Birkeland, K.I.; Hartmann, B.; Holst, J.J.; Holst, R.; Aas, A.-M. Effects of Prebiotics on Postprandial GLP-1, GLP-2 and Glucose Regulation in Patients with Type 2 Diabetes: A Randomised, Double-Blind, Placebo-Controlled Crossover Trial. Diabet. Med. 2021, 38, e14657. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Li, L.; Li, P.; Xu, L. Assessing the Effects of Inulin-Type Fructan Intake on Body Weight, Blood Glucose, and Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Sci. Nutr. 2021, 9, 4598–4616. [Google Scholar] [CrossRef]
- Łoniewski, I.; Szulińska, M.; Kaczmarczyk, M.; Podsiadło, K.; Styburski, D.; Skonieczna-Żydecka, K.; Bogdański, P. Multispecies Probiotic Affects Fecal Short-Chain Fatty Acids in Postmenopausal Women with Obesity: A Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled Study. Nutrition 2023, 114, 112109. [Google Scholar] [CrossRef]
- Rad, Z.A.; Mousavi, S.N.; Chiti, H. A Low-Carb Diet Increases Fecal Short-Chain Fatty Acids in Feces of Obese Women Following a Weight-Loss Program: Randomized Feeding Trial. Sci. Rep. 2023, 13, 18146. [Google Scholar] [CrossRef]

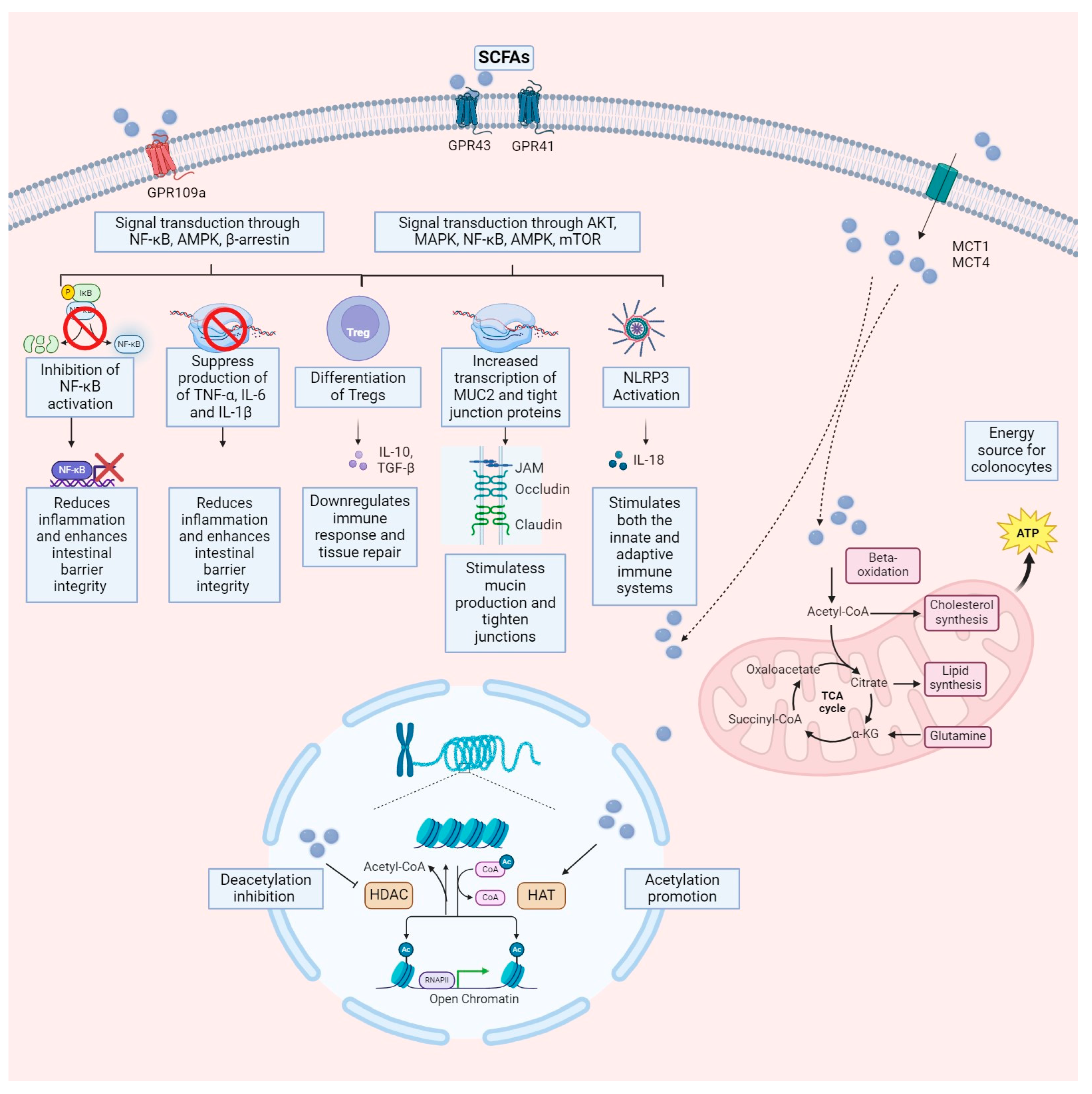
| SCFAs | Metabolic Pathway | Bacteria Involved in the Production |
|---|---|---|
| Acetate | Acetogenesis | Acetobacterium, Acetoanaerobium, Acetogenium, Butyrbacterium, Clostridium, Eubacterium, Pelobacter |
| Carbon fixation | Bacteroides succinogenes, Clostridium butyricum, Syntrophomonas sp. | |
| Propionate | Succinate | Firmicutes (Negativicutes) and Bacteroidetes |
| Propanediol | Lachnospiraceae (Roseburia inulinivorans, Balutia sp.) | |
| Butyrate | Butyryl-CoA: acetate-CoA transferase | Eubacterium, Roseburia, Anaerostipes, Faecalibacterium prausnitzii |
| Butyrate kinase | Coprococcus and Clostridium specific spp. |
| Ref. | Delivery | Year | Groups (n) | Design | Duration | Dosage Butyrate | Drugs (ad) | Improvement |
|---|---|---|---|---|---|---|---|---|
| [66] | enema | 1991 | DC (13) | DB | 2 w | 40 mmol/L | nr | ns |
| [67] | enema | 1992 | UC (10) | SB crossover | 2 w | 100 mmol/L | A-S | s |
| [68] | enema | 1994 | UC (10) | open label | 6 w | 80 mmol/L | A-S | 60% |
| [69] | enema | 1995 | UC (40) | DB-RCT | 6 w | 200 mL/d mix | A-S | s |
| [70] | enema | 1996 | UC (38) | RCT | 6 w | 80 mmol/d | A/S | not impr. |
| [71] | enema | 1996 | UC (47) | DB-RCT | 6 w | 80 mmol/d | nr | not impr. |
| [72] | enema | 1999 | CRP (17) | DB-RCT | 5 w | 80 mmol/d | nr | s |
| [73] | enema | 2000 | UC (30) | RCT | 6 w | 4 gr/d | A | ns |
| [74] | enema | 2000 | APR (20) | RCT crossover | 3 w | 80 mmol/L | nr | s |
| [75] | enema | 2002 | UC (11) | RCT | 8 w | 100 mM | A/S/steroid | s |
| [76] | enema | 2003 | UC (51) | DB-RCT | 6 w | 80 mmol/L | M/steroid | S |
| [77] | oral | 2005 | CD (13) | open label | 8 w | 4 gr/d | A/S | 69% |
| [78] | oral | 2008 | UC (216) | open label | 24 w | 921 mg/d | A + S | 82.4% |
| [79] | enema | 2009 | IBS (11) | DB-RCT | 1 w | 50/100 mmol/L/d | nr | s |
