Overall Polyp Detection Rate as a Surrogate Measure for Screening Efficacy Independent of Histopathology: Evidence from National Endoscopy Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Cohort
Definitions
- (1)
- Screening—PDR as the number of screening procedures where 1 or more polyps were detected divided by the total number of screening colonoscopies in average-risk patients >50 years of age; The screening group did not have prior FOBT.
- (2)
- Surveillance—PDR as the proportion of surveillance colonoscopies in which at least 1 polyp was found;
- (3)
- Diagnostic—PDR as the proportion of diagnostic colonoscopies in which at least 1 polyp was found;
- (4)
- Overall—PDR as the number of procedures where 1 or more polyps were detected over the total number of colonoscopies (irrespective of indication).
2.3. Statistical Analysis
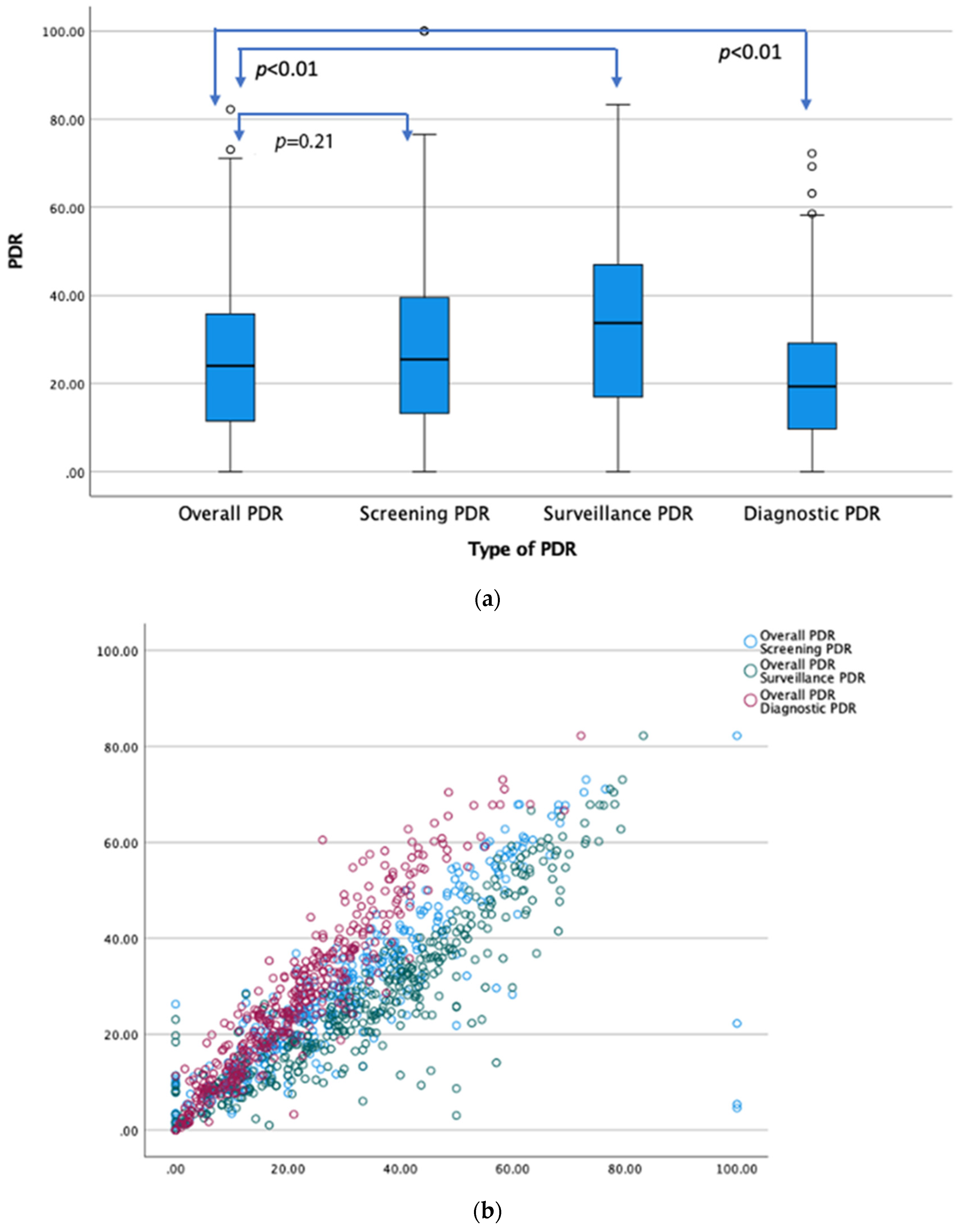
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Keswani, R.N.; Crockett, S.D.; Calderwood, A.H. AGA Clinical Practice Update on Strategies to Improve Quality of Screening and Surveillance Colonoscopy: Expert Review. Gastroenterology 2021, 161, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Bond, J.H.; Winawer, S.; Levin, T.R.; Burt, R.W.; Johnson, D.A.; Kirk, L.M.; Litlin, S.; Lieberman, D.A.; Waye, J.D.; et al. Quality in the Technical Performance of Colonoscopy and the Continuous Quality Improvement Process for Colonoscopy: Recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2002, 97, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Schoenfeld, P.S.; Cohen, J.; Pike, I.M.; Adler, D.G.; Fennerty, M.B.; Lieb, J.G.; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality Indicators for Colonoscopy. Gastrointest. Endosc. 2015, 81, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Butterly, L.F. Colonoscopy: Quality Indicators. Clin. Transl. Gastroenterol. 2015, 6, e77. [Google Scholar] [CrossRef] [PubMed]
- Liem, B.; Gupta, N. Adenoma Detection Rate: The Perfect Colonoscopy Quality Measure or Is There More? Transl. Gastroenterol. Hepatol. 2018, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Holub, J.; Pike, I.M.; Pochapin, M.; Greenwald, D.; Schmitt, C.; Eisen, G. Benchmarking Adenoma Detection Rates for Colonoscopy: Results From a US-Based Registry. Am. J. Gastroenterol. 2021, 116, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, M.M.; Elhanafi, S.; Zuckerman, M.J.; Othman, M.O. Correlation between Adenoma Detection Rate and Novel Quality Indicators for Screening Colonoscopy. A Proposal for Quality Measures Tool Kit. Scand. J. Gastroenterol. 2017, 52, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Delavari, A.; Salimzadeh, H.; Bishehsari, F.; Sobh Rakhshankhah, E.; Delavari, F.; Moossavi, S.; Khosravi, P.; Nasseri-Moghaddam, S.; Merat, S.; Ansari, R.; et al. Mean Polyp per Patient Is an Accurate and Readily Obtainable Surrogate for Adenoma Detection Rate: Results from an Opportunistic Screening Colonoscopy Program. Middle East. J. Dig. Dis. 2015, 7, 214–219. [Google Scholar]
- Murchie, B.; Tandon, K.; Zackria, S.; Wexner, S.D.; O’Rourke, C.; Castro, F.J. Can Polyp Detection Rate Be Used Prospectively as a Marker of Adenoma Detection Rate? Surg. Endosc. 2018, 32, 1141–1148. [Google Scholar] [CrossRef]
- Kaltenbach, T.; Gawron, A.; Meyer, C.S.; Gupta, S.; Shergill, A.; Dominitz, J.A.; Soetikno, R.M.; Nguyen-Vu, T.; Whooley, M.A.; Kahi, C.J. Adenoma Detection Rate (ADR) Irrespective of Indication Is Comparable to Screening ADR: Implications for Quality Monitoring. Clin. Gastroenterol. Hepatol. 2021, 19, 1883–1889.e1. [Google Scholar] [CrossRef] [PubMed]
- Boroff, E.S.; Disbrow, M.; Crowell, M.D.; Ramirez, F.C. Adenoma and Polyp Detection Rates in Colonoscopy According to Indication. Gastroenterol. Res. Pract. 2017, 2017, 7207595. [Google Scholar] [CrossRef] [PubMed]
- Wieszczy, P.; Bugajski, M.; Januszewicz, W.; Rupinska, M.; Szlak, J.; Pisera, M.; Turkot, M.H.; Rupinski, M.; Wojciechowska, U.; Didkowska, J.; et al. Comparison of Quality Measures for Detection of Neoplasia at Screening Colonoscopy. Clin. Gastroenterol. Hepatol. 2023, 21, 200–209.e6. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D. Clinical Outcomes Research Initiative (V3) [Dataset]. NIDDK Central Repository. 2023. [CrossRef]
- Aloysius, M.; Goyal, H.; Nikumbh, T.; Shah, N.J.; Hammoud, G.M.; Mutha, P.; Joseph-Talreja, M.; John, S.; Aswath, G.; Wadhwa, V.; et al. Endoscopic Retrograde Cholangiopancreatography-Related Early Perforations: A Study of Effects of Procedure Duration, Complexity, and Endoscopist Experience. World J. Gastrointest. Endosc. 2023, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Schottinger, J.E.; Jensen, C.D.; Ghai, N.R.; Chubak, J.; Lee, J.K.; Kamineni, A.; Halm, E.A.; Sugg-Skinner, C.; Udaltsova, N.; Zhao, W.K.; et al. Association of Physician Adenoma Detection Rates With Postcolonoscopy Colorectal Cancer. JAMA 2022, 327, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.G.; Rose, T.; Wong, P.; Lane, M.; Frankish, P. Improved Detection of Adenomas and Sessile Serrated Polyps Is Maintained with Continuous Audit of Colonoscopy. BMJ Open Gastroenterol. 2020, 7, e000425. [Google Scholar] [CrossRef] [PubMed]
- Aloysius, M.M.; Nikumbh, T.; Yadukumar, L.; Asija, U.; Shah, N.J.; Aswath, G.; John, S.; Goyal, H. National Trends in the Incidence of Sporadic Malignant Colorectal Polyps in Young Patients (20–49 Years): An 18-Year SEER Database Analysis. Medicina 2024, 60, 673. [Google Scholar] [CrossRef]