| [80] | enema | 2010 | UC (35) | DB-RCT crossover | 20 d | 100 mmol/d | nr | s |
| [81] | oral | 2013 | IBS (66) | RCT | 12 w | 300 mg/d | std | s |
| [82] | oral | 2014 | DC (63) | RCT | 12 month | 300 mg/d | nr | s |
| [83] | oral | 2014 | TD (42) | RCT | 3 d + trip | 1500 mg/d | various | s |
| [84] | enema | 2014 | APR (166) | RCT | 3 w | 1–2–4 gr/d | nr | ns |
| [85] | enema | 2016 | Mix (20) | DB-RCT | 4 w | 600 mmol/L | nr | s |
| [86] | oral | 2017 | DM (40) | DB-RCT | 45 d | 600 mg/d | +inulin | s(+ inulin) |
| [87] | oral | 2020 | UC (39) | Prospective | 12 months | 1 g/d | std | s |
| [64] | oral | 2020 | IBD (49) | DB-RCT | 8 w | 600 mg/d | std | ps |
| [88] | oral | 2020 | DT1 (30) | DB-RCT | 4 w | 4 g/d | nr | ns |
| [89] | oral | 2022 | Ob ped (54) | QB-RCT | 13 months | 20 mg/kg | std | s |
| [90] | oral | 2022 | IBD ped (80) | RCT | 12 w | 150 mg/d | std | ns |
| [91] | oral | 2022 | DT2 (42) | TB-RCT | 6 w | 600 mg/d | nr | s |
| [92] | oral | 2024 | COPD (121) | RCT | 12 w | 300 mg/d | nr | s |
| Ref. | Delivery | Year | Groups (n) | Design | Duration | Dosage Propionate | Formula | Improvement |
|---|---|---|---|---|---|---|---|---|
| [102] | oral | 2015 | Obese (60) | DB-RCT | 24 w | 10 g/d | IPE | s |
| [103] | oral | 2019 | Obese (12) | DB-RCT cross over | 42 d | 20 g/d | IPE | s |
| [104] | oral | 2019 | HovF (20) | RCT | 4 w | 10 g/d | IPE | s |
| [101] | oral | 2020 | MS (36)/Healthy (68) | proof-of-concept | 2 w | 1 g/d | NaP | s |
| [105] | oral | 2022 | ACVD (62) | DB-RCT | 8 w | 1 g/d | propionic acid | s |
| Name of Trial | Type | Identifier/Status | Condition | Intervention | Location |
|---|---|---|---|---|---|
| Combination of Medium Cut-off Dialyzer Membrane and Diet Modification to Alleviate Residual Uremic Syndrome of Dialysis Patients | RCT | NCT04247867/recruiting | Uremic syndrome | Psyllium-inulin/sodium propionate | University medical Centre Ljubljana, Ljubljana, Slovenia |
| The Effect of Combining Medium Cut-Off Dialysis Membrane and Diet Modification on Reducing Inflammation Response | RCT | NCT04260412/recruiting | Uremic syndrome | Psyllium-inulin/sodium propionate | University medical Centre Ljubljana, Ljubljana, Slovenia |
| Ref. | Delivery | Year | Groups (n) | Design | Duration | Dosage Propionate | Formula | Improvement |
|---|---|---|---|---|---|---|---|---|
| [111] | Rectally and intravenous | 2010 | HinsF (6) | open label | 4 times | 60 mmol/L rectal + 20 mmon/Lintravenous | NaAcetate | s |
| [112] | Intravenous | 2012 | Overweight normoglycemic and hyperglycemic subjects (9) | open label | 90 | 140 mmol/L | NaAcetate | no |
| [113] | Proximal and distal colonic | 2016 | Obese (6) | DB-RCT crossover | 3 d | 100–180 mmol/L | Acetate | s |
| [114] | Colonic infusions | 2017 | Obese (12) | DB-RCT crossover | 4 d | 200 mmol/L mix | (acetate, propionate, and butyrate) | s |
| Disease | Supposed Mechanisms of SCFAs Protection/Risk |