- Lieberman, D.; Mascarenhas, R. Adenoma Detection Rate: In Search of Quality Improvement, Not Just Measurement. Gastrointest. Endosc. 2015, 82, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Möschler, O. Software Tools in Endoscopy—Nice to Have or Essential? Visc. Med. 2016, 32, 37–41. [Google Scholar] [CrossRef]
- Murphy, B.; Myers, E.; O’Shea, T.; Feeley, K.; Waldron, B. Correlation between Adenoma Detection Rate and Polyp Detection Rate at Endoscopy in a Non-Screening Population. Sci. Rep. 2020, 10, 2295. [Google Scholar] [CrossRef]
- Anderson, J.C.; Butterly, L.F.; Goodrich, M.; Robinson, C.M.; Weiss, J.E. Differences in Detection Rates of Adenomas and Serrated Polyps in Screening versus Surveillance Colonoscopies, Based on the New Hampshire Colonoscopy Registry. Clin. Gastroenterol. Hepatol. 2013, 11, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Obuch, J.C.; Pigott, C.M.; Ahnen, D.J. Sessile Serrated Polyps: Detection, Eradication, and Prevention of the Evil Twin. Curr. Treat. Options Gastroenterol. 2015, 13, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Barkun, A.N.; Cohen, L.B.; Dominitz, J.A.; Kaltenbach, T.; Martel, M.; Robertson, D.J.; Boland, C.R.; Giardello, F.M.; Lieberman, D.A.; et al. Optimizing Adequacy of Bowel Cleansing for Colonoscopy: Recommendations from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014, 147, 903–924. [Google Scholar] [CrossRef] [PubMed]
- Rai, T.; Navaneethan, U.; Gohel, T.; Podugu, A.; Thota, P.N.; Kiran, R.P.; Lopez, R.; Sanaka, M.R. Effect of Quality of Bowel Preparation on Quality Indicators of Adenoma Detection Rates and Colonoscopy Completion Rates. Gastroenterol. Rep. 2016, 4, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Butterly, L.; Robinson, C.M.; Goodrich, M.; Weiss, J.E. Impact of Fair Bowel Prep on Adenoma and Serrated Polyp Detection: Data from the New Hampshire Colonoscopy Registry Using a Standardized Preparation Quality Rating. Gastrointest. Endosc. 2014, 80, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.J.; Hyun, J.H.; Han, K.S.; Kim, B.C.; Hong, C.W.; Lee, S.-J.; Sohn, D.K. Correlation Between Bowel Preparation and the Adenoma Detection Rate in Screening Colonoscopy. Ann. Coloproctol. 2017, 33, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, A.H.; Thompson, K.D.; Schroy, P.C.; Lieberman, D.A.; Jacobson, B.C. Good Is Better than Excellent: Bowel Preparation Quality and Adenoma Detection Rates. Gastrointest. Endosc. 2015, 81, 691–699.e1. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Ponugoti, P.L. Calculating the Adenoma Detection Rate in Screening Colonoscopies Only: Is It Necessary? Can It Be Gamed? Endoscopy 2017, 49, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Pisegna, J.; Modi, R.; Liang, L.-J.; Atia, M.; Nguyen, M.; Cohen, H.; Ohning, G.; van Oijen, M.; Spiegel, B.M.R. Adenoma Detection Rate Is Necessary but Insufficient for Distinguishing High versus Low Endoscopist Performance. Gastrointest. Endosc. 2013, 77, 71–78. [Google Scholar] [CrossRef]
- Kahi, C.J.; Vemulapalli, K.C.; Johnson, C.S.; Rex, D.K. Improving Measurement of the Adenoma Detection Rate and Adenoma per Colonoscopy Quality Metric: The Indiana University Experience. Gastrointest. Endosc. 2014, 79, 448–454. [Google Scholar] [CrossRef]
- Adler, A.; Wegscheider, K.; Lieberman, D.; Aminalai, A.; Aschenbeck, J.; Drossel, R.; Mayr, M.; Mroß, M.; Scheel, M.; Schröder, A.; et al. Factors Determining the Quality of Screening Colonoscopy: A Prospective Study on Adenoma Detection Rates, from 12,134 Examinations (Berlin Colonoscopy Project 3, BECOP-3). Gut 2013, 62, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Do, A.; Weinberg, J.; Kakkar, A.; Jacobson, B.C. Reliability of Adenoma Detection Rate Is Based on Procedural Volume. Gastrointest. Endosc. 2013, 77, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Gourevitch, R.A.; Morris, M.; Carrell, D.S.; Rose, S.; Shi, Z.; Greer, J.B.; Schoen, R.E.; Mehrotra, A. Endoscopist Factors That Influence Serrated Polyp Detection: A Multicenter Study. Endoscopy 2018, 50, 984–992. [Google Scholar] [CrossRef]
- Penz, D.; Pammer, D.; Waldmann, E.; Asaturi, A.; Szymanska, A.; Trauner, M.; Ferlitsch, M. Association between Endoscopist Adenoma Detection Rate and Serrated Polyp Detection: Retrospective Analysis of over 200,000 Screening Colonoscopies. Endosc. Int. Open 2024, 12, E488–E497. [Google Scholar] [CrossRef] [PubMed]
- Qayed, E.; Vora, R.; Levy, S.; Bostick, R.M. Colonoscopy Procedural Volume Increases Adenoma and Polyp Detection Rates in Gastroenterologytrainees. World J. Gastrointest. Endosc. 2017, 9, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Zorron Cheng Tao Pu, L.; Singh, G.; Rana, K.; Nakamura, M.; Yamamura, T.; Krishnamurthi, S.; Ovenden, A.; Edwards, S.; Ruszkiewicz, A.; Hirooka, Y.; et al. Polyp Detection Rate as a Surrogate for Adenoma and Sessile Serrated Adenoma/Polyp Detection Rates. Gastrointest. Tumors 2020, 7, 74–82. [Google Scholar] [CrossRef]
- Forbes, N.; Boyne, D.J.; Mazurek, M.S.; Hilsden, R.J.; Sutherland, R.L.; Pader, J.; Ruan, Y.; Shaheen, A.A.; Wong, C.; Lamidi, M.; et al. Association Between Endoscopist Annual Procedure Volume and Colonoscopy Quality: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 2192–2208.e12. [Google Scholar] [CrossRef] [PubMed]
- Gessl, I.; Waldmann, E.; Penz, D.; Majcher, B.; Dokladanska, A.; Hinterberger, A.; Szymanska, A.; Ferlitsch, A.; Trauner, M.; Ferlitsch, M. Evaluation of Adenomas per Colonoscopy and Adenomas per Positive Participant as New Quality Parameters in Screening Colonoscopy. Gastrointest. Endosc. 2019, 89, 496–502. [Google Scholar] [CrossRef]