|---|---|
| Inflammatory Bowel Disease | 1. Anti-inflammatory effects: Butyrate, a primary energy source for colonocytes, inhibits NF-κB activation, reducing proinflammatory gene expression. |
| 2. Maintenance of gut barrier integrity: SCFAs promote mucus production and tighten epithelial cell junctions, enhancing the intestinal epithelial barrier. | |
| 3. Modulation of immune responses: SCFAs influence the differentiation and function of regulatory T cells (Tregs), suppressing excessive immune reactions. They engage with receptors like GPR43 and GPR109A to stimulate Treg production. | |
| 4. Tissue repair and healing: SCFAs promote the proliferation and differentiation of epithelial cells, facilitating tissue repair processes within the gut damaged by inflammation in IBD. | |
| Colon Cancer | 1. Protective effects against CRC development: SCFAs exert protective effects against colorectal cancer by regulating gene expression, promoting apoptosis, and inhibiting CRC cell proliferation and metabolism. |
| 2. Anti-inflammatory actions: SCFAs mitigate inflammation in CRC by inhibiting NF-κB activation, decreasing pro-inflammatory cytokine expression, and promoting anti-inflammatory cytokines and regulatory T-cell differentiation. | |
| 3. Potential DNA damage modulation: While SCFAs are anticipated to decrease DNA damage in CRC cells, reports suggest they may exacerbate DNA damage accumulation in some instances, possibly due to disruptions in DNA repair mechanisms. Further evidence is needed. | |
| Disorders of the Gut-Brain Axis | 1. Neuroprotective effects: SCFAs exert neuroprotective effects by influencing brain function, regulating blood flow, and modulating neuroinflammation via interactions with specific receptors and epigenetic modulation. |
| 2. Role in neurodegenerative diseases: Reduced SCFAs levels are implicated in neurodegenerative diseases like Alzheimer’s and Parkinson’s. They contribute to intestinal barrier impairment, the release of pro-inflammatory molecules, and microglial activation, ultimately impacting disease progression. | |
| 3. Gut barrier function and motility: SCFAs promote mucus secretion and strengthen intestinal tight junctions, improving barrier integrity. SCFAs can influence nerve activity, neurotransmitters, and muscle contractions. |
| Disease | Proposed Mechanisms of SCFAs Protection/Risk |
|---|---|
| Obesity | 1. Appetite Regulation: SCFAs can stimulate the release of Peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) from gut endocrine cells. These hormones act centrally in the hypothalamus to signal satiety and decrease appetite. |
| 2. Fat Storage and Metabolism: Increased SCFAs-mediated adipocyte activity might favor fat storage in subcutaneous adipose tissue. SCFAs might enhance brown adipose tissue (BAT) activity, promoting thermogenesis and potentially increasing energy expenditure. | |