- Niv, Y. Polyp Detection Rate May Predict Adenoma Detection Rate: A Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 247–251. [Google Scholar] [CrossRef]
- Williams, J.E.; Holub, J.L.; Faigel, D.O. Polypectomy Rate Is a Valid Quality Measure for Colonoscopy: Results from a National Endoscopy Database. Gastrointest. Endosc. 2012, 75, 576–582. [Google Scholar] [CrossRef]
- Simmons, D.T.; Harewood, G.C.; Baron, T.H.; Bret, P.T.; Wang, K.K.; Enders, F.B.; Ott, B.J. Impact of Endoscopist Withdrawal Speed On Polyp Yield: Implications for Optimal Colonoscopy Withdrawal Time. Gastrointest. Endosc. 2006, 63, AB81. [Google Scholar] [CrossRef]
- Sanchez, W.; Harewood, G.C.; Petersen, B.T. Evaluation of Polyp Detection in Relation to Procedure Time of Screening or Surveillance Colonoscopy. Off. J. Am. Coll. Gastroenterol.|ACG 2004, 99, 1941. [Google Scholar] [CrossRef]
- Ng, S.; Sreenivasan, A.K.; Pecoriello, J.; Liang, P.S. Polyp Detection Rate Correlates Strongly with Adenoma Detection Rate in Trainee Endoscopists. Dig. Dis. Sci. 2020, 65, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Mikoviny Kajzrlikova, I.; Vitek, P.; Chalupa, J.; Kuchar, J.; Platos, J.; Reha, P.; Klvana, P. Correlation between ADR of Screening and All Colonoscopies. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2021, 165, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Scott, F.I.; Espinoza, J.; Laborde, S.; Chambers, M.; Wani, S.; Edmundowicz, S.; Austin, G.; Pell, J.; Patel, S.G. Leveraging Electronic Medical Record Functionality to Capture Adenoma Detection Rate. Sci. Rep. 2022, 12, 9679. [Google Scholar] [CrossRef] [PubMed]
- Gohel, T.D.; Burke, C.A.; Lankaala, P.; Podugu, A.; Kiran, R.P.; Thota, P.N.; Lopez, R.; Sanaka, M.R. Polypectomy Rate: A Surrogate for Adenoma Detection Rate Varies by Colon Segment, Gender, and Endoscopist. Clin. Gastroenterol. Hepatol. 2014, 12, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Hoff, G.; Botteri, E.; Høie, O.; Garborg, K.; Wiig, H.; Huppertz-Hauss, G.; Moritz, V.; Bretthauer, M.; Holme, Ø. Polyp Detection Rates as Quality Indicator in Clinical versus Screening Colonoscopy. Endosc. Int. Open 2019, 7, E195–E202. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.R.-T.; Gralnek, I.M.; Eisen, G.M.; Williams, J.L.; Holub, J.L. Endoscopic Hemostasis Is Rarely Used for Hematochezia: A Population-Based Study from the Clinical Outcomes Research Initiative National Endoscopic Database. Gastrointest. Endosc. 2014, 79, 317–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, Z.L.; Nickel, K.B.; Olsen, M.A.; Vargo, J.J.; Kushnir, V.M. Type of Sedation and the Need for Unplanned Interventions during ERCP: Analysis of the Clinical Outcomes Research Initiative National Endoscopic Database (CORI-NED). Frontline Gastroenterol. 2020, 11, 104–110. [Google Scholar] [CrossRef]