| 3. Metabolic effects: SCFAs activate GPR41 and GPR43 receptors on fat and immune cells, potentially influencing insulin sensitivity, fat metabolism, inflammation, and, thus, weight regulation. | |
| Type 2 Diabetes | 1. Effects on glucose metabolism: SCFAs act as secretagogues for hormones such as GLP-1 and PYY, which enhance satiety and decrease appetite. GLP-1 enhances insulin secretion from the pancreas and reduces glucagon secretion, lowering blood sugar levels. In the liver, SCFAs inhibit glycolysis and gluconeogenesis, promoting glycogen synthesis and fatty acid oxidation. In skeletal muscle and adipose tissue, they improve glucose uptake and glycogen synthesis. |
| 2. Role in intestinal gluconeogenesis (IGN): SCFAs promote IGN production, which is crucial for glucose and energy homeostasis. | |
| 3. Gut Health: SCFAs promote a healthy gut environment, which may be linked to a lower risk of developing diabetes. | |
| Metabolic Dysfunction–Associated Steatotic Liver Disease | 1. Improved Insulin Sensitivity: SCFAs can activate GPR43 on adipocytes and hepatocytes. GPR43 activation can stimulate insulin signaling pathways, leading to increased glucose uptake by these cells and potentially improving overall insulin sensitivity. SCFAs might also suppress gluconeogenesis in the liver. |
| 2. Anti-inflammatory Effects: SCFAs can modulate the activity of immune cells like macrophages in the liver. They might suppress pro-inflammatory cytokine production (e.g., TNF-α, IL-6) and promote the activity of regulatory T cells, creating an anti-inflammatory environment. SCFAs inhibit the NF-κB signaling pathway, a key player in inflammatory responses. | |
| 3. Gut-Liver Axis: SCFAs might also influence Fibroblast Growth Factor (FGF) signaling pathways in the gut-liver axis, potentially impacting bile acid metabolism and hepatocyte function. SCFAs might stimulate the enterohepatic circulation of bile acids. SCFAs-mediated bile acid signaling can activate FXR, a nuclear receptor in the liver, potentially influencing hepatic lipid metabolism and reducing steatosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. https://doi.org/10.3390/life14050559
Facchin S, Bertin L, Bonazzi E, Lorenzon G, De Barba C, Barberio B, Zingone F, Maniero D, Scarpa M, Ruffolo C, et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life. 2024; 14(5):559. https://doi.org/10.3390/life14050559
Chicago/Turabian StyleFacchin, Sonia, Luisa Bertin, Erica Bonazzi, Greta Lorenzon, Caterina De Barba, Brigida Barberio, Fabiana Zingone, Daria Maniero, Marco Scarpa, Cesare Ruffolo, and et al. 2024. "Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications" Life 14, no. 5: 559. https://doi.org/10.3390/life14050559
APA StyleFacchin, S., Bertin, L., Bonazzi, E., Lorenzon, G., De Barba, C., Barberio, B., Zingone, F., Maniero, D., Scarpa, M., Ruffolo, C., Angriman, I., & Savarino, E. V. (2024). Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life, 14(5), 559. https://doi.org/10.3390/life14050559