- Joseph, D.A.; Meester, R.G.S.; Zauber, A.G.; Manninen, D.L.; Winges, L.; Dong, F.B.; Peaker, B.; van Ballegooijen, M. Colorectal Cancer Screening: Estimated Future Colonoscopy Need and Current Volume and Capacity. Cancer 2016, 122, 2479–2486. [Google Scholar] [CrossRef]
- Wang, P.; Berzin, T.M.; Glissen Brown, J.R.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-Time Automatic Detection System Increases Colonoscopic Polyp and Adenoma Detection Rates: A Prospective Randomised Controlled Study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.F.; Chapados, N.; Soudan, F.; Oertel, C.; Linares Pérez, M.; Kelly, R.; Iqbal, N.; Chandelier, F.; Rex, D.K. Real-Time Differentiation of Adenomatous and Hyperplastic Diminutive Colorectal Polyps during Analysis of Unaltered Videos of Standard Colonoscopy Using a Deep Learning Model. Gut 2019, 68, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; McDonald, C.; Guizzetti, L.; Iansavichene, A.; Brahmania, M.; Khanna, N.; Wilson, A.; Jairath, V.; Sey, M. Endoscopy Unit Level Interventions to Improve Adenoma Detection Rate: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 3238–3257. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloysius, M.; Goyal, H.; Nikumbh, T.; Shah, N.; Aswath, G.; John, S.; Bapaye, A.; Guha, S.; Thosani, N. Overall Polyp Detection Rate as a Surrogate Measure for Screening Efficacy Independent of Histopathology: Evidence from National Endoscopy Database. Life 2024, 14, 654. https://doi.org/10.3390/life14060654
Aloysius M, Goyal H, Nikumbh T, Shah N, Aswath G, John S, Bapaye A, Guha S, Thosani N. Overall Polyp Detection Rate as a Surrogate Measure for Screening Efficacy Independent of Histopathology: Evidence from National Endoscopy Database. Life. 2024; 14(6):654. https://doi.org/10.3390/life14060654
Chicago/Turabian StyleAloysius, Mark, Hemant Goyal, Tejas Nikumbh, Niraj Shah, Ganesh Aswath, Savio John, Amol Bapaye, Sushovan Guha, and Nirav Thosani. 2024. "Overall Polyp Detection Rate as a Surrogate Measure for Screening Efficacy Independent of Histopathology: Evidence from National Endoscopy Database" Life 14, no. 6: 654. https://doi.org/10.3390/life14060654
APA StyleAloysius, M., Goyal, H., Nikumbh, T., Shah, N., Aswath, G., John, S., Bapaye, A., Guha, S., & Thosani, N. (2024). Overall Polyp Detection Rate as a Surrogate Measure for Screening Efficacy Independent of Histopathology: Evidence from National Endoscopy Database. Life, 14(6), 654. https://doi.org/10.3390/life14060654







